Abstract
Objective
This study investigated the association between periodontitis severity (exposure) and metabolic syndrome (MetS - outcome), using two criteria for diagnosis of the outcome, since this relationship remains unexplored.
Materials and methods
A case-control study was conducted with 870 individuals: 408 with first MetS diagnosis (cases) and 462 without MetS (controls). Participants’ general information was obtained using a questionnaire and laboratory data was collected from medical records. Periodontitis severity criteria followed the Center for Disease Control and Prevention: none, mild, moderate, and severe. Odds ratios (OR) and 95% confidence intervals (95% CI) were determined by logistic regression analysis.
Results
Findings showed a positive association between moderate and severe periodontitis and MetS: ORadjusted = 1.64 (95% CI: 1.01 to 2.68) and ORadjusted = 1.94 (95% CI: 1.19 to 3.16), respectively, after adjustment for age, sex, schooling level, smoking habit, and cardiovascular disease. The adjusted measurements showed that among individuals with moderate or severe periodontitis, the probability of having MetS was around two times greater than among those without periodontitis, and that the chance was greater among participants with severe periodontitis than those with moderate periodontitis.
Conclusion
An association between the severity of periodontal status and MetS was found, suggesting a possible relationship between the two diseases.
Clinical relevance
MetS influences the etiology of cardiovascular diseases, one of the leading causes of mortality worldwide. The findings suggest that the greater the severity of periodontitis, the greater is the association magnitude with MetS. The health professional needs to recognize that the importance of periodontal disease may play in MetS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is related to cardiovascular disease and diabetes; both recognized among the leading cause of death worldwide [1]. MetS is a group of disorders, such as hypertension, diabetes, obesity, and dyslipidemia. The magnitude of this health problem, and its associated factors, has resulted in the recognition of the importance of MetS as a public policy concern throughout the world justifying more profound investigation on the thematic [2, 3].
Periodontitis, a chronic localized inflammatory disease, has been also associated with MetS. Periodontitis is the second most prevalent oral disease worldwide [4] and the fourth most frequent illness of the Global Disease Burden list and its global burden remains high [5, 6].
Furthermore, reduced high-density lipoprotein levels and increased triglyceride and low-density lipoprotein levels cause a severe pro-inflammatory state (166). All these mechanisms can damage endothelial cells and promote atherogenesis and thrombus formation, thereby increasing the risk of cardiovascular diseases and hypertension. These conditions, when present together, characterize MetS [7,8,9].
The biological plausibility explaining the relationship between periodontitis and MetS is based on the knowledge that periodontal pathogens may trigger a subclinical systemic inflammatory process due to their release of toxic products, such as extracellular vesicles and lipopolysaccharides, resulting in an increase in systemic levels of inflammatory cytokines [10]. Elevated plasma levels of inflammatory mediators, especially tumor-necrosis factor, influence the development of insulin resistance, mainly responsible for obesity and type 2 diabetes and for the alteration of hepatic metabolism, for example the increase in C-reactive protein (CRP) levels and hypertriglyceridemia. Furthermore, reduced high-density lipoprotein levels and increased triglyceride and low-density lipoprotein levels cause a severe pro-inflammatory state [7]. All these mechanisms can damage endothelial cells and promote atherogenesis and thrombus formation, thereby increasing the risk of cardiovascular diseases and hypertension [8]. These conditions, when present together, characterize MetS [9, 11, 12].
However, the relationship between periodontitis severity levels and MetS is still inconclusive. The studies that evaluated this association showed methodologic limitations such as different periodontitis severity definitions, confounding bias, and impossibility to identify the effect of periodontitis levels in the evaluated outcome [13,14,15,16,17,18,19,20].
Recently, a new periodontal disease classification has been developed [21] which has not been used in older studies. Therefore, in the present study, the classification of periodontitis severity levels recommended by the Center for Disease Prevention and Control and American Academy of Periodontology [22, 23] will be employed, along with two internationally recognized criteria for definition of MetS cases [24, 25] to make possible the comparability among the studies. Thereby, the current study investigated the association between periodontitis severity (exposure) and metabolic syndrome (MetS - outcome), comparing two criteria for diagnosis of the outcome.
