Abstract
Objective
The objective of this systematic review was to determine the effectiveness of preoperative oral pregabalin for anxiety control, the most effective dosage regimen, its impact on postoperative pain, and its adverse effects.
Materials and methods
A search was conducted of PubMed/Medline and clinicaltrials.gov (National Library of Medicine, Washington, DC), Scopus, Web of Science, and Cochrane databases for studies published between January 2009 and November 2018, with no language restriction. Based on PRISMA guidelines, the specific question was: is preoperative oral pregabalin effective and safe for anxiety control in patients undergoing surgery? The critical reading of retrieved studies followed questions prepared by the CASPe Network, and their methodological quality was evaluated using the Jadad Scale.
Results
Twelve randomized controlled trials were selected for review. All twelve studies were trials of high quality. A dose of 75 mg preoperative oral pregabalin has been found to reduce anxiety and stabilize intraoperative hemodynamics, although a more significant improvement appears to be achieved with a single dose of 150 mg pregabalin at least 1 h before the surgery. It is not associated with any severe adverse effects.
Conclusion
Preoperative administration of oral pregabalin in a single dose of 150 mg appears to be effective to significantly reduce the anxiety of patients, intraoperative hemodynamic changes, and postoperative pain.
Clinical relevance
These findings suggest that pregabalin is useful and safe for preoperative and intraoperative anxiety control in patients undergoing surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preoperative anxiety has been reported to affect up to one out of six adults due to undergo surgery, and it has been widely selected as model to test the acute anxiolytic effect of various drugs [1]. Components of anxiety include the following: intense feelings of apprehension, fear, or anguish when confronting a perceived threat; a state of irritability that can lead to a loss of concentration capacity; and a set of variable somatic symptoms, including perspiration, palpitations, precordial oppression, fatigue, frequent urination, headaches, myalgias, insomnia, and digestive discomfort [2]. The intensity of preoperative anxiety is influenced by multiple factors, including the expected magnitude of the intervention, the amount of time patients have to adapt to the upcoming event, and personal and family histories of experiences with surgery, besides the propensity of individuals for anxiety [3]. Health care professionals should be aware that routine interventions that appear to be of little importance can pose a major challenge to emotionally vulnerable patients and may affect their recovery.
Benzodiazepines have classically been prescribed for preoperative anxiolysis but are associated with adverse effects (e.g., dizziness, somnolence, respiratory depression), and there is considerable research interest in the development of alternative drugs to treat anxiety, including gabapentinoids [4]. One member of this class of drugs, pregabalin (CASRN: 148553-50-8), is a structural analog of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), to which it is not functionally related. It possesses anticonvulsive, anxiolytic, and antihyperalgesic properties [5]. Pregabalin acts by binding to auxiliary subunit α2-δ of voltage-gated calcium channels in the central nervous system, potentially displacing [3H]-gabapentin and thereby increasing its affinity for this subunit. Activation of these receptors has been implicated in the onset of partial epilepsy seizures, pain, and hypersensitization phenomena [6, 7]. Pregabalin can therefore reduce excitatory neurotransmitters and block hyperalgesia and the sensitization center [6,7,8]. Oral pregabalin is rapidly absorbed, demonstrating linear pharmacokinetics and 90% bioavailability, and it does not bind to plasmatic proteins; it reaches its maximum blood concentration at 1 h and has an elimination half-life of 6 h [9].
Pregabalin is used for pain relief in diabetic neuropathy, postherpetic neuralgia, and focal epileptic seizures. Reports of its effectiveness for acute postoperative pain in minor gynecological surgery, laparoscopic cholecystectomy, amygdalectomy, and third molar surgery [10] have prompted research into its effectiveness against fibromyalgia and generalized anxiety and as co-adjuvant in the multimodal treatment of postoperative analgesia. There have been numerous reports on the use of pregabalin to control preoperative anxiety and reduce postoperative pain and opioid consumption. However, no consensual guidelines have been established on the appropriate dosage regimen.
With this background, we performed a systematic review on the utilization of preoperative oral pregabalin for anxiety control, given the frequency of preoperative anxiety in oral surgery and its relationship with postoperative pain. Our objective was to determine its effectiveness, the optimal dosage regimen, its role in intraoperative hemodynamic changes, and its adverse effects.
