Abstract
Objectives
Periodontal disease is prevalent in patients with chronic kidney disease (CKD) and potentially associated with kidney function decline. However, it is uncertain whether periodontal disease affects the risk of mortality and morbidity in patients with advanced CKD.
Materials and methods
Taiwan’s National Health Insurance Research Database was used to conduct a nationwide population-based cohort study. Propensity score matching procedures were performed to select people with stage 5 CKD and to compare the long-term risk of mortality, end-stage renal disease, and major adverse cardiovascular events (MACE) between people with and without periodontal disease. Multivariable Cox regression analyses were conducted to calculate the adjusted hazard ratio (aHR) with 95% confidence interval (CI) for the outcome of interest.
Results
A total of 8119 subjects with stage 5 CKD were initially included. After matching to demographic and clinical covariates, 1254 subjects with 7099 person-years of follow-up were selected for analyses. Periodontal disease was not associated with long-term risks of all-cause mortality (aHR: 0.77, 95% CI: 0.49–1.22), progression to end-stage renal disease (aHR: 0.91, 95% CI: 0.75–1.10), or MACE (aHR: 1.18, 95% CI: 0.91–1.53). These findings were generally consistent across subgroups of age, sex, comorbid diabetes, uses of systemic antibiotic, and different dental procedures.
Conclusions
Periodontal disease is not a predictor for long-term mortality or morbidity in patients with advanced CKD.
Clinical relevance
These results provide important evidence to elucidate the relationship between periodontitis and critical clinical outcomes of advanced CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a leading cause of premature mortality and disability, affecting approximately 700 million individuals and imposing a huge economic burden on healthcare system worldwide [1]. CKD is projected to increase further due to the aging population and the growing prevalence of diabetes [2]. For patients with CKD, the risks of mortality, cardiovascular events, and hospitalization increase as the glomerular filtration rate (GFR) decreases below 60 mL·min−1 [3]. Patients with pre-dialysis CKD have a particularly high burden of severe symptoms with life expectancies of only 4 to 6 years [4]. However, there are currently few proven treatments in improving the clinical outcomes of pre-dialysis CKD [5, 6].
Periodontal disease is common and under-recognized in patients with CKD [7, 8]. Periodontal disease may cause systemic inflammation and malnutrition, and it has been demonstrated as a potential prognostic factor for health outcomes in the context of renal failure [9, 10]. Studies have shown that periodontal disease is associated with kidney function decline [11,12,13,14,15,16,17], cardiovascular diseases [18], and mortality [12, 19, 20] in CKD patients. However, it is still not fully clarified whether periodontal disease associates with mortality and morbidity in advanced CKD due to several limitations of previous studies, including small patient samples (< 1000 subjects) [13, 14, 16], cross-sectional design [11, 14, 16], unmatched statistical methodology [11,12,13,14,15,16,17, 19,20,21], insufficient confounding adjustment [14, 16], and restriction to specific populations or institutions [12,13,14, 16]. Identifying modifiable risk factors for adverse events of CKD is crucial for reducing its burden, but evidence regarding the association between periodontal disease and CKD prognosis is limited.
Accordingly, Taiwan’s National Health Insurance Research Database (NHIRD) was used to conduct a nationwide population-based cohort study to investigate the putative association of periodontal disease with the long-term risks of mortality, progression to end-stage renal disease (ESRD), and major adverse cardiovascular events (MACE) in patients with advanced CKD. Based on the existing evidence [11,12,13,14,15,16,17,18,19,20], we hypothesized that periodontal disease was associated with greater risks of mortality and morbidity in patients with advanced CKD. The predictors of critical outcomes were also investigated and potentially confounding factors were further controlled using sound analytical methodology.
