Abstract
Objectives
To analyse the residual monomer (MMA) elution of polymethyl methacrylate (PMMA) in distilled water after diverse fabrication methods and aging procedures.
Materials and methods
PMMA specimens (N = 192, PalaXpress; Kulzer, Hanau, Germany) were manufactured (pouring, n = 96/injection, n = 96) and polymerized in water (55°C) without pressure (n = 48) and with 2 bar pressure (n = 48). Specimens were grinded (n = 24) or polished (n = 24) and aged for 12 h in distilled water/37°C (n = 12) or at air/20°C (n = 12) and stored afterwards in distilled water at 37°C. MMA elution was evaluated after 1, 2, 3, 4, 5, 6, 7, 10, 15 days (UV/Vis spectrophotometry). Data were analysed with Kolmogorov-Smirnov, Mann-Whitney-U and Cohen-d test using SPSS (α < 0.5).
Results
The pouring procedure resulted in significantly higher MMA elution than the injection procedure up to 5 days. Polymerization with a pressure of 2 bar reduced the MMA elution significantly for poured specimens. Polishing reduced the MMA elution in comparison to grinding.
Conclusions
The fabrication procedure (pouring/injection) showed the strongest correlation to the MMA elution (r = 0.500), followed by polishing (r = 0.243), the pressure during polymerization (r = 0.109) and the storage medium (r = 0.053).
Clinical relevance
Higher MMA elution may increase the risk of chemical irritations, allergic reactions and hypersensitivities of the oral mucosa. Technicians and dentists should be aware about the elution differences dependent on the fabrication procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the 1930s, polymethyl methacrylate (PMMA) is used for the fabrication of polymeric based dentures [1]. PMMA is still the material of choice for the manufacturing of orthodontic appliances, occlusal splints and complete and partial dentures and relining of complete dentures and tooth-supported or implant-supported overdentures and for provisional dental crowns and bridges [1,2,3,4,5,6]. PMMA combines many advantages, as the easy mode of processing with low costs [3] and the easy contingency to repair. According to the curing reaction, PMMA materials can be allocated to chemical-, light- or heat-activated processes [7]. Although the CAD/CAM technology (Computer Aided Design/Computer Aided Manufacturing) allows the processing of PMMA for fabricating dental restorations [8], the manual processing is actually the most widely used method. The polymerization procedure of methyl methacrylate (MMA) to PMMA can be processed by different physical (light, heating by temperature or microwave energy) and chemical (radical substitution) activation processes [6, 7, 9]. The MMA concentration seems to be dependent on the mixing ratio of monomer to polymer and the method of polymerization [1, 10, 11], as well as on the storage time and storage conditions after fabrication [6, 12, 13].
Despite of the favourable characteristics and wide use of PMMA in different fields of dentistry, there are some drawbacks due to the incomplete conversion of MMA to PMMA. The release of residual MMA can affect the dimension stability of the denture and the mechanical properties [3]. Furthermore, several authors have expressed concerns about its biocompatibility [1, 4, 12, 14]. Here, the release of the unreacted monomeric molecule MMA is in the focus, which may lead to irritations, inflammations, hypersensitizations and allergic reactions of the mucosal tissue [1, 4]. The upper limit (maximum value) for MMA residues is 4.5 wt% for autopolymerising resins on basis of powder-liquid systems, determined in line with the measuring procedures of DIN EN ISO 20195-1 [15]. The release of residual MMA of PMMA-based denture resins has been investigated by various laboratory methods such as infrared spectroscopy, gas chromatography and high-performance liquid chromatography, as well as ultraviolet spectrophotometry [1, 3, 5, 6, 9,10,11,12,13, 16,17,18]. Some limitations are associated to the different laboratory methods. For instance, an overlapping of absorption peaks in complex spectra was stated for the infrared spectroscopy [3]. The high-performance liquid chromatography suffers by the complex preparation of the specimens [3]. For quantitative analytical chemistry, ultraviolet spectrophotometry (UV/Vis) is a widespread and easy optical analysing method [9]. The concentration of MMA in a storage medium can be measured by light absorption since MMA is active in the UV spectrum [9, 19, 20].