Material and methods
Scope of the question
We constructed the following PICO question based on PRISMA (Preferred Reporting Items for Systematic Review and Meta-analysis) guidelines: is preoperative oral pregabalin effective and safe in anxiety control for patients undergoing surgery intervention? P and I (patients and intervention) = patients subjected to surgery under general or local anesthesia receiving a single dose of preoperative pregabalin for anxiety control; C (comparison) = control group of patients not treated with pregabalin; O (outcome) = hemodynamic changes, anxiolytic effect, level of sedation, and drug-related adverse events.
Eligibility criteria
Review inclusion criteria: (a) clinical trial; (b) randomized study; (c) presence of control group and/or group with other medication for the same purpose; and (d) study of patients receiving surgery under general or local anesthesia and administered with preoperative oral pregabalin for anxiety control and/or intraoperative hemodynamic stability. Exclusion criteria were letters to the editor, reviews, systematic reviews, meta-analyses, and case reports.
Search strategy and study selection
A search was conducted of PubMed/Medline and clinicaltrials.gov (National Library of Medicine, Washington, DC), Scopus, Web of Science, and Cochrane databases for studies published between January 2009 and November 2018, with no language restriction. The search strategy was:
(“mouth”[MeSH Terms] OR “mouth”[All Fields] OR “oral”[All Fields]) AND (“pregabalin”[Supplementary Concept] OR “pregabalin”[All Fields]) AND (“anxiety”[MeSH Terms] OR “anxiety”[All Fields]) AND (preoperative[All Fields] OR “preoperative period”[MeSH Terms] OR “preoperative period”[All Fields]) AND (“surgery”[Subheading] OR “surgery”[All Fields] OR “surgical procedures, operative”[MeSH Terms]) OR (“surgical”[All Fields]) AND “procedures”[All Fields] AND (“operative”[All Fields] OR “operative surgical procedures”[All Fields] OR “surgery”[All Fields] OR “general surgery”[MeSH Terms] OR “general”[All Fields]) AND (“surgery”[All Fields] OR “general surgery”[All Fields]).
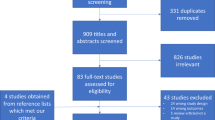
The titles and abstracts of retrieved items were independently examined by two researchers (MITG, FJMM) to select those meeting eligibility criteria. If the abstract included inadequate information for this purpose, the whole article was reviewed before making the final decision. Discrepancies between evaluators were solved by consensus or, when this not possible, by consulting a third examiner (MVOG). A Kappa value of 0.92 was obtained for agreement between the evaluators on the inclusion/exclusion of studies. Search results were cross-verified to eliminate duplicates. The initial search retrieved 84 studies from PubMed, 132 from Scopus, 78 from WOS, and 53 from Cochrane. Out of the ten items selected for meeting eligibility criteria, nine were finally included in the review (see below). Figure 1 depicts the article selection process.
Evaluation of the methodological quality of the study
The critical reading of the retrieved articles addressed the eleven questions proposed by the Spanish Critical Appraisal Skills Program (CASPe) Network [11]. The first three questions rule out articles for which the response is negative, while the remaining eight concern their methodological quality (research design) (Table 1)
The widely used Jadad scale [24] was applied to evaluate the methodological quality of the thirteen retrieved studies, which were all randomized controlled trials (RCTs). This scale evaluates randomization, blinding, and withdrawals and dropouts of patients who fail to complete the course of the trial by answering a 3-point questionnaire. Each question was to be answered with either a yes or a no. Each yes would score a single point, each no zero points, and deduct 1 point in case the method of randomization or blinding is inappropriate. This system allocates trials a score of between zero (very poor) and five (rigorous). Twelve RCTs obtained a score ≥ 3, considered evidence level Ib (evidence from at least one RCT), and were included in the final sample, whereas one obtained a score < 3 and was therefore excluded (Table 2). Consequently, twelve RCTs were finally included in the review.