Materials and methods
A case-control study was conducted by recruiting users of the public health service of the city of Feira de Santana, BA, Brazil, from November 2014 to September 2016. Cases were individuals with first diagnosis of MetS. Controls were defined as individuals without the syndrome. For each case selected, a control was identified and selected randomly from those who sought immunizations, and their companions and neighbors. Those who agreed to take part read and signed an informed consent form and individual anonymity was preserved. This investigation was approved by human subjects Ethics Committee of Feira de Santana State University (number: 928.178) and was conducted in accordance with the Declaration of Helsinki.
Individuals > 18 years were included in the study. Those with systemic changes that needed prior antibiotic prophylaxis to perform periodontal examination, who underwent periodontal treatment or antibiotic therapy within 6 months prior to the examination, with less than 4 teeth, to ensure the validity of the periodontal condition measurements, pregnant women, and HIV positive individuals were excluded from the study.
Sample size
To establish the sample size, estimates were determined: frequency of severe periodontitis, 99% confidence interval, power of 99%, and a ratio of 1:1 between cases and controls. The sample size obtained used a severe periodontitis frequency of 37% for the case group and odds ratio of 2.31 [14]. The minimum estimated sample size with an increase of 20% was 866 individuals, considering the most severe periodontal disease status.
Data collection procedures
All participants were interviewed using a standardized questionnaire to collect socioeconomic-demographic, habits and lifestyle, medical and dental history, and anthropometric data (weight, height, and waist circumference).
Body weight was obtained using an anthropometric digital scale (Filizola, São Paulo, Brazil). Height was recorded directly, using a stadiometer attached to the wall. Body mass index was calculated using the weight and height data [26]. Abdominal circumference (AC) was measured using a tape measure (150 cm - scale 0.5 cm), describing a circle between the iliac crest and the lower costal border held with the individual in an upright position, with a relaxed abdomen and arms beside the body and feet together [27].
Blood pressure (BP) was measured with a calibrated sphygmomanometer and stethoscope (BIC - São Paulo, Brazil). BP was measured three times, with a 1-min pause between measurements, in a calm environment and with correct positioning of the participant. BP was calculated as the arithmetic mean of the last two measurements [28].
Laboratory test results obtained a maximum of 90 days before the oral examination and interview data were obtained from medical records, such as fasting blood glucose, triglycerides, and HDL cholesterol levels. If these data were not available, additional tests were undertaken.
Complete clinical periodontal examination was performed by a single-blinded examiner (ISCEB), dentist, using a Williams probe (Hu-Friedy, Chicago, USA) at six sites per tooth (mesial buccal, mid-buccal, distobuccal, distolingual, med-lingual, and mesial lingual). Periodontal status was assessed by calculation of clinical attachment level, determined as the distance from the cemento-enamel junction to the bottom of the pocket [29]. The pocket depth was registered as the distance between the gingival margin and the most apical depth of the pocket [30]. Gingival recession was determined from the distance between the gingival margin and the cemento-enamel junction [29]. Bleeding upon probing was defined as the presence or absence of bleeding following removal of the probe during the pocket depth measurement [31]. The visible plaque index was determined by the presence of supragingival biofilm deposits on the tooth surface, utilizing a periodontal probe, at four locations per tooth: lingual, buccal, distal, and mesial [32].
Definition of periodontitis severity levels
Individuals were assigned to four groups according to the Center for Disease Prevention and Control and American Academy of Periodontology criteria [22, 23]: mild periodontitis, moderate periodontitis, severe periodontitis, and without periodontitis. Mild periodontitis includes presence of at least 2 interproximal sites with clinical attachment level ≥ 3 mm (not in the same tooth) and at least 2 interproximal sites with probing depth ≥ 4 mm (not in the same tooth) or 1 site with probing depth ≥ 5 mm. Moderate periodontitis includes presence of at least 2 interproximal sites with clinical attachment level ≥ 4 mm (not in the same tooth) or at least 2 interproximal sites with probing depth ≥ 5 mm (not in the same tooth). Severe periodontitis includes presence of at least 2 interproximal sites with clinical attachment level ≥ 6 mm (not in the same tooth) and at least 1 interproximal site with probing depth ≥ 5 mm. Without periodontitis includes individuals not included in the above criteria.