Materials and methods
Source of data
Taiwan’s National Health Insurance program was launched in March 1995 and covered more than 99% of 23.4 million Taiwan residents at the end of 2013. The NHIRD contains comprehensive data of the insured individuals, including demographic characteristics (date of birth, sex, and residential location) and claims data (outpatient and inpatient care, physicians’ primary and secondary diagnoses, medical prescriptions, and treatment procedures). Research articles based on this database have been broadly accepted in prominent scientific journals worldwide [21,22,23,24]. For the protection of personal privacy, a unique identification number is assigned to each beneficiary and enciphered before the data are released for research purposes. In this study, the unique identification number was employed to link all the medical records of each beneficiary. We employed three Longitudinal Health Insurance Databases (LHID2000, LHID2005, and LHID2010), which randomly sampled 1 million subjects from the original NHIRD in the years 2000, 2005, and 2010, respectively. The LHIDs include the most updated medical claims of sampled beneficiaries since 1997. The representativeness of LHIDs has been validated by Taiwan’s National Health Research Institutes [25].
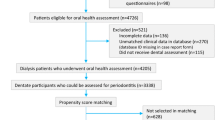
Patient selection
Inclusion criteria were patients aged ≥ 20 years who had a record of CKD using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes and had concurrent uses of erythropoiesis-stimulating agents covered by health insurance between January 1, 2002, and June 30, 2013. (Supplementary Table 1). Only patients with the CKD diagnosis made at least 2 times were included. In the reimbursement regulations of Taiwan’s National Health Insurance, erythropoiesis-stimulating agents can be initiated when non-dialyzed CKD patients have a serum creatinine level > 530 μmol·L−1 (approximately equivalent to stage 5 CKD) and anemia defined by packed-cell volume < 28%, to maintain a packed-cell volume not exceeding 36%. Study has shown that 85% of patients with non-dialyzed stage 5 CKD used erythropoiesis-stimulating agents in Taiwan [4]. Therefore, this selected cohort was highly representative of people with stage 5 CKD. We defined the date of the first prescription of erythropoiesis-stimulating agents as the index date. People receiving renal replacement therapy before the index date were excluded. Patients were further classified into two groups: those with periodontal disease and without periodontal disease (control group). The diagnosis of periodontal disease was defined by the ICD-9-CM codes 523.0, 523.1, 523.3, and 523.4 made within 24 months before the index date. In this study, we considered patients with periodontal disease who had at least two outpatient service claims from board-certified dentists. Similar approaches have been broadly adopted and validated in previous studies [26,27,28]. In Taiwan, dentists considered medical and dental history, findings of periodontal examination, and radiographic analysis to identify patients with periodontal disease in the clinical settings [29]. The periodontal examination typically included measurements of gingival topography (probing depth, recession, and attachment level) and assessments of gingival inflammation, dental plaque and calculus distributions, and subgingival health (bleeding on probing and suppuration) [29]. Patients were excluded from the control group if they had undergone any subgingival curettage or other periodontal procedures within 24 months before the index date.
Covariates
Monthly premium was classified into $0–500, $501–800, and > $800 US dollars. The ICD-9-CM codes of physicians’ diagnoses within 24 months before the index date were used to identify the history of the following coexisting diseases: hypertension, diabetes mellitus, ischemic heart disease, atherosclerosis, cardiac dysrhythmias, heart failure, liver cirrhosis, chronic obstructive pulmonary disease, cerebrovascular disease, dyslipidemia, malignancies, and mental disorders (Supplementary Table 1). These coexisting diseases were chosen based on data availability, physiological plausibility, and the existing literature. Proxies for lifestyle factors were smoking, alcohol abuse, malnutrition, and obesity. We also included the dental procedures performed within 24 months before the index date, including dental scaling, subgingival curettage, periodontal flap surgery, other periodontal procedures, teeth extraction, odontectomy, and emergent dental care. Four types of systemic antibiotics prescribed within 24 months before the index date were included in the analysis: amoxicillin, cephalexin, clindamycin, and metronidazole [30].
Outcome measurement
Primary outcome is all-cause mortality. Secondary outcomes include progression to ESRD, defined as the date patients began dialysis for at least 90 days. According to Taiwan’s Health Insurance reimbursement regulations, patients can undergo long-term dialysis only when their estimated GFR value drops below 5 mL·min−1 per 1.73 m2. Finally, because of the potential association of periodontal disease with cardiovascular diseases and infections [18], we also ascertained the number of patients hospitalized for MACE, acute renal failure, septicemia or sepsis, urinary tract infection, and pyelonephritis (Supplementary Table 1). MACE include acute myocardial infarction, new-onset heart failure, stroke, and cardiac dysrhythmias. The included patients were followed up until December 31, 2013.