Results
Characteristics of reviewed studies
The search found twelve relevant study articles [12,13,14,15,16,17,18,19,20,21,22,23]. All twelve studies were RCTs of high quality. The surgery was conducted under general anesthesia in nine of them [12, 13, 15,16,17, 20,21,22,23], under local anesthesia in two [14, 19], and without anesthesia in one [18]. Four RCTs had 40–80 participants [13, 14, 17, 19, 23] and the other five had 81–120 [12, 15, 16, 18, 20,21,22], with a total age range of 18–65 years. Control groups received a placebo in nine RCTs, including one with an additional control group receiving 0.5 mg alprazolam [18], being administered with 0.3 mg clonidine in the other RCT [13]. Two RCTs include a control group who received i.v dexmedetomidine [21, 22] and a combination of pregabalin and i.v dexmedetomidine [22] (Table 3).
All reviewed RCTs reported the absence of any bias attributable to the characteristics of participants. All except for one study [13] contained a table displaying these variables, including age, sex, ethnicity, ASA classification, weight, and body mass index. The type of surgery was specified in seven studies [12, 14, 15, 20,21,22,23] and its duration in nine [12,13,14, 17, 19,20,21,22,23]. Preoperative variables gathered by three RCTs [17, 21, 22] included the consumption of beta-blockers or calcium-inhibitors and anesthetic risk factors such as the presence of hypertension or diabetes mellitus or a history of myocardial infarction. In addition, Nutt et al. [18] applied a test to evaluate the preoperative anxiety and apprehension of patients. Study populations were divided into two groups in seven trials [13, 14, 16, 17, 19, 21, 23], into three groups in four [12, 15, 18, 22], and into four groups in one [20].
Sample size selection
The sample size was selected to achieve 80% reliability to detect clinically significant results in seven studies [12, 14, 15, 18,19,20, 22] and 90% reliability in three [19, 21, 23], and all seven assumed a type I error α = 0.05 and type II error β = 0.5. The other two studies did not specify the estimation of their sample size.
Dosage and administration guidelines
Pregabalin was administered in a single dose in all reviewed RCTs, at 1 h pre-surgery in eleven studies and at 4 h pre-surgery in one [18]. A dose of 150 mg was selected by Par Veen et al. [13], Rahat et al. [16], Sundar et al. [17], Spreng et al. [19], Nutt et al. [18], Jain et al. [21], and Singh et al. [23] and a dose of 300 mg by Gonano et al. [14]. Finally, Chen et al. [12] used two pregabalin dose groups (150 and 300 mg), Rastogi et al. [15] two pregabalin dose groups (75 and 150 mg); and White et al. [20] three (75, 150, and 300 mg). Vijayan et al. [22] also include a combination of 75 mg pregabalin and dexmedetomidine.
Study outcomes
The main study outcome was the level of preoperative and perioperative anxiety, evaluated using a visual analog scale (VAS) (Table 4). Additional outcomes were perioperative changes in heart rate (HR) and mean arterial pressure (MAP) and in the level of sedation, measured using a VAS or the Ramsay Sedation Score (RSS) [15, 17]. This scale measures sedation on a numerical score of 1–6: 1, anxious, agitated, or restless; 2, co-operative, oriented, and tranquil; 3, responds to command; 4, asleep with brisk response to stimulus; 5, asleep with sluggish response to stimulus; and 6, asleep with no response.
Chen et al. [12] observed a significant decrease in HR versus the placebo group at 1-h post-medication in groups receiving preoperative pregabalin at a dose of 150 mg (P = 0.045) or 300 mg (P < 0.001), with no significant difference between pregabalin groups (P = 0.153), and significantly lower MAP values versus the placebo group in groups treated with 150 mg (P = 0.025) or 300 mg (P = 0.044) pregabalin. At the same time point, the RSS score was higher in the pregabalin groups than in the control group, although statistical significance was not reached, with no significant difference between them.
Par Veen et al. [13] observed a significantly greater decrease (P < 0.01) in HR at 1-h post-medication in patients preoperatively treated with 0.3 mg clonidine versus 150 mg pregabalin, although there was no difference between them in interoperative HR. MAP values were significantly lower (P < 0.01) in the clonidine group at 1-h post-medication, immediately before and induction, but not after intubation. RSS scale scores were also significantly lower in the clonidine versus pregabalin group (P < 0.01) at 1-h post- medication and 1-h post-surgery.