Metabolic syndrome diagnosis
The main criterion chosen for defining the case group, with first diagnosis of MetS, was the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults - NCEP-ATPIII [25]. Participants diagnosed with MetS had at least three of the following components: (1) blood pressure—systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg or prior blood pressure treatment; (2) triglycerides ≥ 150 mg/dL or specific treatment; (3) HDL cholesterol < 40 mg/dL in men and < 50 mg/dL in women or specific treatment; (4) fasting plasma glucose ≥ 100 mg/dL or previous diabetes mellitus or specific treatment; and (5) abdominal circumference ≥ 90 cm in men and in women ≥ 80.
All participants were also defined with MetS using the criterion of The American Heart Association and the National Heart, Lung, and Blood Institute – AHA/NHLBI [24]. It follows the same principle of NCEP-ATPIII criterion: at least three of the previous components, except abdominal circumference ≥ 102 cm in men and in women ≥ 88, according to the parameters of the International Diabetes Federation [33].
Factors investigated
Other epidemiologic, demographic, and biologic data pertaining to the following factors were also collected: sex (male or female), age (in years), race/skin color (white or non-white), place of residence (urban or rural), schooling level (in years of study), marital status (with partner or without partner), current occupation (employed or unemployed/does not work or retired), family income (in minimum salary – MS, < 1 MS or 1 to 2 MS or > 2 MS), number of children (≤ or > 3 children), and household density (number of people per home). Physical activity (yes - weight training, walking, among others, ≥ 3 times a week, or no), alcoholic beverage consumption (yes, ≥ 3 times a week or no), smoking habit (smoker/former smoker in the last 6 months or non-smoker), presence or absence of diseases or conditions, as follows: cardiovascular disease, hypertension, diabetes, body mass index (kg/m2), abdominal obesity (NCEP-ATPIII), systolic and diastolic blood pressure (mmHg), fasting plasma glucose, triglycerides (mg/dL), and HDL cholesterol (mg/dL) were investigated.
Data analysis
Descriptive analysis of the independent variables (mild, moderate, and severe periodontitis) and the covariables was performed to compare case status (individuals with first diagnosis of MetS as the dependent variable) to controls. To further compare case and control groups, bivariate analysis was used, employing Pearson’s Chi-square test for categorical covariables, based on covariable distribution, with a significance level of 5%. Data analysis was performed employing STATA version 14.0 and the Statistical Package for the Social Sciences - SPSS, version 17.0 (SPSS Inc., Chicago, IL, USA).
The selection of confounders and modifier covariables was in accordance with a presumed causality relationship between periodontitis severity level and MetS. Nonetheless, a theoretical conceptual framework was built and the covariables age, sex, schooling level, smoking habit, and cardiovascular disease were selected as confounders. Those covariables of a similar nature, proxi covariables, were represented by only one of them in the modeling in order to avoid overadjustment. Moreover, the presence of modifier covariables was evaluated employing the maximum likelihood ratio test (p < 0.05). For covariables in which there is no identification of effect modification, the presence of confounding covariables was tested employing the backward strategy. A confounder was identified when it produced an alteration of at least 10% in the association measurement.
Unconditional logistic regression analysis estimated the association measurements between periodontitis severity levels and MetS, both crude and adjusted, employing the following statistical models: no periodontitis vs. mild periodontitis and MetS, no periodontitis vs. moderate periodontitis and MetS, and no periodontitis vs. severe periodontitis and MetS. Lastly, the odds ratios (OR) and their 95% confidence intervals (95% CI) were obtained from all possible statistical models. To investigate the goodness of fit of the models employed, the Hosmer-Lemeshow test was used.
