Abstract
Aim
To systematically review the literature to compare the efficacy of air polishing to hand or ultrasonic instrumentation to reduce periodontal inflammation during treatment of residual pockets or supportive periodontal care.
Methods
Electronic searches were performed in five different databases, and two databases were used to capture the “grey literature partially.” Clinical trials that compared the use of an air-polishing device to either conventional scaling and root planing (hand and/or ultrasonic instrumentation) or no treatment during periodontal therapy were included without restriction of year and publication status. The Joanna Briggs Institute instrument for clinical trials was used to appraise the studies critically. The results were submitted to qualitative descriptive analysis. The systematic review protocol was registered in PROSPERO (CRD420220156176).
Results
Electronic searches found 1100 hits published between 2008 and 2019. Thirteen studies were included in the review, out of which four had a follow-up longer than 180 days. Results indicated no differences between the efficacy of air polishing and hand or ultrasonic instruments to reduce periodontal inflammation.
Conclusions
Our findings suggest that there is no difference in the efficacy of air polishing and hand or ultrasonic instrumentation to control biofilm and reduce periodontal inflammation. However, these findings must be carefully interpreted owing to methodological issues, including a short follow-up, and a potential conflict of interest related to industry funding.
Clinical relevance
Air polishing for biofilm control may be used as an alternative to hand and ultrasonic instrumentation to reduce periodontal inflammation during treatment of residual pockets or supportive periodontal care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is a condition associated with the inflammatory destruction of the periodontium that ultimately leads to tooth loss. Periodontitis is clinically characterized by clinical attachment loss (CAL) and bleeding on probing (BOP) accompanied by increased probing pocket depth (PPD) and/or gingival recession [1]. The current paradigm poses that periodontitis results from a polymicrobial dysbiosis with keystone pathogens affecting the virulence of the entire biofilm community [2]. Thus, the inability of the host immune system to eliminate the biofilm insult leads to a complex chronic response with the destruction of bone and periodontal ligament attachment [2]. In addition, some conditions are associated with worsening the ability of the immune system to control the effect of biofilm, including tobacco smoking and diabetes mellitus [1].
The standard periodontitis treatment aims to restore the homeostasis of the immune system by mechanically reducing the microbial load to levels that are compatible with stability and health [3]. This is achieved by (1) professional mechanical biofilm control, (2) training the patient to maintain daily oral hygiene at an adequate level, and (3) performing supportive periodontal therapy to control the biofilm and avoid disease recurrence. Currently, there are several methods available for supra- and subgingival biofilm removal, including hand instruments, sonic and ultrasonic scalers, and air polishing therapy, of which hand instruments and ultrasonic scalers remain as the most commonly used tools for professional biofilm control [4]. However, both hand and ultrasonic instruments are time-consuming and technically demanding and often associated with patient discomfort and pain during and after treatment [5], including hypersensitivity caused by hard tissue loss when scaling the tooth surface [6].
More recently, air polishing devices have become a promising alternative to hand and ultrasonic scalers. An air-polishing device removes biofilm from the tooth surface by spraying compressed air containing water and abrasive particles [5], like glycine, trehalose, erythritol, and sodium bicarbonate. While reducing the clinical time and causing less discomfort and pain for the patients, air polishing devices remove only biofilm, whereas hand and ultrasonic instruments can remove both biofilm and dental calculus. Consequently, it has been suggested that air polishing could be used combined to hand instrumentation during initial periodontal therapy or alone during the treatment of residual pockets after initial therapy or supportive periodontal care. However, there is a gap to be filled in regarding the efficacy of air polishing devices in comparison to hand and ultrasonic instrumentation for the performance of periodontal therapy [7, 8].
Accordingly, this study aimed to systematically review the literature to compare the efficacy of air polishing devices to hand and ultrasonic instrumentation to reduce periodontal inflammation.
Methods
Protocol and registration
This systematic review was performed according to the list of PRISMA (Preferred Reporting Items for Systematic Reviews) recommendations [9] and the Cochrane guidelines [10]. The systematic review protocol was registered in the PROSPERO database under no. CRD420220156176.
Study design and eligibility criteria
This study was a systematic review based on a PICO strategy that aimed to answer the following review question: “Is air-polishing therapy as effective as hand or ultrasonic scaling to reduce periodontal inflammation among patients under periodontal therapy?”, where
-
Population: patients under periodontal therapy;
-
Intervention: air polishing alone or air polishing as an adjunctive therapy;
-
Comparator: manual or ultrasonic scaling;
-
Outcome: main endpoint: probing pocket depth (PPD); secondary endpoints: bleeding on probing (BOP), clinical attachment level (CAL), and plaque index (PI).