Rastogi et al. [15] reported significantly higher (P = 0.03) preoperative RSS scale scores in patients pretreated with pregabalin (75 mg or 150 mg) than in patients receiving placebo, with no significant differences between the pregabalin groups, and significantly higher HR (P = 0.03) and MAP (P = 0.001) values in the control group and 75 mg pregabalin group than in the 150 mg pregabalin group. No group showed a significantly greater decrease in intra-operative HR values.
Sundar et al. [17] observed a significantly higher HR at 1-min post-intubation in controls than in patients receiving 150 mg pregabalin (P = 0.041), but there was no significant difference at 1-, 3-, or 5-min post-intubation. MAP values were significantly lower in the pregabalin group at all time points before anesthesia induction (P = 0.021), reaching a significance of P = 0.001 at 5-min post-intubation. There was no significant difference (P = 0.053) between groups in VAS anxiety score at 6-, 12-, or 24-h post-surgery.
Rahat et al. [16] reported that MAP values were significantly (P = 0.01) lower and HR values even more significantly lower (P = 0.001) at 1 h post-medication in the 150 mg pregabalin versus placebo group. VAS-anxiety scores were also significantly lower (P = 0.03) in the patients receiving 150 mg pregabalin than in those administered with placebo.
White et al. [20] found no significant difference in post-operative VAS-anxiety score among groups receiving 150 mg pregabalin, 300 mg pregabalin, or placebo. However, VAS-sedation scores were significantly higher (P = 0.01) in the 300-mg pregabalin group than in the control group during the pre-induction period and at 90- and 120-min post-surgery.
Gonano et al. [14] reported a significantly lower (P = 0.003) VAS-anxiety score immediately before anesthesia induction in patients receiving 300-mg pregabalin than in controls, although no significant between-group difference was observed during the first 24-h post-surgery.
Spreng et al. [19] described a significant decrease in anxiety at 1-h pre-surgery in the 150-mg pregabalin group versus controls (P = 0.001) and a positive correlation between preoperative anxiety and postoperative pain at 120 min after its administration.
Nutt et al. [18] observed a significant reduction in VAS-anxiety score at 2.5-h post-medication in patients receiving 150-mg pregabalin (P = 0.014) or 0.5-mg alprazolam (P = 0.018) than in those administered with a placebo. The statistical significance of this anxiolytic effect was higher between 2.5- and 4-h post-medication in the alprazolam group (P = 0.01) but not in the pregabalin group. They also found a significantly higher (P < 0.01) VAS-sedation score versus the placebo group in the pregabalin group between 2.5- and 4-h post-medication and in the alprazolam group at 2-h post-medication (P < 0.01).
Jain et al. [21] reported that mean intraoperative HR was significantly higher (P = 0.036) in 150-mg premedicated pregabalin group compared with dexmedetomidine group. They also found MAP values were significantly lower (P = 0.025) in dexmedetomidine group intraoperatively. However, these changes in HR and MAP were not significant statistically intragroup when comparing with baseline (immediately before induction of general anesthesia).
Vijayan et al. [22] describe a significant reduction in mean HR in all three groups intraoperatively compared with preoperative period. Comparison intergroups showed a significant decreased HR in group D (i.v. dexmedetomidine 1 μg.kg−1) compared with Group P (oral pregabalin 150 mg) (P = 0.001-0.045) and compared with group C (combination dexmedetomidine (0.5 μg.kg−1)/ pregabalin 75 mg) (P = 0.009–0.047) during intraoperative period. They also observed mean MAP was to be significantly lower in Group D compared with Groups P and C at all intraoperative time intervals (Group D vs. Group P: P = 0.000–0.037 and Group D vs. Group C: P = 0.000–0.024). There was no difference in mean MAP between Groups P and C in the intraoperative period. Postoperative Ramsay sedation score value was significantly higher (P < 0.05) in Group D comparing with Group P and Group C. VAS sedation score in Group D was significantly higher than in Groups P and C at 60 min after extubation (postoperative) (P = 0.0001).
