Abstract
Objectives
The purpose of this experimental in vivo investigation was to evaluate the influence of modifying the implant surface by adding a monolayer of multi-phosphonate molecules on the development of experimental peri-implantitis.
Material and methods
Eight beagle dogs received 5 tests and 5 control implants each following a split-mouth design 3 months after premolar and molar extraction. On the most mesial implant of each side, a 3-mm buccal dehiscence was artificially created. Experimental peri-implantitis was induced by silk ligatures over a 4-month period; after ligature removal, peri-implantitis was left to progress for another 4 months without plaque control. Clinical, histological, and radiographic outcomes were evaluated.
Results
Radiographically, both implant groups showed a similar bone loss (BL) at the end of the induction and progression phases. BL measured on the histological sections of the test and control groups was 3.14 ± 0.42 mm and 3.26 ± 0.28 mm, respectively; the difference was not statistically significant (p > 0.05). The remaining buccal bone to implant contact (bBIC) percentage of the test and control groups was 59.38 ± 18.62 and 47.44 ± 20.46%, respectively; the difference, however, was not statistically significant (p > 0.05). Bone loss observed at dehiscent sites compared to non-dehiscent ones showed no statistically significant difference (p > 0.05).
Conclusions
Addition of a monophosphonate layer to a moderately rough implant surface did not affect development of experimental peri-implantitis.
Clinical relevance
Influence of implant surface on peri-implantitis may condition implant selection by the clinician, especially on patients with disease risk factors. In that sense, monophosphate layer implants do not show higher peri-implantitis risk than control implants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental implants have demonstrated long-term predictable success due to the attainment of a bone-to-implant interface termed osseointegration; the latter was described as direct contact between the living bone and the surface of a load-carrying implant [1, 2]. The biological process to reach osseointegration has been studied in different experimental in vivo models, and changes of the surface microtopography have shown to enhance bone healing with faster and more predictable osseointegration [3]. Recently, studies have investigated how changes at the nano-scale level or the surface chemistry may further enhance the bone response [4,5,6,7]. One of these attempts has been to add a monolayer of permanently bound multi-phosphonic acid molecules to a standard moderately rough implant surface (SurfLink®, Nano Bridging Molecules, Gland, Switzerland). The aim was to mimic a bone surface and develop nano-bridging molecules between the bone and the implant surface with the objective to increase the BIC and improve the stability of osseointegration. In vitro results and preclinical in vivo studies using this chemically modified surface have shown increased velocity of osseointegration [8] and a higher bone to implant contact (BIC) [9]. However, when implants with this modified surface were placed in patients and compared to similar implants with a standard moderately rough surface, clinical performance was similar [10].
The current challenge in implant dentistry, however, is not to increase the percentage of osseointegration, since this is a highly predictable outcome; rather, it is to develop a strong bond between the implant surface and the bone that is resistant to inflammation and bone resorption. Peri-implantitis was recently defined at the World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions [11] as a plaque-associated pathological condition that causes inflammation and progressive bone loss in peri-implant tissues; it is a frequent condition that affects about 20% of the patients that have been restored with an implant-supported prosthesis in function for more than 5 years [12]. Pathogenesis of peri-implantitis has been linked to the following risk factors, history of periodontitis, lack of oral hygiene, and lack of compliance with regular recalls (ref). An additional series of indicators with still controversial impact on its incidence do also exist; one of these factors is the implant surface. Several studies have shown that rougher surfaces increase biofilm deposition; the latter leads to a higher risk of developing inflammation and bone resorption [13, 14]. What is less clear is whether modifications in the chemical composition of the implant surface will develop a stronger bond between the bone and the implant surface and thus, reduce the incidence of bone resorption, in spite of the presence of bacteria-induced inflammation.
The aim of the present investigation was then to evaluate this modified implant surface using a well-validated experimental peri-implantitis preclinical experimental model. The objective was to assess the impact of this surface on clinical, radiological, and histological outcomes during the initiation and progression of experimental peri-implantitis.
Material and methods
Study design and randomization
The study was designed as a preclinical split-mouth randomized controlled trial comparing two implants with identical macro-design but different surface characteristics. The study protocol consisted on four interventions: (i) tooth extraction, (ii) implant placement, (iii) ligature-induced peri-implantitis, and (iv) euthanasia followed by histological processing and evaluation. The experimental sites were randomly allocated to either test or control according to a computer-generated randomization list (IBM SPSS Statistics® V20. JM. Domenech). Randomization sequence was generated using a blocking, balanced restricted randomization, stratified by hemi-mandible and implant position (P1‑P5). Allocation to the treatment was concealed by the mean of sealed envelopes containing the implant type which were opened during the surgical procedure once the flaps were raised and the bone was exposed (Fig. 1).