Statistical analysis
According to Schoenfeld’s formula for sample size estimation of proportional hazards regression models [31], at least 112 events are needed to attain a power of 0.8 assuming an alpha level of 0.05 and hazard ratio (HR) of mortality 1.7 [19]. Of note, a total of 179 deaths occurred during the study period (87 and 92 in periodontal disease and control groups, respectively), which has met the requirement of sample size. Matching procedures with propensity score were conducted to balance the distribution of age, sex, monthly premium, coexisting diseases, and lifestyle factors between CKD patients with and without periodontal disease. A non-parsimonious multivariable logistic regression model was applied to estimate a propensity score for subjects with or without periodontal disease. We matched subjects with periodontal disease to controls (case-control ratio 1:1) using a greedy matching algorithm within a tolerance limit of 0.05 and without replacement [32]. The distributions of baseline characteristics in propensity score–matched samples were compared between subjects with and without periodontal disease by using standardized difference [33]. The cumulative incidences of mortality and morbidity were illustrated with the Kaplan-Meier method and compared between groups using log-rank tests. Adjusted hazard ratio and 95% confidence interval (CI) of CKD outcomes associated with periodontal disease were calculated by multivariable Cox proportional hazards regression models. Stratified analyses were also conducted by age, sex, comorbid diabetes, uses of systemic antibiotic, and different dental procedures to examine the outcomes of patients with periodontal disease within these strata. Finally, we applied stepwise backward variable elimination processes with an entry probability of 0.05 and removal probability of 0.1 to identify the independent factors associated with all-cause mortality, progression to ESRD, and MACE. A two-sided level of 0.05 was considered statistically significant. All the statistical analyses were conducted using Statistics Analysis System (SAS), Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
After the matching process, a total of 627 matched pairs were selected for analyses (Fig. 1). The follow-up time was median 5.4 years (interquartile range 2.8–8.5 years), and the cumulative time at risk of all matched subjects was 7099 person-years. Table 1 shows the baseline characteristics of the included subjects with and without periodontal disease. The distributions of demographics, coexisting diseases, and lifestyle factors were well balanced after propensity score matching. Of note, people with periodontal disease were more likely to receive systemic antibiotics and dental procedures compared with control group (Supplementary Table 2).
Periodontal disease and all-cause mortality
One-year, 3-year, and 5-year cumulative incidences of all-cause mortality were 2.9% (95% CI: 1.5–4.3), 6.1% (4.1–8.1), and 10.2% (7.7–12.7) for people with periodontal disease, and 3.6% (2.2–5.0), 8.5% (6.1–10.9), and 12.9% (10.0–15.8) for controls (Fig. 2a). The duration between the index date and all-cause mortality was median 4.0 years (interquartile range 1.3–6.1) for people with periodontal disease and 2.8 years (1.1–5.3) for controls. The association between periodontal disease and all-cause mortality was non-significant either in the univariate model (crude HR: 0.87, 95% CI: 0.65–1.17) or in the multivariable model (adjusted HR: 0.77, 95% CI: 0.49–1.22) (Table 2). Similarly, there was no association between periodontal disease and mortality in the subgroups of age, sex, comorbid diabetes, uses of systemic antibiotics, or dental scaling (Table 3). Supplementary Table 3 shows the risk of all-cause mortality in patients with periodontal disease who had various dental treatments.
Periodontal disease and progression to ESRD
One-year, 3-year, and 5-year cumulative incidences of onset of ESRD were 59.9% (95% CI: 56.0–63.8), 82.6% (79.5–85.7), and 88.2% (85.3–91.1) for people with periodontal disease, and 61.4% (57.5–65.3), 82.9% (79.6–86.2), and 87.7% (84.6–90.8) for controls (Fig. 2b). The duration between the index date and onset of ESRD was median 0.6 year (interquartile range 0.2–1.2) for people with periodontal disease and 0.5 year (0.2–1.0) for controls. The association of periodontal disease with ESRD was non-significant either in the univariate model (crude HR: 1.00, 95% CI: 0.88–1.13) or in the multivariable model (adjusted HR: 0.91, 95% CI: 0.75–1.10) (Table 2). Supplementary Table 4 shows the risk of ESRD in periodontally diseased patients undergoing various dental procedures.