Several studies about the release of residual MMA of prosthetic dentures or orthodontic resins were performed to determine the influence of manufacturing mode or storage conditions by gas chromatography [3, 12] or high-performance liquid chromatography [1, 13, 14, 18]. To the authors’ best knowledge, there is no report about the influence on the MMA elution of dental prostheses subjected to different manufacturing procedures and post-manufacturing storage conditions based on UV/Vis spectrophotometry. Hence, the study focused on the quantitative determination of residual MMA elution in distilled water of conventional PMMA for removable dentures after diverse fabrication methods using the UV/Vis spectrophotometry.
The null hypotheses of this investigation stated that there are no differences for MMA elution between the fabrication procedures and between the post-fabrication conditions.
Materials and methods
Specimen fabrication
Rectangular specimens (edge length = 14 × 12 × 2.5 mm, N = 192) were manufactured by two different fabrication procedures using PalaXpress denture resin (composition powder: methylmethacrylate-coploymer; composition liquid: methylmethacrylate, dimethacrylate; company: Kulzer, Hanau, Germany). The study design is displayed in Fig. 1.
-
1.
Group P (n = 96): Pouring procedure: By using replicas with the above given specimen’s size, a silicon mould (silicon: Kontursil, SILADENT Dr. Böhme & Schöps GmbH, Goslar, Germany, automatic mixing device: Dreve Dentamid GmbH, Unna, Germany) was manufactured (Fig. 2). PalaXpress was manually mixed according to the manufacturer’s instruction for the pouring procedure (10 g powder and 7 ml liquid). After the dough was homogenous and bubble-free, it was poured into the silicone mould (Fig. 2). After 7 min, the mould was given into the pressure pot (Palamat elite, Kulzer) with 55°C water for 30 min. Half of the specimens were polymerized with a pressure of 2 bar (group P: n = 48), and the other half without pressure (group N: n = 48).
-
2.
Group I (n = 96): Injection procedure: By using replicas of modelling wax (Carvex Holland BV, Haarlem, Netherlands) with the same specimen’s size than the pouring specimens, a polymerization dental flask was prepared (Fig. 3). The wax replicas were therefore fixed on a type 4 plaster socket (Premium pico-rock 280, Picodent Dental Produktions- und Vertriebs-GmbH, Wipperfürth, Germany), and the socket was embedded in type 3 plaster (Pico crema soft, Picodent Dental Produktions- und Vertriebs-GmbH). Wax injection canals were added, and the first counterpart was made by silicon (Kontursil, SILADENT Dr. Böhme & Schöps GmbH), followed by the plaster counterpart (Fig. 3). After the plaster counterpart was consolidated, the wax was boiled out (Wapo-Ex 12, Wassermann Dentalmaschinen GmbH, Hamburg, Germany) and wax residues were washed out with hot water. The plaster socket was then isolated (Aislar, Kulzer), the parts of the flask were closed and the PalaXpress was manually mixed according to the manufacturer’s instruction for the injection procedure (mixed ratio of 30 g powder and 15 ml liquid). After the dough was homogenous and bubble-free, it was poured into the filling cylinder in the metal cartridge (Fig. 3). After the dough reached a dull surface, the metal cartridge was closed and placed in the injecting device (Palajet, Kulzer) under pressure. After 5 min, the metal cartridge was removed and given into the pressure vessel (Palamat elite, Kulzer) with 55°C water for 30 min. Half of the specimens were polymerized under this conditions with a pressure of 2 bar (group P: n = 48), and the other half without pressure (group N: n = 48).
For the post-fabrication procedures, firstly the specimens were mechanically processed with constant water irrigation (Abramin, Struers, Ballerup, Denmark) using silicate carbide (SiC) paper (P) 500 under a grinding pressure of 2.5–3.0 bar to get identically shaped specimen with a final thickness of 2.00 ± 0.05 mm. According to the procedure, the specimens were further divided into two groups:
-
1.
Group G (n = 24): Grinding: Specimens were grinded on both sides with SiC P500 to obtain flat surfaces.
-
2.
Group P (n = 24): Polishing: Specimens were grinded on both sides with SiC P500 to obtain flat surfaces and polished on one side with SiC P1200, P2000 and P4000 on the other for 30 s each.
After grinding or polishing, the specimens were ultrasonically cleaned (Ultrasonic cleaner T-14, L&R Manufacturing Company, Keamy, USA) in distilled water for 2 min to remove all polishing residues.
Afterwards, one of two storing conditions was chosen for 12 h:
-
1.
Group W (n = 12): Water (post-polymerization at 37°C in distilled water in an incubator ) (Hera Cell 150, Heraeus Kulzer, Hanau, Germany) and
-
2.