Inclusion criteria comprised randomized controlled trials that compared the use of an air-polishing device to either conventional scaling and root planing (using hand instruments and/or ultrasonic devices) or no treatment during periodontal therapy (active phase or supportive therapy) without restriction of year and publication status. Exclusion criteria included studies not using an air-polishing device during periodontal therapy; studies on patients with a systemic commitment (pregnancy, diabetes); studies on dental implants; review articles; letters to the editor/editorials; books/book chapters; abstracts; as well as case reports and series.
Sources of information and search
Cochrane, Embase, PubMed (including MEDLINE), Scopus, and Web of Science databases were used as primary study sources. OpenGrey and ProQuest were used to capture the “grey literature partially.” A manual search was also performed through a systematized analysis of the reference list of the eligible articles. All steps were performed to minimize selection and publication biases.
The MeSH (Medical Subject Headings), DeCS (Health Sciences Descriptors), and Emtree (Embase Subject Headings) resources were used to select appropriate search descriptors. The Boolean operators “AND” and “OR” were used to enhance the research strategy through several combinations (Appendix Table 5). The search strategy included the following descriptors: “Air-Powder,” “Air polishing,” “Air Abrasion,” “Air Abrasion,” “Dental,” “Abrasive Powder,” “Tooth Polishing,” “Dental Polishing,” “Periodontal,” “Periodontitis,” and “Periodontal Diseases.” The bibliographic search ended in June 2020.
Study selection
All references were managed in EndNote X8 (Thomson Reuters, New York, NY, USA). Duplicate references were excluded. Before the assessment, reviewers were calibrated by discussing the eligibility criteria and examining 20% of the retrieved articles to estimate the level of agreement between both reviewers. Only when a kappa > 0.80 was achieved, the reviewers were allowed to start the study selection.
Titles, abstracts, and keywords were screened based on the inclusion and exclusion criteria by two reviewers independently. Lists were compared, and in case of disagreement, a consensus was reached by discussion. Assessment of the full articles identified in the initial screening was performed by the same two reviewers. In addition to the electronic search, a hand search was performed in the reference list of all included studies by the same reviewers.
Data collection
After study selection, predefined data collection worksheets were employed for the assessment of each selected publication. The following data were elicited from the studies: identification (author, year, study location), sample characteristics (population, age), study design, periodontal data (definition, endpoints), characteristics of the therapy (active or supportive, frequency, follow-up), air polishing characteristics (machine, power), manual instrumentation (hand or ultrasound), adverse effects, secondary outcomes, primary results, and source of funding. Whenever necessary, the authors were contacted by e-mail to clarify potential doubts regarding the study methodology or results.
Risk of individual bias of the studies
The Joanna Briggs Institute (JBI) Critical Appraisal Tools for use in JBI Systematic Reviews for randomized clinical trials [11] was used to assess the risk of bias and the individual methodological quality of the selected studies. The same two reviewers assessed each domain independently regarding their potential risk of bias, as recommended by the PRISMA statement [9].
The following criteria were evaluated: (Q.1)Was true randomization used for assignment of participants to treatment groups? (Q.2) Was allocation to treatment groups concealed? (Q.3) Were treatment groups similar at the baseline? (Q.4) Were participants blind to treatment assignment? (Q.5) Were those delivering treatment blind to treatment assignment? Q.6) Were outcomes assessors blind to treatment assignment? Q.7) Were treatment groups treated identically other than the intervention of interest? Q.8) Was follow-up complete and if not, were differences between groups in terms of their follow-up adequately described and analyzed? (Q.9) Were participants analyzed in the groups to which they were randomized? (Q.10) Were outcomes measured in the same way for treatment groups? (Q.11) Were outcomes measured in a reliable way? Q.12) Was appropriate statistical analysis used? (Q.13) Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial. In addition to the JBI instrument questions, we also assessed whether the study provided a sample size calculation based on previous studies and had a follow-up longer than 180 days.
Each study was categorized according to the percentage of positive answers to the questions corresponding to the assessment tool. The risk of bias was considered High when the study obtained up to 49% of “yes” answers, Moderate when the study obtained 50% to 69% of “yes” answers, and Low when the study reached more than 70% of “yes” scores.