Singh et al. [23] observed a significant decrease (P < 0.01) in HR in the 150-mg pregabalin group compared with placebo group from 2 min after laryngoscopy and at all intraoperative times after. However, changes in HR were not statistically significant neither in pregabalin group nor placebo group when comparing with preoperative time (just before induction of anesthesia). They also found a significant increase of MAP among the groups when comparing preoperative and intraoperative (P < 0.05) values, and intergroup comparison showed a highly significant lower MAP value in 150-mg pregabalin group at all intraoperative times (P < 0.001). Finally, they reported a lower score in VAS anxiety scale in pregabalin group comparing with placebo group 60 min after premedication, but it was not statistically significant.
Adverse effects
All except for two of the reviewed RCTs gathered data on drug-related adverse effects, which were never severe in any study group. The most frequent adverse events were dizziness, somnolence, vomiting, and nausea. None of the articles included in this systematic review reported respiratory depression associated with pregabalin as a side effect.
Chen et al. [12] reported that dizziness at 1-h post-medication was more frequent in the control group than in the 150-mg pregabalin group (P = 0.038) or 300-mg pregabalin group (P = 0.010). Veen et al. [13] found no difference in the frequency of adverse effects between pregabalin and clonidine groups. Sundar et al. [17] observed no significant differences in the frequency of nausea between premedicated and control groups and reported no cases of dizziness or vomiting. Rahat et al. [16] found a higher prevalence of dizziness in the pregabalin group versus controls (P = 0.01) but no significant between-group differences in the frequency of nausea and vomiting. In comparison with controls, White et al. [20] observed a similar frequency of adverse effects in the 150-mg pregabalin group but a significantly higher frequency of dizziness and difficulty to awaken in the 300-mg pregabalin group (P < 0.05). Spreng et al. [19] found no significant differences between premedicated and control groups in the frequency of adverse effects, which were most commonly dizziness, nausea, and vomiting. In the study by Nutt et al. [18], the most frequent adverse events in the pregabalin and alprazolam groups were fatigue and dizziness, with no significant between-group differences. Jain et al. [21] reported a significant higher incidence of nausea in pregabalin group during postoperative period. Neither vomiting nor dizziness was reported in any group. Vijayan et al. [22] found no significant difference in the incidence of side effects among the three groups except for three patients in dexmedetomidine group who had bradycardia associated with hypotension in comparison with none in the other groups. Finally, Singh et al. [23] reported no significant difference in incidence of side effects among groups.
Concerning the time when side effects caused by pregabalin such as nausea or vomiting are reported, all the articles included make this summary at the end of surgery. Therefore, this might be considered a bias since general anesthetics might also cause this side effect by itself.
Discussion
In this review of data on the effectiveness and safety of oral pregabalin to control preoperative anxiety, all studies found a positive correlation between its pre-operative administration at a dose of ≥ 150 mg and a lower VAS anxiety score in comparison with controls. Likewise, the sedation level (VAS or RSS score) was higher in patients pre-medicated with pregabalin at a dose of ≥ 150 mg, and the difference with controls was statistically significant in all except one RCT. Results confirmed that the minimum effective pregabalin regimen for anxiety control and sedation is 150 mg administered in a single oral dose. Mild side effects (e.g., dizziness or nausea) were more frequent at higher pregabalin doses.
The studies that analyzed MAP and HR values [12, 15,16,17,18, 21,22,23] found an improvement in patients receiving preoperative pregabalin, which was statistically significant in those receiving a dose ≥ 150 mg. Rastogi et al. [15] and Sundar et al. [17] observed that these stabilizing hemodynamic effects were more marked at 1 h after pregabalin administration and gradually decreased over the next 3 h. On the other hand, Jain et al [21] and Vijayan et al. [23] reported a longer maintenance of hemodynamic effects even until postoperative time for dexmedetomidine groups.
MAP and HR values were always recorded before the drug/placebo administration and again immediately before surgery, except for one study [12] that measured them only before the intervention. All of these studies excluded patients under antihypertensive medication or whose MAP and HR values were abnormal before the drug/placebo administration. Other RCTs [17, 21, 22] also excluded patients with diabetes mellitus or previous myocardial infarction that could affect the hemodynamic data.