Group A (n = 12): Air (post-polymerization at 20°C at air without water-storage).
Artificial aging
For artificial aging, each specimen was placed separately in a laboratory glass tube with 4 ml of distilled water as storage liquid using a standardized laboratory analysing pipette (Eppendorf, Hamburg, Germany). The test tubes were sealed with paraffin foil (Parafilm, Bemis, Neeneh, USA), placed in test tube racks and stored in an incubator (Hera Cell 150, Heraeus Kulzer, Hanau, Germany) at 37°C. Measurements were performed after 1 day (24 h), 2 days, 3 days, 4 days, 5 days, 6 days, 7 days, 10 days and 15 days.
Absorption maximum of pure MMA solution
A spectrophotometer (Lambda 35 UV-Vis Perkin Elmer, Perkin Elmer Inc., Waltham, USA) and quartz cells (UV-Vis Spectroscopy cells, Perkin Elmer Lab Solutions, Rodgau, Germany) were used to determine the absorption maximum of MMA and to measure the absorption of residual MMA of each specimen eluted in the storage liquid.
The spectrophotometer calculates the absorbance (A) by measurement of the transmission of the MMA of the specimen (I) in relation to the transmission of the pure storage liquid distilled water (I0). For all measurements, pure distilled water was used. The absorbance was calculated according to the following formula:
For the determination of the absorption maximum for MMA, the absorbance of ten samples of pure MMA (Lot MKBX9911V, Sigma-Aldrich, Darmstadt, Germany) was measured in quartz cells at the wavelength spectrum of 200–500 nm. The absorbance maximum was determined to be at a wavelength of 220 nm. This wavelength was used for the following determination of the MMA concentration of the specimens.
Calibration curve
For the calibration curve, a standard solution was created by mixing 10 μl of pure MMA in 9990 μl of distilled water (1000 ppm of MMA). This solution was stored for 12 h at 37°C in the incubator (Hera Cell 150, Heraeus Kulzer) to improve the interaction between the molecules. Five further solutions were created from this standard solution with the following concentrations: 10 ppm (100 μl standard solution + 9900 μl distilled water), 20 ppm (200 μl standard solution + 9800 μl distilled water), 30 ppm (300 μl standard solution + 9700 μl distilled water), 40 ppm (400 μl standard solution + 9600 μl distilled water) and 50 ppm (500 μl standard solution + 9500 μl distilled water). Three aliquots of each solution was measured in quartz cells with the spectrophotometer at 220 nm and the absorbance values for the five concentrations recorded. By determining the inclination (m) of the calibration line, the MMA concentration of the specimens’ elutions (CMMA) can be calculated according the formula:
Determination of the MMA content in the storage solution
For UV/Vis spectrophotometry of the specimens, an aliquot of the storage liquid solution from the glass tubes of each sample was transferred to quartz cells and measured at 220 nm wavelength. All absorbance values were recorded in the UV WinLab Software (Perkin Elmer, Perkin Elmer Inc., Waltham, USA) for calculation of CMMA. After performing the spectrophotometry, the storage solution of each specimen was returned to the test tube and the samples were sealed again and returned to the incubator to continue the aging process.
Data analysis
Normality of data distribution was tested with the Kolmogorov-Smirnov test. Descriptive statistics (minimum, median, maximum) were calculated, and data were analysed by Mann-Whitney U test. Cohen-d test was used to determine the correlation effect between the fabrication or post-fabrication procedures and the MMA elution. The level of significance was set at 0.05. All data were analysed with the computer software SPSS (SPSS version 25.0; IBM SPSS Inc., New York, USA).
Results
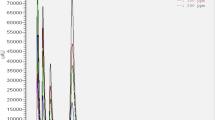
Descriptive statistics of MMA elution for the different fabrication procedures, post-processing methods and aging durations are given in Table 1. In addition, Fig. 4 depicts the specific boxplots for all test intervals. Kolmogorov-Smirnov test indicated a violation of the assumption of normality for 22 of 144 groups (15.3%). In view of the single test parameters, the fabrication procedure (pouring/injection) showed the strongest correlation to the MMA elution (r = 0.500), followed by the polishing (r = 0.243), the pressure during polymerization (r = 0.109) and the storage medium (r = 0.053).