Summary measures and syntheses of results
Initially, a descriptive analysis of all articles included in this systematic review was carried out. In addition to the descriptive analysis, we attempted to perform a meta-analysis to compare the effect of air polishing to conventional instrumentation on the primary (PPD) and secondary (BOP, CAL, PI) endpoints at different follow-up periods. However, due to the low number of studies with a follow-up longer than 180 days, we were not able to achieve at least three studies per type of treatment (singular use or adjunctive to manual or ultrasonic scaling), which, therefore, precluded the performance of a clinically relevant meta-analysis.
Results
Study selection
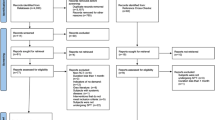
During the first phase of study selection, 1110 hits were found among the seven electronic databases. After removing duplicates, 733 articles remained for the analysis of titles and abstracts. Searches were updated in June 2020, but no new article fulfilled the inclusion criteria. Twenty studies were considered eligible for full-text analysis. At the full-text reading stage, one study was not found, whereas six studies did not meet the inclusion criteria and were, therefore, eliminated. Appendix Table 6 shows the studies excluded after full-text reading with the reasons for exclusion. The references of the 13 potentially eligible studies were evaluated carefully, and no additional article was selected. Finally, 13 studies were included for qualitative analysis. Figure 1 reproduces the process of search, identification, inclusion, and exclusion of articles.
Characteristics of eligible studies
The studies were published between the years of 2008 and 2019, and they were performed in Germany [4, 12], India [13], Sweden [14], Switzerland [6, 15, 16], South Korea [5], USA [17], Greece [18], Turkey [19], and China [20, 21]. Air polishing was compared to hand scaling in 10 studies [4,5,6, 15,16,17,18,19,20,21] and to ultrasonic scaling in three studies [12,13,14]. While eight studies evaluated the effect of air polishing to treat residual pockets after initial periodontal therapy, five studies [12, 15, 16, 18, 20] used it during supportive therapy. Ten studies compared the use of air polishing alone, while three studies [5, 19, 21] evaluated air polishing as an adjunctive to hand and ultrasonic instruments. Glycerin powder was used in 10 studies, while erythritol powder was used in two [5, 15], and trehalose powder in only one [12]. Nine studies used the air-polishing device for subgingival biofilm removal using a subgingival nozzle [5,6,7, 12, 14,15,16, 18, 21], whereas three studies did not provide information on the nozzle used for subgingival biofilm removal [4, 13, 19]. The remaining study used a supragingival nozzle to remove supragingival biofilm [20]. The average age ranged between 18 and 70 years among the group of patients. Only four studies had a follow-up up to or longer than 180 days [12, 16, 18, 21] (Table 1). All studies reported that they followed ethical criteria and applied terms of consent to all patients. Only one study [17] reported to follow the CONSORT guidelines. No study mentioned whether it was registered in clinical trial databases. Of the 13 studies, seven have been funded by the industry [4, 6, 12, 14,15,16,17]. More details are found in Table 1 for studies with a follow-up longer than 180 days and in Table 2 for the remaining studies.
Risk of individual bias of the studies
Two eligible studies [12, 17] had low risk of bias, while eight studies [5, 6, 13, 14, 16, 18,19,20] had a moderate risk of bias, and three a high risk of bias [4, 15, 21]. Of the studies with a follow-up longer than 180 days, one had a low risk of bias [12], two a moderate risk [16, 18], while one had a high risk of bias [21]. Table 3 shows detailed information on the risk of bias assessment among the studies. Items 1 and 2 were considered unclear when tossing a coin was used as the randomization method. In item 4, none of the studies informed about the participants blinding. All studies were marked as “no” in item 5 because they did not blind the operators. Item 6 was considered “unclear” when no information about blinding the clinical assessor was provided. In item 9, all 13 studies were marked as “no” because they reported neither participant dropout nor provided intention to treat analysis. Item 13 was considered as “unclear” because the articles did not follow a guideline to fulfill the RCT designs and did not mention any specific method to do so. Additionally, seven studies did not provide a sample size calculation based on results from previous findings, and the vast majority (nine studies) did not have a follow-up longer than 180 days.