In accordance with the pharmacokinetics of pregabalin and alprazolam, they were always administered at 1-h pre-surgery except for one study [18], in which 150 mg pregabalin or 0.5 mg alprazolam was administered at 4 h before surgery to estimate the duration of their analgesic and anxiolytic effects and to determine the maximum effectiveness peak. This study [18] obtained greater anxiolytic effects and longer duration of sedation levels with 0.5 mg alprazolam, although it was more frequently associated with somnolence and dizziness in comparison with 150 mg pregabalin. In another study [25], no difference in anxiolytic effect was found between 75 or 150 mg oral pregabalin and 5 mg diazepam, but adverse events were more frequent in the diazepam group. Clonidine is an antihypertensive drug that acts on the central nervous system and is used in combination with other drugs to treat attention-deficit hyperactivity disorder, and an 0.3 mg dose was reported [13] to have a superior effect on anxiety and hemodynamic changes in comparison with 150 mg pregabalin; however, clonidine has more contraindications and drug interactions and exerts no analgesic effects.
Dexmedetomidine is a newer α2-agonist which causes a decrease in mean MAP and HR when used preoperatively comparing with pregabalin [21, 22]. It seems to improve hemodynamic stability during the intraoperative period and raise sedation level because of its hypnotic effects. In contrast, dexmedetomidine premedicated groups reported more incidence of bradycardia as side effect.
Five studies studied the association with postoperative pain [13, 14, 17, 19, 20]. No significant between-group difference in opioid and/or analgesic consumption or VAS-pain score was observed during the recovery period with the exception of one of these studies [19], which also found a statistically significant correlation between preoperative anxiety and postoperative pain. In a systemic review and meta-analysis [2], greater anxiety about a dental visit was found to be closely associated with a worse experience of pain during the procedure, suggesting that special efforts are needed to improve the comfort of patients especially prone to anxiety during their treatment.
The limitations of the present systematic review include differences among the trials in the type of surgery, the dose of pregabalin, and the anesthetic technique used for the surgery.
Conclusion
Preoperative oral pregabalin can be effective to significantly reduce the anxiety of surgery patients and control hemodynamic changes, with no severe adverse effects. A dose of 75 mg oral pregabalin has been found to reduce anxiety and stabilize intraoperative hemodynamics, although a more significant improvement appears to be achieved with a single dose of 150 mg pregabalin at least 1 h before the surgery.
References
Bovaira M, Herrero Babiloni A, Jovaní M, Peñarrocha-Diago M, González-Lemonnier S, Peñarrocha-Oltra D (2017) Preoperative anxiety and its influence on patient and surgeon satisfaction in patients receiving dental implant surgeries performed under intravenous conscious sedation. Int J Oral Maxillofac Implants 32:912–918
Lin C-S, Wu S-Y, Yi C-A (2017) Association between anxiety and pain in dental treatment: a systematic review and meta-analysis. J Dent Res 96:153–162
Armfield JM, Heaton LJ (2013) Management of fear and anxiety in the dental clinic: a review. Aust Dent J 58:390–407
Banchs RJ, Lerman J (2014) Preoperative anxiety management, emergence delirium, and postoperative behavior. Anesthesiol Clin 32:1–23
Chizh BA, Göhring M, Tröster A, Quartey GK, Schmelz M, Koppert W (2007) Effects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteers. Br J Anaesth 98:246–254
Ha K-Y, Carragee E, Cheng I, Kwon S-E, Kim Y-H (2011) Pregabalin as a neuroprotector after spinal cord injury in rats: biochemical analysis and effect on glial cells. J Korean Med Sci 26:404–411
Shneker BF, McAuley JW (2005) Pregabalin: a new neuromodulator with broad therapeutic indications. Ann Pharmacother 39:2029–2037