Impact of fabrication procedure (pouring/injection)
For measurement up to 5 days, the pouring procedure resulted for all subgroups in significantly higher MMA elution than the injection procedure (p < 0.001 to p = 0.001). No differences were found for group PPA (at 6 days, 10 day and 15 days), for group PPW (at 7 days, 10 days and 15 days) and for group PGA plus NPA (at 15 days). All other groups showed for pouring procedure significant higher MMA elution than for injection (p < 0.001 to p = 0.045).
Impact of pressure during polymerization (with/without pressure)
Within the group of pouring procedure, no differences could be observed for the impact of pressure when specimens are grinded and water-stored. Groups polymerized at 55°C without pressure resulted in significantly higher MMA elution for group NGA (at 4 days, 6 days, 7 days, 10 days and 15 days; p = 0.001 to p = 0.045), for group NPW (at 6 days, 7 days, 10 days and 15 days; p < 0.001) and for group NPA (at 7 days and 10 days; p = 0.003 to p = 0.008).
Within the group of injection procedure, no differences could be observed for the impact of pressure when specimens were polished and water-stored. For polished specimens after air-storage, the polymerization at 55°C/2 bar showed significantly higher MMA elution at 1 day, 5 days, 6 days, 7 days, 10 days and 15 days (p < 0.001 to p = 0.024). For grinded specimens, the polymerization at 55°C without pressure showed significantly higher MMA elution at 1 day, 2 days, 3 days, 6 days and 7 days (p < 0.001 to p = 0.006) after water-storage and at 2 days (p = 0.003) after air-storage.
Impact of polishing (grinding/polishing)
Within the group of pouring and polymerization at 55°C without pressure a significantly higher MMA elution was observed for grinded specimens, independent from the storage medium for all measurement days (p < 0.001 to p = 0.014), and also for polymerization at 55°C/2 bar, when specimens were water-stored (p < 0.001 to p = 0.045). For specimens polymerized at 55°C/2 bar and air-stored, significantly higher MMA elution was found for grinding at 1 day, 2 days, 3 days, 6 days, 10 days and 15 days (p < 0.001 to p = 0.024).
Within the group of injection and polymerization at 55°C without pressure, significantly higher MMA elution was found for grinding after water-storage (at 1 day, 2 days, 3 days, 4 days, 6 days, 7 days and 15 days; p < 0.001 to p = 0.020) and after air-storage at 15 days (p = 0.024). For polymerization at 55°C/2 bar, no differences were found between grinding and polishing for water-stored groups. For air-storage, grinding resulted in higher MMA elution at 7 days (p = 0.002), while higher MMA elution was observed for polishing at 1 day (p = 0.004) and 10 days (p = 0.001).
Impact of storage medium (air 20°C/water 37°C)
Within the group of pouring, significant differences were found between air-storage and water-storage. Higher MMA elution was found after air-storage for groups NGA (at 1 day, 2 days, 3 days and 4 days; p < 0.001 to p = 0.045), NPA (1 day, 2 days and 3 days; p < 0.001 to p = 0.006), PGA (1 day, 2 days and 6 days; p = 0.001 to p = 0.020) and PPA (1 day, 2 days, 3 days, 4 days, 6 days and 15 days; p < 0.001 to p = 0.020). Higher MMA elution was found after water-storage for group NGW (15 days; p = 0.012), NPW (10 days; p = 0.008 and 15 days; p < 0.001) and PGW (7 days, 10 days and 15 days; p < 0.001 to p = 0.017).
Within the group of injection, significantly higher MMA elution was found after air-storage for groups NPA (1 days, 2 days and 3 days; p = 0.002 to p = 0.006), PGA (1 day, 2 days, 3 days, 5 days and 6 days; p < 0.001 to p = 0.024) and PPA (1 day, 2 days, 3 days, 4 days, 5 days, 6 days, 7 days and 15 days; p < 0.001 to p = 0.033). Higher MMA elution was found after water-storage for group NGW (10 days; p = 0.033).
Discussion
PMMA is still the most widely used material for the fabrication of dentures. Although PMMA prostheses can nowadays also be manufactured using CAD/CAM technology, conventional fabrication with manual mixing of powder and liquid continues to be of great importance. In the standardized industrial production of PMMA blanks for CAD/CAM, the manufacturing process is intended to reduce the total MMA elution for dentures. Opposed to this, for the manual mixing of powder and liquid, there are exact specifications of the mixing ratio by the manufacturer, but the manufacturing and polymerization process stays user dependent.