Experimental sample
A total of 8 healthy adult female beagle dogs of 72 months of age (weight between 12 and 15 kg) were used in this investigation, in full compliance with the ARRIVE guidelines [15]. All experimental animals were acquired from the Service of Animal Experimentation of the University of Cordoba, Spain. Then, they were housed in the Animal Experimentation Service Facility of the Rof Codina Foundation (Lugo, Spain) and after an adaptation/quarantine period of 3 weeks, the experimental segment of the study took place from May 2016 to June 2017. The Ethical Committee of the Rof Codina Foundation (Lugo, Spain) approved the study protocol (AELU001/04/16). All the experiments were performed according to Spanish and European regulations on use and care of research animals, being the dogs monitored daily during the study by a veterinarian accredited in laboratory animal science. These animals were maintained in a group kennel with outdoor and indoor areas, with a controlled temperature of 18 ± 2 °C with natural light and air renewal. The animals were fed using a granulated dog food, previously wetted in water, with individual bowls and free supply of water.
Study devices
All implants had the same surface and macroscopic design (C1 implants, MIS®); implants of the test group received an additional covalently bonded phosphonate layer that created a nanometer thin molecular nanolayer of monophosphonate molecules (Nano Bridging Molecules, Gland, Switzerland) (Fig. 2g).
Clinical stages of implant placement. a Baseline situation. b Teeth hemisection prior to extraction. c Suture after teeth extraction. d Healed crest 3 months after extractions. e Bone crest after flap raising. f Implant osteotomies. g Identical macroscopic design of test and control implants. h Buccal bone dehiscence on most mesial implant. i Implant placement. j Abutment placement. k Suture after implant placement. l Radiographic control after implant placement
Surgical procedures
All surgical interventions were performed under sterile conditions, in an animal-operating theater. First, pre-medication with medetomidine (20 μg/kg/i.m., Domtor, Esteve, Barcelona, Spain) and pain control with morphine (0.4 mg/kg/i.m., Morfina Braun 2%, B. Braun Medical, Barcelona, Spain) was administered, and then general anesthesia was induced by propofol (3–5 mg/kg/i.v., Propovet®, Abbott Laboratories, Kent, UK), and maintained with a concentration of 2.5–4% of isoflurane (Isoba-vet®, Schering-Plough, Madrid, Spain). During anesthesia, the animals were cared by a veterinarian doctor (B or C category), who continuously monitored the animals with electrocardiography, capnography, pulsioxymetry, and non-invasive blood pressure. Prophylactic cefazolin (20 mg/kg/i.v., Kurgan, Normon, Madrid, Spain) and cefovezin (8 mg/kg/s.i.d./s.c., Convenia, Zoetis, Madrid, Spain) were administered intraoperatively. At the end of the intervention, atipamezole (50 mg/kg/i.m., Esteve, Barcelona, Spain) was administered to revert the effects of medetomidine. Postoperative pain was controlled by administration of morphine (0.2 mg/kg/i.m./6 h, Morfina Braun 2% B. Braun Medical, Barcelona, Spain) and meloxicam as anti-inflammatory and analgesic treatment (0.2 mg/kg/i.m./SID, Metacam, Boehringer Ingelheim, Barcelona, Spain) for 5 days.
The surgical protocol used in this study has been reported in detail in a recent publication from our research group [16]. In brief:
Phase 1: tooth extraction
Extraction of the mandibular 2nd, 3rd, and 4th premolars and the 1st molar (PM2-M1) after being hemisected in both jaws using forceps and root elevators within a flapless procedure was conducted. Prophylactic administration of cefazolin (20 mg/kg/i.v., Kurgan, Normon, Madrid, Spain) and cefovezin (8 mg/kg/s.i.d./s.c., Convenia, Zoetis, Madrid, Spain) was performed intraoperatively (Fig. 2 a‑d).
Phase 2: implant placement
Three months after tooth extraction, full-thickness flaps were elevated bilaterally in each hemi-mandible. After randomization and implant allocation, a total of 40 controls and 40 test implants were installed. In each hemi-mandible, five implants with 3 mm height platform-switching healing abutments were placed. In the most mesial location at both sides, buccal bone was removed prior to implant placement, resulting in a dehiscence defect (approximately 3 mm wide and 3 mm high) with the buccal implant surface exposed. Mucoperiosteal flaps were then repositioned and primary wound closure was achieved with absorbable suture (Coated Vicryl™ Raptide, Ethicon, US, LLC 2014) (Fig. 2 e‑k). The animals were subsequently enrolled in an oral hygiene program consisting in tooth cleaning three times a week with gauzes embedded in chlorhexidine oral rinse 0.12% (Perio-Aid Treatment®, Dentaid, Cerdanyola del Valles, Spain) during the first 2 weeks and subsequently, three times a week with toothbrush and chlorhexidine gel.