Brawek B, Löffler M, Dooley DJ, Weyerbrock A, Feuerstein TJ (2008) Differential modulation of K(+)-evoked (3)H-neurotransmitter release from human neocortex by gabapentin and pregabalin. Naunyn Schmiedebergs Arch Pharmacol 376:301–307
Bockbrader HN, Radulovic LL, Posvar EL, Strand JC, Alvey CW, Busch JA, Randinitis EJ, Corrigan BW, Haig GM, Boyd RA, Wesche DL (2010) Clinical pharmacokinetics of pregabalin in healthy volunteers. J Clin Pharmacol 50:941–950
Ben-Menachem E (2004) Pregabalin pharmacology and its relevance to clinical practice. Epilepsia 45(Suppl 6):13–18
Cabello López JB (2015) Lectura crítica de la evidencia clínica. Barcelona, Elsevier
Chen W, Huang H, Yang C, Hu X, Bao F, Jiang H (2019) Preoperative low-dose and high-dose pregabalin and cardiovascular response to endotracheal intubation: a prospective, randomized, single-blind, controlled study in China. Clin Ther 41:68–77
Parveen S, Negi DS, Kumar R, Bagwan MC (2016) Oral clonidine vs oral pregabalin premedication to attenuate pressor response to direct laryngoscopy in patients undergoing laparoscopic cholecyste. J Clin Diagn Res 10:UC21–UC25
Gonano C, Latzke D, Sabeti-Aschraf M, Kettner SC, Chiari A, Gustorff B (2011) The anxiolytic effect of pregabalin in outpatients undergoing minor orthopaedic surgery. J Psychopharmacol 25:249–253
Rastogi B, Gupta K, Gupta PK, Agarwal S, Jain M, Chauhan H (2012) Oral pregabalin premedication for attenuation of haemodynamic pressor response of airway instrumentation during general anaesthesia: a dose response study. Indian J Anaesth 56:49–54
Rahat Dahmardeh A, Moosavi A, Nasir-Al-Din Tabatabaei SM, Ordoni Avval J, Sistanizad M (2018) The effect of a single dose oral pregabalin on hemodynamic changes and duration of analgesia after spinal anesthesia in orthopedic surgeries of tibial fractures. Iran J Pharm Res 17(Suppl):2–7
Sundar AS, Kodali R, Sulaiman S, Ravullapalli H, Karthekeyan R, Vakamudi M (2012) The effects of preemptive pregabalin on attenuation of stress response to endotracheal intubation and opioid-sparing effect in patients undergoing off-pump coronary artery bypass grafting. Ann Card Anaesth 15:18–25
Nutt D, Mandel F, Baldinetti F (2009) Early onset anxiolytic efficacy after a single dose of pregabalin: double-blind, placebo- and active-comparator controlled evaluation using a dental anxiety model. J Psychopharmacol 23:867–873
Spreng UJ, Dahl V, Raeder J (2011) Effect of a single dose of pregabalin on post-operative pain and pre-operative anxiety in patients undergoing discectomy. Acta Anaesthesiol Scand 55:571–576
White PF, Tufanogullari B, Taylor J, Klein K (2009) The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg 108:1140–1145
Jain A, Sinha R, Pandey S, Sahu V (2019) Comparative evaluation of dexmedetomidine and pregabalin as premedication agent to attenuate adverse hemodynamic and stress response in patients undergoing laparoscopic cholecystectomy. Anesth Essays Res 13:608–614
Vijayan NK, Talwar V, Dayal M (2019) Comparative evaluation of the effects of pregabalin, dexmedetomidine, and their combination on the hemodynamic response and anesthetic requirements in patients undergoing laparoscopic cholecystectomy: a randomized double-blind prospective study. Anesth Essays Res 13:515–521
Singh D, Yadav JS, Jamuda BK, Singh P (2019) Oral pregabalin as premedication on anxiolysis and stress response to laryngoscopy and endotracheal intubation in patients undergoing laparoscopic cholecystectomy: a randomized double-blind study. Anesth Essays Res 13:97–104
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Jokela R, Ahonen J, Tallgren M, Haanpää M, Korttila K (2008) A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain 4:106–112
Funding
The work was supported by the Master of Oral Surgery and Implant Dentistry, School of Dentistry, University of Granada, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals carried out by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Torres-González, M.I., Manzano-Moreno, F.J., Vallecillo-Capilla, M.F. et al. Preoperative oral pregabalin for anxiety control: a systematic review. Clin Oral Invest 24, 2219–2228 (2020). https://doi.org/10.1007/s00784-020-03352-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03352-y