Periodontal disease and MACE
One-year, 3-year, and 5-year cumulative incidences of MACE were 17.4% (95% CI: 14.5–20.3), 30.6% (26.9–34.3), and 40.4% (36.1–44.7) for people with periodontal disease, and 16.4% (13.5–19.3), 30.1% (26.4–33.8), and 36.8% (32.7–40.9) for controls (Fig. 2c). The duration between the index date and MACE was median 1.5 years (interquartile range 0.5–3.9) for people with periodontal disease and 1.3 years (0.3–3.2) for controls. The association of periodontal disease with MACE was non-significant either in the univariate model (crude HR: 1.06, 95% CI: 0.89–1.27) or in the multivariable model (adjusted HR: 1.18, 95% CI: 0.91–1.53) (Table 2). Supplementary Table 5 shows the MACE risk of periodontal disease patients with different dental procedures.
Periodontal disease and infection
There was no association between periodontal disease and the risk of hospitalizations for septicemia or sepsis (adjusted HR: 0.86, 95% CI: 0.63–1.17), urinary tract infection (adjusted HR: 1.21, 95% CI: 0.85–1.72), pyelonephritis (adjusted HR: 0.94, 95% CI: 0.28–3.14), or acute renal failure (adjusted HR: 0.74, 95% CI: 0.46–1.20) (Table 2).
Independent factors associated with mortality and morbidity
Supplementary Table 6 shows the results of backward variable elimination processes. Age and diabetes were both associated with the risk of all-cause mortality, progression to ESRD, and MACE. Of note, prior receipts of odontectomy and emergent dental care were linked to higher risks of ESRD (adjusted HR: 5.54, 95% CI: 2.44–12.58) and MACE (adjusted HR: 1.31, 95% CI: 1.06–1.63), respectively.
Discussion
This study found no evidence to support an association between periodontal disease and long-term risks of mortality, progression to ESRD, or MACE in patients with stage 5 CKD. Our results also suggested that periodontal disease was not associated with the risk of hospitalizations for sepsis or urinary tract infection in advanced CKD patients. Our study has two strengths to evaluate the relationship between periodontal disease and prognosis of advanced CKD. First, a nationwide dataset was used to increase the generalizability of analytical results. Second, we conducted robust statistical analyses using propensity score matching to balance the distribution of baseline characteristics. Our results provide important evidence to elucidate the relationship between periodontitis and important clinical outcomes of advanced CKD patients.
To our knowledge, this is the first study to focus on the putative impact of periodontal disease on patients with pre-dialysis CKD, who have a particularly high burden of symptoms among populations of kidney disease [4]. In addition, propensity score matching analyses were used to minimize potential confounding effect from periodontitis-related systemic comorbidities, which have not been performed in previous studies [11,12,13,14,15,16,17, 19,20,21, 34]. Compared with previous cross-sectional studies [11, 14, 16], we used a longitudinal data with a follow-up interval up to 12 years to better examine the temporal relationship and causality. Our study found no evidence to support the hypothesis that periodontitis is an important risk factor for mortality, kidney function decline, and cardiovascular events of CKD, agreeing with one recent report [34] but not the others [11,12,13,14,15,16,17, 19,20,21]. Prior studies claimed a relationship between periodontal disease and mortality or kidney function decline in patients with old age [12, 13, 16] and diabetes mellitus [15, 20]. However, our subgroup analyses did not reveal such an association.
There might be several reasons why we did not identify an effect of periodontitis on mortality and kidney function as reported in prior studies [11,12,13,14,15,16,17, 19,20,21]. First, the etiology of CKD might vary among the included patients and possibly affect the CKD progression and mortality risk [35]. Second, the association between periodontal status and CKD outcomes might be attenuated by improvements in the medical care of CKD and periodontitis in Taiwan during the follow-up period [29, 36]. Third, our analyses have adjusted for a variety of periodontitis-related comorbidities (such as diabetes, ischemic heart disease, and atherosclerosis) in the models, which might conceal effects of periodontitis, which are (if present) only minor. Fourth, the disease in stage 5 CKD might be too advanced for periodontal disease to exert its detrimental effect on the selected outcomes [4].