Phase 3: experimental peri-implantitis
After 3 months of healing, the oral hygiene regime was interrupted, and 4-0 silk ligatures were placed submarginally around the neck of each implant following to the method described by Lindhe et al. [17]. Ligatures were replaced each month for 4 months (induction phase) (Fig. 3 a‑e). During the three first months, platform-switching healing abutments were covering the implants; however, on the last month of induction, these abutments were replaced by platform-matching abutments to increase progression of the disease (Fig. 3 f, g). Thereafter, the ligatures were removed, and the animals were left for 4 additional months without plaque control (progression phase) (Fig. 3 h, i). Every month of the induction period and at the end of the experiment, clinical and radiographical variables were recorded.
Clinical stages of experimental peri-implantitis. a 3 months after implant placement (visit baseline). b Submarginal placement of silk ligatures. Start of induction phase. c 1 month after ligature placement. Change of ligatures. d 2 months after ligature placement. Change of ligatures. e 3 months after ligature placement. Change of abutments and change of ligatures. f Abutment change diagram; from platform-switching design, to platform-matching design. g Clinical abutment change. h 4 months after ligature placement, 1 month with platform-matching abutments. Ligatures were removed in this visit. End of induction phase and start of progression phase. i 8 months after ligature placement visit, 4 months after ligature removal. End of progression phase
Phase 4: euthanasia
At the end of the progression phase, the experimental animals were first sedated with medetomidine (30 μg/kg/i.m., Esteve, Barcelona, Spain) and then euthanized with an intravenous overdose of sodium pentobarbital (40–60 mg/kg/i.v., Dolethal, Vetoquinol, France). Subsequently, the lower jaws were dissected and retrieved with intact soft tissues and fixed in buffered 10% formaldehyde solution. Previous to fixation, the 80 implants were retrieved with intact soft tissues and individually separated using a band saw.
Clinical outcome variables
Clinical measurements were obtained from 6 sites per implant (mesio-buccal, buccal, disto-buccal, mesio-lingual, lingual, and disto-lingual) by means of a PCPUNC15 periodontal probe (Hu-Friedy Co., Chicago, IL, USA). Measurements were performed before ligature placement and then once every month during the experimental peri-implantitis period.
The following clinical outcome variables were recorded by one calibrated examiner (JS):
-
Modified gingival index (GI)
-
Probing depth (PD) measured from the mucosal margin (M) to the bottom of the pocket (BP)
-
Recession (Rec) measured from the top of the implant abutment (A) to the mucosal margin (M)
Radiographical analysis
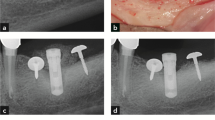
Periapical X-rays were taken during each visit of the study to assess bone loss and progression of the peri-implantitis (Fig. 4). Interproximal bone levels were measured at each implant, from the implant shoulder to the first visible bone to implant contact. The mean radiographic bone level was calculated averaging the mesial and distal measurements. All radiographs were measured by the same calibrated examiner (J.S.) using a computer image analysis software (Image J., National Institutes of Health, Bethesda, MD) after calibrating the images using the previously known distance (length of the implant) to compensate for image distortion and magnification.
Radiographic assessment. a Baseline visit, 3 months after implant placement. Ligatures were placed during this visit. b 1 month after ligature placement. Change of ligatures. c 2 months after ligature placement. Change of ligatures. d 3 months after ligature placement. Change of abutments and change of ligatures. e 4 months after ligature placement, 1 month with platform-matching abutments. Ligatures were removed in this visit. f 8 months after ligature placement visit, 4 months after ligature removal
Histological processing
Using a randomization protocol, half of the blocks containing the implant and the surrounding hard and soft tissues were dissected and processed for ground sectioning following the method described by Donath and Breuner [18]. The samples were dehydrated in a graded series of ethanol solutions and embedded in a light-curing resin (Technovit 7200 VLC; Heraeus-Kulzer GMBH, Werheim, Germany). From each specimen, one central bucco-lingual section through the implant was sectioned using a band saw (Exakt Apparatebau, Norderstedt, Germany) and subsequently polished mechanically using 1200 and 4000 grit silicon carbide papers (Struers, Copenhagen, Denmark), obtaining specimens with a thickness of approximately 50 μm. The slides were stained according to the Levai Laczkó method [19]. The other half of the specimens were prepared for decalcification following the method “fracture technique” as described in [20].