Synthesis of results
Table 4 displays the main clinical findings of the studies included in the review. Eleven studies evaluated probing pocket depth [4, 5, 12, 14,15,16,17,18,19,20,21] (Appendix Table 7), while five studies evaluated clinical attachment level [5, 12, 15, 19, 21] (Appendix Table 8). Eight articles measured plaque index [4, 13, 15,16,17,18,19,20] (Appendix Table 9). Finally, bleeding on probing was measured by 11 studies [4,5,6, 12, 14,15,16,17, 19,20,21] (Appendix Table 10).
For the primary endpoint of this study, PPD data, four studies with a follow-up longer than 180 days [12, 16, 18, 21] provided a comparison between air polishing and hand and ultrasonic instruments. Three studies used air polishing alone to treat residual periodontal pockets (PPD ≥ 4 mm) during supportive therapy [12, 16, 18], while one study used air polishing as an adjunctive therapy to hand instrumentation during initial periodontal therapy [21]. Three studies indicated that air polishing achieved similar PPD results to hand instrumentation after 180 days [12, 16, 21], while one study showed greater improvement of PDD among participants treated with hand instrumentation [18] (Table 4).
As displayed in Table 4, no differences were observed between manual or ultrasonic scaling and air polishing for any of the secondary clinical parameters evaluated (BOP, CAL, PI), irrespective of the evaluation time.
In addition to the clinical parameters, seven studies performed microbiological analyses [5,6,7, 14, 16, 18, 20], seven evaluated patient’s comfort [5,6,7, 12, 14,15,16], and three carried out efficacy analysis on clinical professional time [6, 15, 18], whereas VSC level was evaluated in one study [19], and GCF volume and cytokines in another [21]. Regarding the microbiological analyses VSC level and GCF volume, air polishing had similar results than manual or ultrasonic scaling did; however, it showed better results regarding the patients’ comfort and the time spent by the professional (Table 4). Finally, 12 studies did not observe any adverse effect related to the use of air polishing, while one study did not report information about it [19] (Table 4).
Discussion
This systematic review aimed to evaluate the efficacy of air polishing compared to manual and ultrasonic scaling for reduction of periodontal inflammation. Our findings suggest no differences between the efficacy of air polishing and manual or ultrasonic instruments to reduce probing pocket depth (PPD) during treatment of residual periodontal pockets or supportive periodontal care. Similar results were also observed for the secondary endpoints, namely, BOP, CAL and PI. In addition, air polishing seems to be safe, more comfortable for patients, and to reduce the length of the clinical appointment.
Prior to further discussions, let us examine the limitations and strengths of our review. The main limitation of this review relates to the lack of a standard protocol for air polishing, which included the use of different powders, among others. Additionally, none of the included studies had a follow-up longer than 1 year, a methodological issue that should be overcome in future studies, as there is a common relapse in RCT’s on periodontal therapy after the first year of treatment completion. Additionally, as few studies provided a sample size calculation, one may speculate whether the lack of difference between therapies was not related to underpowered studies. A similar thought would also apply to studies that presented problems related to randomization or blinding. However, one should bear in mind that the lack of difference between therapies was not a phenomenon limited to “high-risk-of-bias” studies, as those with a higher methodological quality achieved similar results. Regardless of the limitations, our study has some strengths that should be further stressed. Firstly, this systematic review comprises a thorough literature search, which included the grey literature without restriction of time, language, and publication status. Besides, the “Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews Checklist for Randomized Controlled Trials” was rigorously applied by the authors to assess the risk of bias, in addition to two other questions to assess the quality of the studies. Finally, according to the Oxford Center for Evidence-Based Medicine, our study indicates a good level of evidence to support our findings [22].
In the treatment of periodontitis, biofilm control is critical to prevent or to arrest disease progression. After a treatment phase, including non-surgical and surgical approaches, patients usually follow supportive periodontal treatment [23]. A previous systematic review revealed that air polishing and ultrasonic instrumentation showed similar clinical efficacy, while air polishing showed a preferable level of comfort than ultrasonic instrumentation [8], supporting our findings. Furthermore, we also observed similar results when comparing air polishing to hand instrumentation alone (Tables 1, 2, and 4). Results are not surprising since most periodontal treatments performed in practice aim to restore the homeostasis between the biofilm and the host immune system by mechanically reducing the microbial load to levels that are compatible with health. What can be deduced from our findings is that air polishing, manual scaling, and ultrasonic scaling are all clinically efficient in controlling the biofilm to levels compatible with periodontal inflammation reduction measured by plaque accumulation, bleeding, pocket depth, and attachment level.