Studies have shown that intensive periodontal therapy may improve clinical periodontal parameters and reduce circulating inflammatory response in ESRD patients [37, 38]. A clinical trial further showed that non-surgical periodontal treatment may benefit the kidney function, assessed by GFR using cystatin C, in patients with chronic periodontitis [39]. However, our analyses did not demonstrate an association between CKD outcomes and periodontal therapy or dental scaling. The current evidence supporting the therapeutic effect of periodontal care on kidney function and survival in CKD patients is still insufficient.
Whether periodontitis negatively influences kidney function in people without CKD remains an issue of great debate, with increased risks reported in some studies [12, 13, 17] but not in others [34]. Two studies focused on the elderly population and both reported a significant association between periodontal disease and kidney function decline [12, 13]. In contrast, the evidence regarding the relationship between periodontal disease and kidney function is conflicting among non-elderly people [17, 34]. The discrepant findings might result from the different measures of kidney function (formulas based on only serum creatinine levels or combination of serum creatinine and cystatin C levels) and potential measurement errors of periodontal disease due to increased tooth loss in elderly population.
There are limitations to our study. First, our data did not contain information about physical measures and biochemical laboratory measures that were not covered by NHIRD. Therefore, our analyses could not further adjust for GFR values [3], periodontal metrics, severity of periodontal disease [40], and level of inflammatory markers [9, 10], which might affect the risk of mortality and morbidity in CKD patients. There might exist patients with minor forms of periodontal disease or with a slower progression rate in the control group. Second, our analyses did not consider the pathophysiology of CKD and causes of death due to the difficulty of data acquisition. Third, our dataset did not include patients with periodontal disease who did not seek conventional dental care due to mild symptoms. Fourth, it is possible that our analyses could not detect a subtler effect of periodontal disease on CKD prognosis, which might be overridden by the significant comorbidities of CKD. Fifth, our results may not be applicable to non-Asian populations [41]. Finally, our cohort was followed up merely until the end of 2013 due to the regulations of NHIRD. Nevertheless, a previous study has shown that over half events of ESRD occurred within 4 years after the diagnosis of CKD [42]. In our study, patients were followed up for median 5.4 years, and reliable estimated results can still be obtained in the context of survival analysis.
In conclusion, this study found no evidence for an association between periodontal disease and long-term mortality, progression to ESRD, or MACE in patients with stage 5 CKD in a general population examined over 12 years. Our analyses showed that periodontal disease is not an independent risk factor for mortality and morbidity in patients with advanced CKD. However, these results should be interpreted with caution because the periodontal metrics were not available. Future studies should focus on populations with early-stage CKD rather than predominantly pre-dialysis patients.