Histomorphometry
Histomorphometry was carried out using a Nikon Eclipse Ti microscope (Nikon, Heidelberg, Germany) equipped with image analysis software (Q-500MC; Nikon). One bucco-lingual section per implant was analyzed.
The following landmarks were identified on both the buccal and lingual sides of each implant (Fig. 5 a) [16]:
-
Implant shoulder (IS),
-
The most coronal level of bone in contact with the implant (fBIC),
-
Bone crest, defined as the most coronal point of the bone (BC).
Linear measurements in millimeters were calculated by drawing a line along the long axis of the implant, from IS to fBIC (i.e., defect length (DL)) and from IS to BC (i.e., bone crest distance (BCD)). The intraosseous defect (ID) linear variable was calculated between DL and BCD.
The amount of BIC was measured as the proportion of the total implant surface in direct contact with mineralized tissue on both the buccal and lingual aspects (% BIC). The percentage of denuded implant surface (% BL) was measured as a percentage of the DL implant surface on both the buccal and lingual aspects (Fig. 5 b).
All histometric measurements were evaluated by one calibrated investigator blinded to the specific experimental conditions (G.A.). The calibration test consisted on repeated evaluation of the defect length (IS-fBIC) of the first section of each animal. Intra-examiner intra-class correlation coefficient was 0.998 (95% confidence intervals: 0.997–1.000).
Statistical analysis
Data from clinical, radiographic, and histological analysis were expressed in means (± SD), considering the dog as the statistical unit of analysis (n = 8). The Shapiro-Wilk test data was used to test normality of the data. For the longitudinal measurements (clinical and radiographic), comparisons between experimental and control implants were analyzed using the two-way ANOVA test and compared using general linear model with intragroup comparisons. Bonferroni post hoc analysis was further performed to evaluate differences between the time intervals. For histomorphometric analysis, comparisons were made by means of Mann-Whitney U test for independent samples. Differences were considered statistically significant when p was < 0.05. This statistical analysis was performed using the software SPSS (SPSS® 20.0, SPSS Inc., Chicago, IL, USA). Data from dehiscence implants (most mesial implants) was analyzed independently.
Results
Clinical observations
There were no adverse events in any experimental animal during all phases of the study. All implants were osseointegrated and were available for assessment all over the experimental phase.
Bleeding on probing was absent at baseline visit; then, it increased significantly in both groups over the experiment. Almost all explored sites bled during the induction and progression phases regardless of the group (Table 1). Probing depths were shallow at the baseline evaluation for both the test and control implants (2.33 ± 0.15 mm and 2.30 ± 0.18 mm, respectively). During the induction phase, an increase in probing depth was observed in both implant groups; the maximum value was reached at the end of induction phase (4.77 ± 0.51 mm and 4.61 ± 0.55 mm, respectively). After removing the ligatures, probing depth did not increase in any of the groups during the progression phase (4.53 ± 0.2 and 4.38 ± 0.5 mm, respectively) (Table 2). During both the induction and progression phases, the position of the mucosal margin in relation to the top of the abutment (clinical recession) did not change substantially (Table 3); it was about 1.5 mm in both groups at all study visits. There were no statistically significant differences between the implant groups for any of the clinical variables measured.
Radiographic analysis
A progressive increase in radiographic bone loss was observed all over the experimental peri-implantitis experiment. At the end of the induction, bone loss in the test group was 2.53 ± 0.39 mm, while in the control group, it was 2.55 ± 0.30. Bone loss developed during the progression phase, although the change was less marked (test: 0.21 ± 0.32 mm vs. control: 0.15 ± 0.3 mm). The higher increase in bone level change (bone loss) was observed in the test and control groups between the third (1.94 SD = 0.23 vs. 1.94 SD = 0.26 mm respectively) and the fourth month of the induction period (3.00 SD = 0.35 vs. 2.87 SD = 0.26 mm respectively); it coincided with the abutment change (Fig. 4). Once ligatures were removed, radiographic bone loss continued, but to a slower pace, being approximately 35% of the implant surface bone loss in both groups (Table 4). The only statistically significant difference between groups was observed at the baseline visit, with test implants showing slightly higher bone remodeling than control implant (0.47 SD = 0.09 vs. 0.32 SD = 0.16 mm respectively), although these differences do not have any clinical relevance.