Results
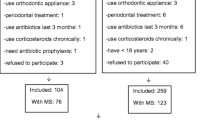
The study consisted of 924 individuals who accepted to participate. However, 54 subjects withdrew from participation during the exam period, representing a loss rate of 5.8%. The final sample was 870 individuals, 284 (32.6%) men and 586 (67.4%) women, with a mean ± standard deviation of age of 52.8 ± 14.8 (median of 53.0 years) and age limits of 18-89 years.
The criterion of choice for defining the first diagnosis of MetS was the NCEP-ATPIII. Thus, 408 individuals (46.89%) made up the case group (296 women and 112 men), while 462 (53.11%) comprised the control group (290 women and 172 men). The sample was also classified for a diagnosis of MetS using the AHA/NHLBI criterion: 494 (56.78%) comprised the case group (155 men and 339 women) and 376 individuals (43.22 %) comprised the control group (129 men and 247 women).
General characteristics of individuals are presented in Table 1, classified using both definition criteria for MetS: NCEP-ATPIII and AHA/NHLBI. Covariables that were rarely observed were not incorporated into Table 1. Differences between cases and controls, statistically significant, for both diagnostic criteria definitions included age (p < 0.01), schooling level (p < 0.01), race/skin color (p < 0.01), current occupation (p < 0.01), family income (p = 0.01), number of children (p < 0.01), smoking habit (p < 0.05), and periodontitis severity (p < 0.05) (Table 1).
Other factors related to or part of the diagnosis of MetS showed a statistically significant difference (p ≤ 0.05) between the comparison groups, for both the case definition criteria. Factors, such as cardiovascular disease, hypertension, diabetes, body mass index, abdominal obesity, and others, showed a higher occurrence for the group of individuals with the first diagnosis of MetS (Table 1).
The distribution of severity levels of periodontitis also varied considerably (Table 1). The frequency of severe periodontitis was higher than moderate among the cases (48.80% and 47.40%), for both diagnostic criteria of MetS (NCEP-ATPIII and AHA/NHLBI, respectively). The occurrence of moderate periodontitis was also higher in the cases than in controls. On the other hand, the frequency of mild periodontitis was very low, and individuals with this periodontal condition were not identified in the control group when the participants were classified according to AHA/NHLBI diagnosis of MetS. The frequency of subjects with no periodontitis was slightly < 17%, both in cases and in controls.
Some clinical parameters showed statistical difference among the comparison groups (Table 2). The average number of teeth present was greater in the control groups (14.65 ± 7.23, in both the case groups, and 18.50 ± 7.76 and 19.37 ± 7.50, in the control groups, NCEP-ATPIII and AHA/NHLBI, respectively), whereas the average clinical attachment level was higher in the case groups (3.59 ± 1.36 and 3.55 ± 1.33, in the case groups, and 3.24 ± 1.38 and 3.22 ± 1.42, in the control groups, NCEP-ATPIII and AHA/NHLBI, respectively). Also, the number of teeth ≥ 30% with clinical attachment level ≥ 5 mm (265 (65.00%) and 316 (64.00%), in the case group, and 228 (49.40%) and 177 (47.10%), in the control groups, NCEP-ATPIII and AHA/NHLBI, respectively) occurred in the case groups (unpublished data).
According to crude association measurements (Table 3), severe and moderate periodontitis were positively associated with MetS for both criteria used to define MetS. The association measurements ranged from ORcrude = 2.02 to 2.35; 95% CI: 1.27 to 3.71. In the final model using unconditional logistic regression analysis, the adjustment measurements using the selected confounders, age, sex, schooling level, smoking habit, and cardiovascular disease, also confirmed positive association with a decrease in the magnitude in comparison to the crude ones (Table 3).