References
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 392:1789–1858. https://doi.org/10.1016/S0140-6736(18)32279-7
O’Sullivan ED, Hughes J, Ferenbach DA (2017) Renal aging: causes and consequences. J Am Soc Nephrol 28:407–420. https://doi.org/10.1681/ASN.2015121308
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351:1296–1305. https://doi.org/10.1056/NEJMoa041031
Hung SC, Chang YK, Liu JS, Kuo KL, Chen YH, Hsu CC, Tarng DC (2015) Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. Lancet Diabetes Endocrinol 3:605–614. https://doi.org/10.1016/S2213-8587(15)00123-0
Cody JD, Hodson EM (2016) Recombinant human erythropoietin versus placebo or no treatment for the anaemia of chronic kidney disease in people not requiring dialysis. Cochrane Database Syst Rev 2016:CD003266. https://doi.org/10.1002/14651858.CD003266.pub3
Campbell KL, Ash S, Bauer JD (2008) The impact of nutrition intervention on quality of life in pre-dialysis chronic kidney disease patients. Clin Nutr 27:537–544. https://doi.org/10.1016/j.clnu.2008.05.002
Palmer SC, Ruospo M, Wong G, Craig JC, Petruzzi M, De Benedittis M, Ford P, Johnson DW, Tonelli M, Natale P, Saglimbene V, Pellegrini F, Celia E, Gelfman R, Leal MR, Torok M, Stroumza P, Frantzen L, Bednarek-Skublewska A, Dulawa J, Del Castillo D, Bernat AG, Hegbrant J, Wollheim C, Schon S, Gargano L, Bots CP, Strippoli GF, ORALD Investigators (2016) Patterns of oral disease in adults with chronic kidney disease treated with hemodialysis. Nephrol Dial Transplant 31:1647–1653. https://doi.org/10.1093/ndt/gfv413
Borawski J, Wilczyńska-Borawska M, Stokowska W, Myśliwiec M (2007) The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant 22:457–464. https://doi.org/10.1093/ndt/gfl676
Ioannidou E, Swede H, Dongari-Bagtzoglou A (2011) Periodontitis predicts elevated C-reactive protein levels in chronic kidney disease. J Dent Res 90:1411–1415. https://doi.org/10.1177/0022034511423394
Yoshihara A, Iwasaki M, Miyazaki H, Nakamura K (2016) Bidirectional relationship between renal function and periodontal disease in older Japanese women. J Clin Periodontol 43:720–726. https://doi.org/10.1111/jcpe.12576
Ioannidou E, Hall Y, Swede H, Himmelfarb J (2013) Periodontitis associated with chronic kidney disease among Mexican Americans. J Public Health Dent 73:112–119. https://doi.org/10.1111/j.1752-7325.2012.00350.x
Chen YT, Shih CJ, Ou SM, Hung SC, Lin CH, Tarng DC, Taiwan Geriatric Kidney Disease (TGKD) Research Group (2015) Periodontal disease and risks of kidney function decline and mortality in older people: a community-based cohort study. Am J Kidney Dis 66:223–230. https://doi.org/10.1053/j.ajkd.2015.01.010
Grubbs V, Vittinghoff E, Taylor G, Kritz-Silverstein D, Powe N, Bibbins-Domingo K, Ishani A, Cummings SR, Osteoporotic Fractures in Men (MrOS) Study Research Group (2016) The association of periodontal disease with kidney function decline: a longitudinal retrospective analysis of the MrOS dental study. Nephrol Dial Transplant 31:466–472. https://doi.org/10.1093/ndt/gfv312
Ausavarungnirun R, Wisetsin S, Rongkiettechakorn N, Chaichalermsak S, Udompol U, Rattanasompattikul M (2016) Association of dental and periodontal disease with chronic kidney disease in patients of a single, tertiary care centre in Thailand. BMJ Open 6:e011836. https://doi.org/10.1136/bmjopen-2016-011836
Chang JF, Yeh JC, Chiu YL, Liou JC, Hsiung JR, Tung TH (2017) Periodontal pocket depth, hyperglycemia, and progression of chronic kidney disease: a population-based longitudinal study. Am J Med 130:61–69. https://doi.org/10.1016/j.amjmed.2016.08.024
Yoshihara A, Sugita N, Iwasaki M, Wang Y, Miyazaki H, Yoshie H, Nakamura K (2017) Relationship between renal function and periodontal disease in community-dwelling elderly women with different genotypes. J Clin Periodontol 44:484–489. https://doi.org/10.1111/jcpe.12708