Our findings also revealed that side or adverse effects related to the use of air polishing devices are rare and usually include non-serious events, such as increased tooth sensitivity or painless gingival erosion. None of the studies observed any adverse effect related to the use of air polishing for subgingival biofilm removal. Thus, it seems to be a safe therapy to be used as part of the routine periodontal care. On a similar note, a previous study demonstrated that air polishing with glycine powder resulted in fewer areas of gingival erosion compared to hand instrumentation or air polishing with sodium bicarbonate [4]. However, sifting through the evidence regarding the safety of air-polishing devices reveals that other rare complications may occur, such as subcutaneous emphysema and abrasion of the root cementum.
A critical finding of our study relates to the industry’s involvement in the studies using air-polishing devices. Out of 13 RCT’s on the topic, seven were funded by the industry, and in some, authors were involved in the development of the powder used in the air-polishing device. This fact should raise caution to the reader when interpreting our findings since it is not possible to determine whether studies with negative results have been subjected to publication bias.
Conclusion
Our findings suggest that there is no difference between the efficacy of air polishing and hand or ultrasonic instrumentation to control biofilm and to reduce periodontal inflammation. In addition, air polishing seems to be a safe, comfortable, and less time-consuming tool to be incorporated in the periodontal care combined to hand instruments during initial periodontal therapy, or alone for treatment of residual inflamed pockets and supportive periodontal therapy. However, these findings must be carefully interpreted owing to a potential conflict of interest related to industry funding and a moderate quality of the evidence. In addition, randomized clinical trials with a follow-up period longer than 12 months with a higher methodological quality, including a properly calculated sample size, are required to assess the stability of periodontal parameters treated with air polishing.
References
Nascimento GG, Leite FRM, Scheutz F, López R (2017) Periodontitis: from Infection to Inflammation. Curr Oral Health Rep 4(4):301–308
Belibasakis GN, Mylonakis E (2015) Oral infections: clinical and biological perspectives. Virulence 6(3):173–176. https://doi.org/10.1080/21505594.2015.1025191
Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL Jr, Socransky SS (1997) The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol 24(5):324–334. https://doi.org/10.1111/j.1600-051x.1997.tb00765.x
Petersilka G, Faggion CM Jr, Stratmann U, Gerss J, Ehmke B, Haeberlein I, Flemmig TF (2008) Effect of glycine powder air-polishing on the gingiva. J Clin Periodontol 35(4):324–332. https://doi.org/10.1111/j.1600-051X.2007.01195.x
Park EJ, Kwon EY, Kim HJ, Lee JY, Choi J, Joo JY (2018) Clinical and microbiological effects of the supplementary use of an erythritol powder air-polishing device in non-surgical periodontal therapy: a randomized clinical trial. J Periodontal Implant Sci 48(5):295–304. https://doi.org/10.5051/jpis.2018.48.5.295
Möene R, Decaillet F, Andersen E, Mombelli A (2010) Subgingival plaque removal using a new air-polishing device. J Periodontol 81(1):79–88. https://doi.org/10.1902/jop.2009.090394
Flemmig TF, Hetzel M, Topoll H, Gerss J, Haeberlein I, Petersilka G (2007) Subgingival debridement efficacy of glycine powder air polishing. J Periodontol 78(6):1002–1010. https://doi.org/10.1902/jop.2007.060420
Zhang J, Liu J, Li J, Chen B, Li H, Yan F (2019) The clinical efficacy of subgingival debridement by ultrasonic instrumentation compared with subgingival air polishing during periodontal maintenance: a systematic review. J Evid Based Dent Pract 19(4):101314. https://doi.org/10.1016/j.jebdp.2019.02.001
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64(4):401–406. https://doi.org/10.1016/j.jclinepi.2010.07.015
Tufanaru CMZ, Aromataris E, Campbell J, Hopp L (2017) Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z (eds) Joanna Briggs Institute Reviewer's Manual. Joanna Briggs Institute, Australia
Kruse AB, Akakpo DL, Maamar R, Woelber JP, Al-Ahmad A, Vach K, Ratka-Krueger P (2019) Trehalose powder for subgingival air-polishing during periodontal maintenance therapy: A randomized controlled trial. J Periodontol 90(3):263–270. https://doi.org/10.1002/JPER.17-0403