Histological findings
Histological bone loss was observed in all sections. Typical peri-implantitis bone resorption lacunae lesions were frequently observed. The bone loss pattern in the buccal aspect was more pronounced leading to predominantly supra-osseous lesions, whereas in the lingual aspect, bone loss was less evident and presence of intraosseous lesions was frequently observed. In some sections, images compatible with active bone modeling and remodeling were observed (bone areas intensively stained), probably resulting from healing after ligature removal (Fig. 5 a). The supracrestal soft tissues were predominantly separated from the implant surface. The connective tissue covering the bone was very thin and, in some sections, the bone was directly exposed to the peri-implant pocket (Fig. 5 a, b).
Histometric results
Thirty-nine specimens were evaluated after undecalcified ground sectioning. From these, 7 corresponded to the implants with experimental dehiscence lesion (3 tests and 4 controls) and 32 (16 tests and 16 controls) to the rest.
Non-dehiscence implants
In both implant groups, defect length (DL) was higher in the buccal aspect, while formation of intraosseous defects (ID) was more marked in the lingual aspects. In the buccal aspect, the test implants had lower mean DL and ID values compared to control ones (DL = 3.14 ± 0.42 mm and 3.26 ± 0.28 mm, ID = 0.42 ± 0.5 mm vs. 0.63 ± 0.62 mm, respectively), but these differences were not statistically significant (Table 5). The percentage of buccal bone loss (BL) was similarly lower in test compared with the control implants, although differences were without statistical significance (28.13 ± 4.61% vs. 29.73 ± 2.78%, respectively). The remaining buccal bone to implant contact (% BIC) was also superior in the test vs. the control implants (59.38 ± 18.62 and 47.44 ± 20.46, respectively). When both buccal and lingual aspects were considered together, only minor differences were noted (56.39 ± 13.41 and 52.82 ± 13.54, respectively) (Table 6).
Dehiscence vs. non-dehiscence implants
Behavior of the test compared to the control implants in the dehiscence implant sites was similar. When both test and control implants from these dehiscence sites were compared with the rest of the non-dehiscence sites, dehiscence implants showed increased buccal DL (3.35 ± 0.68 vs. 3.20 ± 0.35), decreased buccal ID (0.37 ± 0.5 vs. 0.53 ± 0.56), and slightly more bucco-lingual BL% (28.24 ± 8.58 vs. 27.21 ± 3.79). Remaining % BIC was also slightly lower compared to non-dehiscence implants (47.95 ± 5.99 vs. 54.61 ± 13.15) (Table 7). Differences between dehiscence and non-dehiscence implants were not statistically significant (p > 0.05).
Discussion
The present preclinical in vivo investigation was designed to address the clinical, radiographical, and histological behavior of a new implant surface treatment based on a monolayer of multi-phosphonate molecules, compared to a standard moderately rough implant surface, when exposed to experimental peri-implantitis, combining the traumatic effect of ligature placement and bacterial challenge due to the lack of hygienic measures during both the induction and progression phases. The test implants had a similar clinical and radiological behavior when compared to the control implant group. In both test and control implant groups, there was a significant increase in probing depths and radiographic bone loss, mainly during the ligature-induced peri-implantitis period (induction phase) (2.53 ± 0.39 mm and 2.55 ± 0.3 mm, respectively). After ligature removal, progression of the disease continued, both clinically and radiographically, although at a much lesser pace (0.21 ± 0.32 mm and 0.15 ± 0.3 mm, respectively). This radiological bone loss during the induction phase of experimental peri-implantitis was comparable to the results reported by Albouy [13], with 3.00 mm for a turned surface and 3.27 mm for rough surface implants. During the progression phase, these authors also reported radiographic bone loss (0.03 mm) similar to the present study in the turned surface implants and some moderately rough surface implants, while reporting a higher radiographic bone loss associated with a specific rough surface microtopography (1.47 mm) [21]. These results clearly indicate that factors other than surface roughness may influence the development and progression of experimental peri-implantitis.
The histological results corroborated the clinical and radiographical data, depicting that the novel implant surface had lower buccal bone loss and higher remaining bone to implant contact at the end of the experimental peri-implantitis period, when compared to the control implants, although these differences were not statistically significant. Implants with modified surface based on coating with a monolayer of multi-phosphonate molecules have been shown in preclinical models to increase bone to implant contact and removal torque after osseointegration [9]. The hypothesis of this investigation was based on this likely increased bone response that would reduce the incidence and progression of peri-implantitis. However, this significant added value was not demonstrated in human clinical trials, in which these chemically modified surface implants did not perform significantly better than control implants, but proved to be safe and achieved a high degree of osseointegration [10]. In the present study, although the phosphonate surface treatment showed better buccal bone to implant contact (BIC), when compared to control implants (50.4% vs. 47.4%), this difference did not imply a higher resistance to experimental peri-implantitis, since the defect length values were similar in both groups (3.14 vs. 3.26). This expected effect on early osseointegration, with increased BIC percentages, did not result in preventing bone resorption in response to the combination of the trauma of ligature placement and the biofilm-derived inflammation, what indicates that implants with monophosphonate coating did not have a higher resistance to peri-implantitis when compared with control implants. In terms of clinical relevance, these results may provide clinicians with relevant information on their choice of implants. If this choice is based on the search for an improved early bone response, this surface treatment has shown an improved response, when compared to controls. However, if the choice is based on the search for an implant more resistant to peri-implantitis, this coated implant did not demonstrate an added value when compared to controls.