Lertpimonchai A, Rattanasiri S, Tamsailom S, Champaiboon C, Ingsathit A, Kitiyakara C, Limpianunchai A, Attia J, Sritara P, Thakkinstian A (2019) Periodontitis as the risk factor of chronic kidney disease: mediation analysis. J Clin Periodontol 46:631–639. https://doi.org/10.1111/jcpe.13114
Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D’Aiuto F, Bouchard P, Chapple I, Dietrich T, Gotsman I, Graziani F, Herrera D, Loos B, Madianos P, Michel JB, Perel P, Pieske B, Shapira L, Shechter M, Tonetti M, Vlachopoulos C, Wimmer G (2020) Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol 47:268–288. https://doi.org/10.1111/jcpe.13189
Palmer SC, Ruospo M, Wong G, Craig JC, Petruzzi M, De Benedittis M, Ford P, Johnson DW, Tonelli M, Natale P, Saglimbene V, Pellegrini F, Celia E, Gelfman R, Leal MR, Torok M, Stroumza P, Bednarek-Skublewska A, Dulawa J, Frantzen L, Ferrari JN, Del Castillo D, Bernat AG, Hegbrant J, Wollheim C, Gargano L, Bots CP, Strippoli GF, ORAL-D Study Investigators (2015) Dental health and mortality in people with end-stage kidney disease treated with hemodialysis: a multinational cohort study. Am J Kidney Dis 66:666–676. https://doi.org/10.1053/j.ajkd.2015.04.051
Sharma P, Dietrich T, Ferro CJ, Cockwell P, Chapple IL (2016) Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol 43:104–113. https://doi.org/10.1111/jcpe.12502
Dai YX, Tai YH, Chen CC, Chang YT, Chen TJ, Chen MH (2020) Bidirectional association between alopecia areata and major depressive disorder among probands and unaffected siblings: a nationwide population-based study. J Am Acad Dermatol 82:1131–1137. https://doi.org/10.1016/j.jaad.2019.11.064
Dai YX, Tai YH, Chang YT, Chen TJ, Chen MH (2020) Association between major depressive disorder and subsequent autoimmune skin diseases: a nationwide population-based cohort study. J Affect Disord 274:334–338. https://doi.org/10.1016/j.jad.2020.05.070
Tai YH, Chang CC, Yeh CC, Sung LC, Hu CJ, Cherng YG, Chen TL, Liao CC (2020) Long-term risk of stroke and poststroke outcomes in patients with heart failure: two nationwide studies. Clin Epidemiol 12:1235–1244. https://doi.org/10.2147/CLEP.S261179
Wu YM, Kuo HC, Li CC, Wu HL, Chen JT, Cherng YG, Chen TJ, Dai YX, Liu HY, Tai YH (2020) Preexisting dementia is associated with increased risks of mortality and morbidity following major surgery: a nationwide propensity score matching study. Int J Environ Res Public Health 17:8431. https://doi.org/10.3390/ijerph17228431
National Health Insurance Research Database. Data subsets. Available from: https://nhird.nhri.org.tw/en/Data_Subsets.html. Accessed January 6, 2021.
Lee CY, Chang CC, Lin CS, Yeh CC, Hu CJ, Wu CZ, Chen TL, Liao CC (2020) Risk of dementia in patients with periodontitis and related protective factors: a nationwide retrospective cohort study. J Clin Periodontol 47:1428–1436. https://doi.org/10.1111/jcpe.13372
Lin HW, Chen CM, Yeh YC, Chen YY, Guo RY, Lin YP, Li YC (2019) Dental treatment procedures for periodontal disease and the subsequent risk of ischaemic stroke: a retrospective population-based cohort study. J Clin Periodontol 46:642–649. https://doi.org/10.1111/jcpe.13113
Lee YL, Hu HY, Chou P, Chu D (2015) Dental prophylaxis decreases the risk of acute myocardial infarction: a nationwide population-based study in Taiwan. Clin Interv Aging 10:175–182. https://doi.org/10.2147/CIA.S67854
Chan CL, You HJ, Lian HJ, Huang CH (2016) Patients receiving comprehensive periodontal treatment have better clinical outcomes than patients receiving conventional periodontal treatment. J Formos Med Assoc 115:152–162. https://doi.org/10.1016/j.jfma.2015.10.017
Lockhart PB, Tampi MP, Abt E, Aminoshariae A, Durkin MJ, Fouad AF, Gopal P, Hatten BW, Kennedy E, Lang MS, Patton LL, Paumier T, Suda KJ, Pilcher L, Urquhart O, O’Brien KK, Carrasco-Labra A (2019) Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: a report from the American Dental Association. J Am Dent Assoc 150:906–921. https://doi.org/10.1016/j.adaj.2019.08.020