Generally, it is known that MMA can lead to mucosal irritations and sensitization of tissues at a certain level [21]. Several investigations have already dealt with the different manufacturing processes and the resulting difference in MMA elution [21, 22]. Prostheses can be injected under pressure or be poured into a hollow mould. This is normally followed by post-polymerization, which takes place in a water bath with or without pressure application. After this post-polymerization, the PMMA restoration is finished and polished. It is obvious that these procedures could have a decisive influence on MMA elution. In order to increase biocompatibility, the technician and dentist should be aware of the specific impact of the manufacturing processes. To the best of the authors' knowledge, there are no data in the scientific literature available to examine specifically the effects of these different fabrication procedures and post-fabrication processes on the MMA elution using the UV/Vis spectrophotometry as economically priced measuring method.
The first null hypothesis stating that there are no differences for MMA elution between the fabrication procedures (pouring/injection) is rejected. The present investigation showed that the injection procedure of the PMMA specimens with pressure resulted in a lower MMA elution, even significantly up to day 5. Looking at the first day of measurement, for example, it is noticeable that the MMA elution for the injection of the PMMA material was between 11.3 and 55.6 ppm and for the injection procedure significantly lower between 5.0 and 11.5 ppm. For the fabrication procedure, the highest correlation was found to the values of MMA elution. The difference of both methods can be attributed to the different powder/liquid mixing ratio. For the pouring group, a mixing ratio of 10 g powder/7 ml liquid (1.42:1) was used and for the injection group 30 g powder/15 ml liquid (2:1). These mixing ratios are necessary to ensure the correct dough consistency for the respective process. Different studies aimed to investigate the influence of the powder/liquid mixing ratio on the residual monomer content and stated lower values for increasing powder amount [23, 24]. However, the optimization of the powder/liquid mixing ratio is subjected to the processing related limitations [23]. A further reason for the differences between pouring and injection may be the exclusion of oxygen during the injection process of the dough in the flask, while the pouring of the dough in the moulds was performed under oxygen access. Generally, oxygen is a polymerization inhibitor [23, 25] and may therefore have a negative impact on the monomer conversion rate of the pouring group.
After the fabrication of the PMMA specimens, the subsequent post-fabrication procedures also had a decisive influence on the MMA release. There was an impact of pressure during polymerization (with/without pressure), an impact of polishing (grinding/polishing) and an impact of storage medium (air 20°C/water 37°C) with significant differences between the test groups. The second null hypothesis stating that there are no differences for MMA elution between the post-fabricating handling can therefore be rejected. For all post-fabricating factors, the polishing process showed the highest correlation to the values of MMA elution, followed by the pressure during the polymerization. The storage condition after the polishing/grinding process showed the weakest correlation.
In the present investigation, the MMA elution was highest in the first measurement days and decreased in the further course of the study procedure. After the measurement, the elution-water was poured back in the test tube. Theoretically, this would result in a constant or increasing value for the MMA content in the elution-water. On basis of the present results, it may be suggested that a part of the MMA dissolved by a possible evaporation [24, 26] as MMA is volatile at room temperature [27]. Furthermore, a possible hydrolysis of MMA to methacrylic acid [5] or an oxidation to formaldehyde [28] was stated. Due to the different absorption maximum for both molecules (formaldehyde: 175 nm, methacrylic acid: 205–215 nm), both could not be measured at 220 nm [19].
Continuous diffusion processes from the PMMA in the fluid environment dissolve the MMA monomers. After polishing, a lower MMA elution was observed. The polishing process also minimizes the surface roughness. As a result, the diffusion surface decreases as well as the MMA elution. Polishing or sealing of PMMA resins leads to lower MMA release [29]. According to the clinical routine, polishing of removable prostheses can only be performed on the side that is not in contact with the mucosa. Although the polishing leads to lower MMA release, the polishing of the contact side to the mucosa should be avoided as it may result in an incongruent surface and probably a less-fitting restoration. The specimens of the present investigation were grinded with P500 on both sides to always achieve the same specimen geometry/dimension and to flatten the specimen surface. In consequence, the present in vitro results are not directly comparable with the procedure for manufacturing of in vivo dentures.