In the model using the diagnosis of MetS according to NCEP-ATPIII, the association measurements for moderate periodontitis were ORadjusted = 1.64 and 95% CI: 1.01 to 2.68 and for severe periodontitis: ORadjusted = 1.94 and 95% CI: 1.19 to 3.16. Likewise, for the model using the diagnosis of MetS according to AHA/NHLBI, the adjusted measurements exhibited a decrease compared to the unadjusted ones: ORadjusted = 1.74 and 95% CI: 1.08 to 2.80 and ORadjusted = 1.90 and 95% CI: 1.19 to 3.03, moderate and severe periodontitis, respectively.
The Hosmer-Lemeshow statistical test confirmed the goodness of fit of the regression models employed and the good quality of them, given that the p value ranged from 0.29 to 0.49 and the null hypothesis was rejected.
Discussion
This study showed a positive association between moderate and severe periodontitis and MetS, for both MetS definition criteria. It also demonstrated that the more severe periodontitis is, the greater the magnitude of the positive association between periodontitis and MetS. Some of these findings are in agreement with those of two studies [15, 17]. However, Fukui et al. defined periodontitis severity levels using percentage of probing depth, and Li et al. employed periodontal attachment loss. Both criteria, based on a single periodontal clinical parameter, proved to be fragile, for example, compromising the accuracy of the association measurements and resulting in wide confidence intervals [15].
Other findings regarding the association only between severe periodontitis and MetS are in agreement with several studies [14, 18, 19], one study only in men [20] and then, other study using only panoramic radiographic bone loss to define periodontitis severity [16]. In contrast, only one investigation did not confirm this association [13].
This study’s findings demonstrated that relationship may exist between the levels of periodontitis severity and MetS. Among those individuals with severe periodontitis, the probability of having MetS was always greater (approximately twofold) in comparison to those with moderate periodontitis (approximately one-and-a-half fold). The strong magnitude of this association agrees with the conclusions of a systematic review and meta-analysis, which estimated an OR of 1.7 to 2.1 [34].
These findings need to be evaluated with cautious since the difference between those with moderate and severe periodontitis was little. Therefore, it has been shown that chronic inflammatory diseases have the capacity to increase systemic inflammation, with greater inflammation increasing the risk, also providing a justification for a direct relationship between inflammation and MetS [1]. The biological plausibility can be explained by pathogenic factors such as systemic dissemination of periodontopathogic microorganisms and/or their toxic products from periodontal tissues or adipose tissues, oxidative stress, proatherogenic lipoproteins, abdominal obesity, cross-reaction, and molecular mimicry [34].
An important issue to be highlighted in the present study refers to the criterion used to stratify periodontitis according to the severity level, allowing comparability across studies [22, 23]. Four previous studies have used this definition criterion [14, 18,19,20], but only two studies classified individuals according to mild level of periodontitis severity, and did not join this stratum to the group without periodontitis [18, 19].
In this study, we used two internationally known criteria to define MetS, NCEP-ATPIII, and AHA/NHLBI, as there is a lack of consensus regarding which one best represents MetS. The NCEP-ATPIII criterion for defining the first MetS has been the most widely used in previous studies [13, 14, 18, 19, 34]. The AHA/NHLBI criterion has also been used in recent previous studies evaluating severity of periodontitis and MetS [18,19,20]. For this criterion, the waist circumference value is lower than that used by the NCEP-ATPIII, explaining the higher frequency of diagnosis of MetS.
Other aspects of this study’s method that strengthened our findings include sample size. Minimum sample size is an important issue in studies evaluating a biological gradient of an exposure [35]. The sample size of the present investigation had enough power to evaluate the association between periodontitis severity and MetS, as demonstrated by the precise association measurements of narrow 95% CIs that ranged from 1.01 to 3.16.
Complete periodontal examinations were performed in this study, as were in only three previous studies [15, 18, 19]. A complete exam helps to avoid classification errors, such as an underestimate or overestimate of the magnitude and/or presence of periodontitis [22].