Schoenfeld DA (1983) Sample-size formula for the proportional-hazards regression model. Biometrics 39:499–503
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424. https://doi.org/10.1080/00273171.2011.568786
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107. https://doi.org/10.1002/sim.3697
Wangerin C, Pink C, Endlich K, Rettig R, Stracke S, Nauck M, Völzke H, Kocher T, Holtfreter B (2019) Long-term association of periodontitis with decreased kidney function. Am J Kidney Dis 73:513–524. https://doi.org/10.1053/j.ajkd.2018.10.013
Reichel H, Zee J, Tu C, Young E, Pisoni RL, Stengel B, Duttlinger J, Lonnemann G, Robinson BM, Pecoits-Filho R, Fliser D (2020) Chronic kidney disease progression and mortality risk profiles in Germany: results from the Chronic Kidney Disease Outcomes and Practice Patterns Study. Nephrol Dial Transplant 35:803–810. https://doi.org/10.1093/ndt/gfz260
Lin CM, Yang MC, Hwang SJ, Sung JM (2013) Progression of stages 3b-5 chronic kidney disease--preliminary results of Taiwan national pre-ESRD disease management program in Southern Taiwan. J Formos Med Assoc 112:773–782. https://doi.org/10.1016/j.jfma.2013.10.021
Fang F, Wu B, Qu Q, Gao J, Yan W, Huang X, Ma D, Yue J, Chen T, Liu F, Liu Y (2015) The clinical response and systemic effects of non-surgical periodontal therapy in end-stage renal disease patients: a 6-month randomized controlled clinical trial. J Clin Periodontol 42:537–546. https://doi.org/10.1111/jcpe.12411
Wehmeyer MM, Kshirsagar AV, Barros SP, Beck JD, Moss KL, Preisser JS, Offenbacher S (2013) A randomized controlled trial of intensive periodontal therapy on metabolic and inflammatory markers in patients with ESRD: results of an exploratory study. Am J Kidney Dis 61:450–458. https://doi.org/10.1053/j.ajkd.2012.10.021
Graziani F, Cei S, La Ferla F, Vano M, Gabriele M, Tonetti M (2010) Effects of non-surgical periodontal therapy on the glomerular filtration rate of the kidney: an exploratory trial. J Clin Periodontol 37:638–643. https://doi.org/10.1111/j.1600-051X.2010.01578.x
Holmlund A, Holm G, Lind L (2006) Severity of periodontal disease and number of remaining teeth are related to the prevalence of myocardial infarction and hypertension in a study based on 4,254 subjects. J Periodontol 77:1173–1178. https://doi.org/10.1902/jop.2006.050233
Naorungroj S, Slade GD, Divaris K, Heiss G, Offenbacher S, Beck JD (2017) Racial differences in periodontal disease and 10-year self-reported tooth loss among late middle-aged and older adults: the dental ARIC study. J Public Health Dent 77:372–382. https://doi.org/10.1111/jphd.12226
Ekart R, Ferjuc A, Furman B, Gerjevič Š, Bevc S, Hojs R (2013) Chronic kidney disease progression to end stage renal disease: a single center experience of the role of the underlying kidney disease. Ther Apher Dial 17:363–367. https://doi.org/10.1111/1744-9987.12079
Funding
This work was supported by the grants from Shuang Ho Hospital (109IIT-02), Taipei Medical University, New Taipei City, Taiwan, and Ministry of Science and Technology (MOST109-2314-B-038-024), Taipei, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was reviewed and approved by the Institutional Review Board of Taipei Medical University and Taipei Veterans General Hospital in Taiwan (TMU-JIRB-N202010040; IRB-TPEVGH-2013-04-005E).
Consent to participate
Written informed consent was waived by the Institutional Review Board (chair: Professor Chung-Ming Chen of Taipei Medical University and Professor Shung-Tai Ho of Taipei Veterans General Hospital).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 41 kb)
Rights and permissions
About this article
Cite this article
Tai, YH., Chen, JT., Kuo, HC. et al. Periodontal disease and risk of mortality and kidney function decline in advanced chronic kidney disease: a nationwide population-based cohort study. Clin Oral Invest 25, 6259–6268 (2021). https://doi.org/10.1007/s00784-021-03924-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-03924-6