Simon CJ, Munivenkatappa Lakshmaiah Venkatesh P, Chickanna R (2015) Efficacy of glycine powder air polishing in comparison with sodium bicarbonate air polishing and ultrasonic scaling - a double-blind clinico-histopathologic study. Int J Dent Hyg 13(3):177–183. https://doi.org/10.1111/idh.12133
Wennström JL, Dahlen G, Ramberg P (2011) Subgingival debridement of periodontal pockets by air polishing in comparison with ultrasonic instrumentation during maintenance therapy. J Clin Periodontol 38(9):820–827. https://doi.org/10.1111/j.1600-051X.2011.01751.x
Hägi TT, Hofmanner P, Salvi GE, Ramseier CA, Sculean A (2013) Clinical outcomes following subgingival application of a novel erythritol powder by means of air polishing in supportive periodontal therapy: a randomized. controlled clinical study. Quintessence Int 44(10):753–761. https://doi.org/10.3290/j.qi.a30606
Müller N, Moene R, Cancela JA, Mombelli A (2014) Subgingival air-polishing with erythritol during periodontal maintenance: randomized clinical trial of twelve months. J Clin Periodontol 41(9):883–889. https://doi.org/10.1111/jcpe.12289
Flemmig TF, Arushanov D, Daubert D, Rothen M, Mueller G, Leroux BG (2012) Randomized controlled trial assessing efficacy and safety of glycine powder air polishing in moderate-to-deep periodontal pockets. J Periodontol 83(4):444–452. https://doi.org/10.1902/jop.2011.110367
Kargas K, Tsalikis L, Sakellari D, Menexes G, Konstantinidis A (2015) Pilot study on the clinical and microbiological effect of subgingival glycine powder air polishing using a cannula-like jet. Int J Dent Hyg 13(3):161–169. https://doi.org/10.1111/idh.12104
Caygur A, Albaba MR, Berberoglu A, Yilmaz HG (2017) Efficacy of glycine powder air-polishing combined with scaling and root planing in the treatment of periodontitis and halitosis: A randomised clinical study. J Int Med Res 45(3):1168–1174. https://doi.org/10.1177/0300060517705540
Lu H, He L, Zhao Y, Meng H (2018) The effect of supragingival glycine air polishing on periodontitis during maintenance therapy: a randomized controlled trial. PeerJ 6:e4371. https://doi.org/10.7717/peerj.4371
Tsang YC, Corbet EF, Jin LJ (2018) Subgingival glycine powder air-polishing as an additional approach to nonsurgical periodontal therapy in subjects with untreated chronic periodontitis. J Periodontal Res 53(3):440–445. https://doi.org/10.1111/jre.12532
Phillips B, Ball C, Sackett D, D. B (2009) Oxford Centre for Evidence-based Medicine – Levels of Evidence. Oxford Centre for Evidence-based Medicine,
Axelsson P, Nystrom B, Lindhe J (2004) The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol 31(9):749–757. https://doi.org/10.1111/j.1600-051X.2004.00563.x
Dosumu EB, Arowojolu MO, Savage KO (2002) Alveolar bone regeneration pattern following surgical and non-surgical treatment in juvenile periodontitis. West Afr J Med. 21(4):272–275
Michalowicz BS, Hodges JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, Buchanan W, Bofill J, Papapanou PN, Mitchell DA, Matsoane S, Tschida PA (2006) Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 355(18):1885–1894
Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, Stamilio DM, Appleby D, Clothier B, Sammel MD, Jeffcoat M (2010) Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS). Am J Obstet Gynecol. 202(2):147.e1–147.e8
Jaramillo A, Arce R, Contreras A, Herrera JA (2012) Effect of periodontal therapy on the subgingival microbiota in preeclamptic patients. Biomedica. 32(2):233–238
López NJ, Quintero A, Casanova PA, Martínez B (2014) Routine prophylaxes every 3 months improves chronic periodontitis status in type 2 diabetes. J Periodontol. 85(7):e232–e240
Funding
This study was also financed in part by CAPES (Coordination for the Improvement of Higher Education Personnel–Brazilian Ministry of Education)-Finance Code 001. We are also thankful for the support of CNPq (Council for Scientific and Technological Development–Brazilian Ministry of Science, Technology and Innovations)-Finance Code 307808/2018-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 64 kb)
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Nascimento, G.G., Leite, F.R.M., Pennisi, P.R.C. et al. Use of air polishing for supra- and subgingival biofilm removal for treatment of residual periodontal pockets and supportive periodontal care: a systematic review. Clin Oral Invest 25, 779–795 (2021). https://doi.org/10.1007/s00784-020-03762-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03762-y