In this study, evaluation of confounding covariables was possible due to a large number of participants. An objective was to neutralize these confounding covariables, since they can affect both exposure and outcome. The conceptual framework for this investigation was in accordance with knowing that both periodontitis and MetS are found with greater frequency with increasing age [14, 36, 37]. Furthermore, both diseases occur more frequently in smokers [38, 39], and both have a relationship with cardiovascular disease [40, 41]. Regarding the socioeconomic status of the individuals, as represented by schooling level, the frequency of both periodontitis and MetS is higher for the lowest status, that is, fewest years of education [35, 42]. To avoid overadjustment of the models, schooling level represented proxy covariables, such as race/skin color, current occupation, and family income.
This study also had limitations. First, it was not possible to exclude the possibility of residual confounders represented by factors that were not measured, such as genetics [14, 18, 43]. The observational case-control design is another limitation, which does not allow for measurement of the temporality between exposure and outcome. In relation to medication usage and diet, although all individuals received medications to control diabetes and hypertension, and when necessary, received diet counseling, it cannot be sure that everyone followed the orders correctly. Another limitation is the small number of the participants defined with mild periodontitis. The association measurement for this stratum was impossible to estimate, likely due to most individuals in this study having more severe periodontal conditions due to lower oral health awareness and limited access to professional dental care.
Despite these limitations, this investigation contributes to the available evidence of a possible relationship between severity of periodontitis and MetS, as severe periodontitis had a stronger association with MetS than moderate periodontitis, for both criteria used to diagnose the outcome, reinforcing the results. The findings of this investigation also indicate that further study is needed to determine if the relationship between periodontitis severity and MetS is a result of severity of systemic inflammation.
References
Dregan A et al (2014) Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 130(10):837–844
Crowson CS et al (2013) Rheumatoid arthritis and cardiovascular disease. Am Heart J 166(4):622–628.e1
Corrado E et al (2010) An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb 17(1):1–11
Marcenes W et al (2013) Global burden of oral conditions in 1990-2010: a systematic analysis. J Dent Res 92(7):592–597
Vos T et al (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2163–2196
Tonetti MS et al (2017) Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol 44(5):456–462
Chen S et al (2014) Hyperlipidemia causes changes in inflammatory responses to periodontal pathogen challenge: implications in acute and chronic infections. Arch Oral Biol 59(10):1075–1084
Schmitt A et al (2015) Periodontitis and arterial stiffness: a systematic review and meta-analysis. J Clin Periodontol 42(11):977–987
Genco RJ et al (2005) A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol 76(Suppl 11S):2075–2084
Lin D et al (2003) Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun 71(9):5163–5168
Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132(6):2169–2180
Khosravi R et al (2013) Tumor necrosis factor-α and interleukin-6: potential interorgan inflammatory mediators contributing to destructive periodontal disease in obesity or metabolic syndrome. Mediat Inflamm 2013:728987
Borges P et al (2007) Prevalência e características associadas à síndrome metabólica em nipo-brasileiros com e sem doença periodontal. Cad Saúde Públ 23:657–668
D’Aiuto F et al (2008) Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J Clin Endocrinol Metab 93(10):3989–3994
Li P et al (2009) Relationship of metabolic syndrome to chronic periodontitis. J Periodontol 80(4):541–549
Nesbitt MJ et al (2010) Association of periodontitis and metabolic syndrome in the Baltimore Longitudinal Study of Aging. Aging Clin Exp Res 22(3):238–242
Fukui N et al (2012) Periodontal status and metabolic syndrome in middle-aged Japanese. J Periodontol 83(11):1363–1371
Gomes-Filho IS et al (2016) Severity of periodontitis and metabolic syndrome: is there an association? J Periodontol 87(4):357–366