Other attempts have been made to develop implants with a lesser susceptibility to peri-implantitis by modifying the implant surface, mainly through the addition of coatings with antimicrobial effect. Similar to the results of this investigation, addition of hydroxyapatite (OH-AP) coatings did not confer a significantly lesser susceptibility when compared to conventional moderately rough surface implants [22,23,24]. The only surface modification that has shown resistance to bacterial hurdle is silver coatings. Godoy-Gallardo et al. reported that silver-coated implants (3.2 ± 0.7 mm of histological bone loss) and silanized-coated implants (3.2 ± 0.7 mm) had less bone loss when compared with conventional titanium implants (3.9 ± 1.0 mm) [25]. The results from the present investigation reported a similar degree of histological bone loss (3.14 ± 0.42 in test vs. 3.26 ± 0.28 in control) compared with the coated surface implants, although the long-term behavior of these coated surfaces is uncertain once the metallic ions have been fully released. Similarly, other investigators have tested glass/n-Ag-coated titanium abutments using the experimental peri-implantitis model. The histological bone loss observed in implants covered with the biocide-coating abutment (1.32 mm) was about twice lower than control abutments (3.47 mm), in spite of having a higher roughness [26]. Abutments, however, have shown different bone response dependent not only on their surface, but also on their also configuration, mainly its height and the type of implant to abutment connection, with longer abutments showing a lesser marginal bone loss [27] and tighter implant to abutment and platform-switching connections being associated with lesser bone loss [28, 29]. In the present study, the results of the bone loss associated with the change of abutment from platform-switching to a platform-matching abutment carried out between the 3rd and 4th months visit of the induction phase clearly showed a significant impact in the progression of the experimental peri-implantitis, with twice the radiographic bone loss which was twice (1 mm) compared with the previous month (0.5 mm).
This investigation also aimed to evaluate the possible influence of a buccal dehiscence, as a locus of minor resistance to experimental peri-implantitis. Implants with a buccal dehiscence of 3 mm did not show a different pattern of bone loss when exposed to experimental peri-implantitis compared with implants fully covered with bone. It was not the purpose to compare the impact of the chemically modified implant surface, since only 3 implants were available with the monophosphonate molecule treatment, which in fact revealed a very similar behavior compared with the control implants (n = 4). These results were comparable with those reported in a clinical trial reporting similar outcomes when comparing bone level changes in implants presenting bone dehiscence defects compared with implants where similar dehiscence defects had been regenerated using guided bone regeneration (GBR) [30]. Nevertheless, buccal bone wall thickness has been reported as a relevant factor for preventing peri-implant bone loss, with an established critical bone wall thickness of 1.5 mm to prevent inflammatory complications [31].
The results obtained in the present investigation should be interpreted with caution due to the inherent limitations of this experimental model, since ligature-induced peri-implantitis allows for a reproducible peri-implant disease initiation and progression but the possible traumatic effect of the ligatures does not occur in the biofilm-induced inflammatory peri-implant disease. Also the higher bone metabolism of the experimental animals compared to humans may distort the results. In addition, due to the fact that histological sample was split in half to perform subsequent histological soft tissue analysis, the number of implants was reduced to 50%, resulting in a small sample size, which may have reduced the possibility to reach statistical significance in the comparisons between the test and control groups. Within these clear limitations, the results from the present experimental in vivo investigation allow us to conclude that (i) addition of a monophosphonate layer to a moderately rough implant, although attaining a lesser buccal bone loss, did not influence the initiation and progression of peri-implantitis; (ii) buccal dehiscence existing at the time of implant placement did not increase the bone loss pattern; and (iii) the change from switching-platform to matching-platform abutments increased the bone loss progression during the induction of experimental peri-implantitis.
References
Albrektsson T, Branemark PI, Hansson HA, Lindstrom J (1981) Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand 52:155–170. https://doi.org/10.3109/17453678108991776
Schroeder A, van der Zypen E, Stich H, Sutter F (1981) The reactions of bone, connective tissue, and epithelium to endosteal implants with titanium-sprayed surfaces. J Maxillofac Surg 9:15–25. https://doi.org/10.1016/s0301-0503(81)80007-0
Wennerberg A, Albrektsson T, Andersson B (1996) Bone tissue response to commercially pure titanium implants blasted with fine and coarse particles of aluminum oxide. Int J Oral Maxillofac Implants 11:38–45
Vignoletti F, Johansson C, Albrektsson T, De Sanctis M, San Roman F, Sanz M (2009) Early healing of implants placed into fresh extraction sockets: an experimental study in the beagle dog. De novo bone formation. J Clin Periodontol 36:265–277. https://doi.org/10.1111/j.1600-051X.2008.01363.x
Esposito M, Coulthard P, Thomsen P, Worthington HV (2005) The role of implant surface modifications, shape and material on the success of osseointegrated dental implants. A Cochrane systematic review. Eur J Prosthodont Restor Dent 13:15–31
Rossi F, Lang NP, De Santis E, Morelli F, Favero G, Botticelli D (2014) Bone-healing pattern at the surface of titanium implants: an experimental study in the dog. Clin Oral Implants Res 25:124–131. https://doi.org/10.1111/clr.12097
Shah FA, Nilson B, Branemark R, Thomsen P, Palmquist A (2014) The bone-implant interface-nanoscale analysis of clinically retrieved dental implants. Nanomedicine 10:1729–1737. https://doi.org/10.1016/j.nano.2014.05.015
Viornery C, Guenther HL, Aronsson BO, Pechy P, Descouts P, Gratzel M (2002) Osteoblast culture on polished titanium disks modified with phosphonic acids. J Biomed Mater Res 62:149–155. https://doi.org/10.1002/jbm.10205
von Salis-Soglio M, Stubinger S, Sidler M, Klein K, Ferguson SJ, Kampf K, Zlinszky K, Buchini S, Curno R, Pechy P, Aronsson BO, von Rechenberg B (2014) A novel multi-phosphonate surface treatment of titanium dental implants: a study in sheep. J Funct Biomater 5:135–157. https://doi.org/10.3390/jfb5030135
Esposito M, Dojcinovic I, Buchini S, Pechy P, Aronsson BO (2017) Safety and efficacy of a biomimetic monolayer of permanently bound multiphosphonic acid molecules on dental implants: 3 years post-loading results from a pilot quadruple-blinded randomised controlled trial. Eur J Oral Implantol 10:43–54
Schwarz F, Derks J, Monje A, Wang HL (2018) Peri-implantitis. J Clin Periodontol 45(Suppl 20):S246–S266. https://doi.org/10.1111/jcpe.12954
Dreyer H, Grischke J, Tiede C, Eberhard J, Schweitzer A, Toikkanen SE, Glockner S, Krause G, Stiesch M (2018) Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodontal Res 53:657–681. https://doi.org/10.1111/jre.12562
Albouy JP, Abrahamsson I, Berglundh T (2012) Spontaneous progression of experimental peri-implantitis at implants with different surface characteristics: an experimental study in dogs. J Clin Periodontol 39:182–187. https://doi.org/10.1111/j.1600-051X.2011.01820.x
Carcuac O, Abrahamsson I, Derks J, Petzold M, Berglundh T (2020) Spontaneous progression of experimental peri-implantitis in augmented and pristine bone: a pre-clinical in vivo study. Clin Oral Implants Res 31:192–200. https://doi.org/10.1111/clr.13564
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160:1577–1579. https://doi.org/10.1111/j.1476-5381.2010.00872.x
Sanz-Esporrin J, Blanco J, Sanz-Casado JV, Munoz F, Sanz M (2019) The adjunctive effect of rhBMP-2 on the regeneration of peri-implant bone defects after experimental peri-implantitis. Clin Oral Implants Res 30:1209–1219. https://doi.org/10.1111/clr.13534
Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C (1992) Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res 3:9–16. https://doi.org/10.1034/j.1600-0501.1992.030102.x
Donath K, Breuner G (1982) A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J Oral Pathol 11:318–326. https://doi.org/10.1111/j.1600-0714.1982.tb00172.x
Jeno L, Geza L (1975) A simple differential staining method for semi-thin sections of ossifying cartilage and bone tissues embedded in epoxy resin. Mikroskopie 31:1–4
Berglundh T, Lindhe J, Jonsson K, Ericsson I (1994) The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol 21:189–193. https://doi.org/10.1111/j.1600-051x.1994.tb00302.x