Pham T (2018) The association between periodontal disease severity and metabolic syndrome in Vietnamese patients. Int J Dent Hyg 16(4):484–491
Kim OS et al (2018) The severity of periodontitis and metabolic syndrome in Korean population: the Dong-gu study. J Periodontal Res 53(3):362–368
Caton JG et al (2018) A new classification scheme for periodontal and peri-implant diseases and conditions-introduction and key changes from the 1999 classification. J Periodontol. 2018 Jun;89 Suppl 1:S1-S8. J Clin Periodontol 45(S20)
Page RC, Eke PI (2007) Case definitions for use in population-based surveillance of periodontitis. J Periodontol 78(7):1387–1399
Eke PI et al (2012) Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83(12):1449–1454
Alberti KG et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645
Grundy SM et al (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112(17):2735–2752
Gorman A et al (2012) Changes in body weight and adiposity predict periodontitis progression in men. J Dent Res 91(10):921–926
Chan DC et al (2003) Waist circumference, waist-to-hip ratio and body mass index as predictors of adipose tissue compartments in men. QJM 96(6):441–447
Andrade JP (2010) VI Diretrizes Brasileiras de Hipertensão. Arq Bras Cardiol 95:1–51
Ramfjord SP (1959) Indices for prevalence and incidence of periodontal disease. J Periodontol 30(1):51–59
Pihlstrom BL, Ortiz-Campos C, Mchugh RB (1981) A randomized four-years study of periodontal therapy. J Periodontol 52(5):227–242
Ainamo J, Bay I (1975) Problems and proposals for recording gingivitis and plaque. Int Dent J 25(4):229–235
López NJ, Smith PC, Gutierrez J (2002) Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Periodontol 73(8):911–924
(IDF), I.D.F. The Internacional Diabetes Federation consensus worldwide definition of metabolic syndrome. 2005 [cited 2016 10 February]
Nibali L et al (2013) Clinical review: association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab 98(3):913–920
Rothman KJ, Greeland S, Lash TL (2011) Modern epidemiology, 3rd edn. Artmed, Porto Alegre, p 888
Ford ES, Giles WH, Dietz WH (2002) Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287(3):356–359
Razzouk L, Muntner P (2009) Ethnic, gender, and age-related differences in patients with the metabolic syndrome. Curr Hypertens Rep 11(2):127–132
Duane B (2014) Further evidence that periodontal bone loss increases with smoking and age. Evid Based Dent 15(3):72–73
Lee JA et al (2017) Impact of combined lifestyle factors on metabolic syndrome in Korean men. J Public Health (Oxf) 39(1):82–89
Kurl S et al (2016) Metabolic syndrome and the risk of sudden cardiac death in middle-aged men. Int J Cardiol 203:792–797
Stewart R, West M (2016) Increasing evidence for an association between periodontitis and cardiovascular disease. Circulation 133(6):549–551
Lee WY et al (2005) Effects of smoking, alcohol, exercise, education, and family history on the metabolic syndrome as defined by the ATP III. Diabetes Res Clin Pract 67(1):70–77
Perri R et al (2012) MicroRNA modulation in obesity and periodontitis. J Dent Res 91(1):33–38
Acknowledgments
Thanks to all those who contributed in some way to the data collection, especially the undergraduate and graduate students.
Funding
The work was supported by the Research Support Foundation of the State of Bahia (FAPESB), Salvador, Bahia, Brazil. And also by The National Council for Scientific and Technological Development (CNPq), Brasilia, Brazil, and Feira de Santana State University, Bahia, Brazil.
Author information
Authors and Affiliations
Contributions
I. S. Gomes-Filho, I. S. C. E. Balinha, S. S. da Cruz, S. C. Trindade, E. M. M. Cerqueira, J. S. Passos-Soares, J. M. F. Coelho, M. I. P. Vianna, A. M. Hintz, T. S. Costa, P. P. dos Santos, A. C. M. G. Figueiredo, and I. C. O. da Silva contributed to the formulation of the question under investigation, conception of the study, design, data acquisition, data analysis and interpretation, and draft and critical revision of the manuscript. A. M. T. Ladeia, F. A. Scannapieco, M. L. Barreto, and P. M. Loomer contributed to the analysis, interpretation, and critical revision of the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomes-Filho, I.S., Balinha, I.d.S.C.E., da Cruz, S.S. et al. Moderate and severe periodontitis are positively associated with metabolic syndrome. Clin Oral Invest 25, 3719–3727 (2021). https://doi.org/10.1007/s00784-020-03699-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03699-2