Carcuac O, Abrahamsson I, Albouy JP, Linder E, Larsson L, Berglundh T (2013) Experimental periodontitis and peri-implantitis in dogs. Clin Oral Implants Res 24:363–371. https://doi.org/10.1111/clr.12067
Martins MC, Abi-Rached RS, Shibli JA, Araujo MW, Marcantonio E Jr (2004) Experimental peri-implant tissue breakdown around different dental implant surfaces: clinical and radiographic evaluation in dogs. Int J Oral Maxillofac Implants 19:839–848
Tillmanns HW, Hermann JS, Tiffee JC, Burgess AV, Meffert RM (1998) Evaluation of three different dental implants in ligature-induced peri-implantitis in the beagle dog. Part II. Histology and microbiology. Int J Oral Maxillofac Implants 13:59–68
Madi M, Zakaria O, Noritake K, Fuji M, Kasugai S (2013) Peri-implantitis progression around thin sputtered hydroxyapatite-coated implants: clinical and radiographic evaluation in dogs. Int J Oral Maxillofac Implants 28:701–709. https://doi.org/10.11607/jomi.2891
Godoy-Gallardo M, Manzanares-Cespedes MC, Sevilla P, Nart J, Manzanares N, Manero JM, Gil FJ, Boyd SK, Rodriguez D (2016) Evaluation of bone loss in antibacterial coated dental implants: an experimental study in dogs. Mater Sci Eng C Mater Biol Appl 69:538–545. https://doi.org/10.1016/j.msec.2016.07.020
Lopez-Piriz R, Sola-Linares E, Granizo JJ, Diaz-Guemes I, Enciso S, Bartolome JF, Cabal B, Esteban-Tejeda L, Torrecillas R, Moya JS (2012) Radiologic evaluation of bone loss at implants with biocide coated titanium abutments: a study in the dog. PLoS One 7:e52861. https://doi.org/10.1371/journal.pone.0052861
Blanco J, Pico A, Caneiro L, Novoa L, Batalla P, Martin-Lancharro P (2018) Effect of abutment height on interproximal implant bone level in the early healing: a randomized clinical trial. Clin Oral Implants Res 29:108–117. https://doi.org/10.1111/clr.13108
Galindo-Moreno P, Fernandez-Jimenez A, O’Valle F, Monje A, Silvestre FJ, Juodzbalys G, Sanchez-Fernandez E, Catena A (2015) Influence of the crown-implant connection on the preservation of peri-implant bone: a retrospective multifactorial analysis. Int J Oral Maxillofac Implants 30:384–390. https://doi.org/10.11607/jomi.3804
Monje A, Pommer B (2015) The concept of platform switching to preserve peri-implant bone level: assessment of methodologic quality of systematic reviews. Int J Oral Maxillofac Implants 30:1084–1092. https://doi.org/10.11607/jomi.4103
Jung RE, Herzog M, Wolleb K, Ramel CF, Thoma DS, Hammerle CH (2017) A randomized controlled clinical trial comparing small buccal dehiscence defects around dental implants treated with guided bone regeneration or left for spontaneous healing. Clin Oral Implants Res 28:348–354. https://doi.org/10.1111/clr.12806
Monje A, Chappuis V, Monje F, Munoz F, Wang HL, Urban IA, Buser D (2019) The critical peri-implant buccal bone wall thickness revisited: an experimental study in the beagle dog. Int J Oral Maxillofac Implants 34:1328–1336. https://doi.org/10.11607/jomi.7657
Acknowledgments
The authors would like to express their appreciation to the personnel of the Rof Codina research facilities, for their invaluable support with the care of the animals. Also, the authors would like to acknowledge the support with the histological processing made by Fernando Muñoz’s research team.
Funding
This study was partially funded through a research contract between the University Complutense of Madrid and MIS Implants (Israel).
Author information
Authors and Affiliations
Contributions
• Javier Sanz-Esporrin: data retrieval, data analysis, help in surgical procedures, and writing the manuscript.
• Riccardo Di Raimondo: helped in surgeries in dogs.
• Rafael Pla: helped in surgeries in dogs.
• Fernando Luengo: helped in surgeries in dogs.
• Fabio Vignoletti: surgeries in dogs, protocol design, and manuscript editing.
• Javier Núñez: surgeries in dogs and manuscript editing.
• Georgios Antonoglou: histomorphometry measurements and draft preparation.
• Juan Blanco: surgeries in dogs, protocol design, and manuscript editing.
• Mariano Sanz: protocol design and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 219 kb)
Rights and permissions
About this article
Cite this article
Sanz-Esporrin, J., Di Raimondo, R., Pla, R. et al. Experimental peri-implantitis around titanium implants with a chemically modified surface with a monolayer of multi-phosphonate molecules: a preclinical in vivo investigation. Clin Oral Invest 25, 3789–3800 (2021). https://doi.org/10.1007/s00784-020-03708-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03708-4