By applying a pressure of 2 bar during the polymerization at 55°C, possible porosities within the PMMA resin could be minimized. Pronounced porosities may result in a higher surface, followed by a higher MMA diffusion [10, 29]. In addition, the water-storage at 37°C reduces the MMA elution. Different authors stated a lower MMA elution after water-storage [2, 5, 20, 26]. However, as a limitation of the present study, the water-storage was performed at 37°C, while the air-storage was at 20°C. Hence, the influence of the temperature could also be accountable for the MMA reduction of the specimen before starting the measurement. The higher temperature accelerates not only the diffusion of MMA in the liquid environment but also the post-polymerization by increasing the reaction kinetics [2, 5, 7], thus, in turn, leads to lower MMA monomers in the PMMA resin.
The UV/Vis spectrophotometry method used in the present investigation is a simple and inexpensive measuring method, which has already been described in the literature [9, 19, 20, 30] and could be seen as an approach in the present investigation. With this test method, the MMA accumulation in the test medium distilled water can be calculated by using the absorbance parameter. The correlation of the absorption, which is proportional to the molar concentration, is determined by the Lambert-Beer’s law. However, as standard method to determine MMA concentrations in liquids, the use of chromatographic methods such as gas chromatography is recommended according to DIN EN ISO 20195-1 [15] and is superior for measuring MMA values quantitatively. Hereby, the MMA release in the solvents acetone and methanol is measured after the extraction under predefined conditions. Compared with the UV/Vis spectrophotometry, the gas chromatography and high-performance liquid chromatography (HPLC) allow additionally the simultaneous analysis of multiple components. A comparison between the values of the present study and values obtained by analyses according to DIN EN ISO 20195-1 [15] is difficult as MMA has a lower solubility in water than in acetone or methanol. Therefore, the choice of the measurement method is a limitation of the present study. Further investigations of the residual monomer elution of PMMA would be necessary, especially for the direct comparison of UV/Vis spectrophotometry and HPLC.
On basis of the different test methods in literature, it is also difficult to state a threshold value to avoid the occurrence of irritations, inflammations, hypersensitizations and allergic reactions of the mucosa. As mentioned in the “Introduction,” the upper limit (maximum value) for MMA residues is 4.5 wt% for autopolymerising resins on basis of powder-liquid systems, when determined according to the methods of DIN EN ISO 20195-1 [15].
By virtue of the different analysing methods, specimen designs and general test conditions, the comparison of the results of prior investigations with the results of the present study is difficult as the following sections presents. Baker et al. performed an in vivo study to measure the residual MMA release in saliva for up to one week by gas chromatography after using different solvents and stated values with a maximum concentration of 45 μg/ml [26]. Kedjarune et al. investigated heat-curing and autopolymerising resins on basis of an in vitro test design in view of the MMA release in saliva by gas chromatography after further use of solvents and in view of the cytotoxicity [24]. After 24 h, the MMA concentration for autopolymerising resins was between 8.52 ± 19.05 and 65.11 ± 15.60 μg/ml saliva and decreased at the measurement point of 48 h. By calculating the ppm values on basis of the specific density of MMA, it could be stated that the group of pouring in the present study was in the same value range (11.33 to 55.61 ppm). Stafford et al. measured the content of MMA in distilled water by UV/Vis spectrophotometry and stated values from 0.08 up to 0.31% dependent on the orthodontic resin used [20]. Lamb et al. measured the loss of residual monomer from autopolymerising acrylic resin immersed in water by UV/Vis spectrophotometry and found a complete (99%) diffusion after 14 days storage at 22°C in distilled water by a kinetic measurement procedure [19]. Ayaz et al analysed the residual monomer of different PMMA-based denture resins processed by autoclave and conventional water bath techniques [9]. As extraction solvent methanol was used instead of distilled water, the measured values of residual MMA are between 0.0170 ± 0.0043 and 0.0664 ± 0.0178% dependent on the manufacturing procedure.
Conclusions
On basis of the present in vitro study to analyse the impact of fabrication procedures on the MMA elution of PMMA materials for dentures, it could be stated that:
-
1.
The fabrication procedure (pouring/injection) showed the strongest correlation to the MMA elution. The procedure of injection should be preferred to reduce the MMA elution.
-
2.
The polishing, the pressure during polymerization and the storage medium showed a weaker correlation. Nevertheless, also in view of the bacterial adherence, the upper side of the dentures should be polished. The dentures should also be manufactured under pressure of 2 bar to avoid airlocks.
References
Ica RB, Ozturk F, Ates B, Malkoc MA, Kelestemur U (2014) Level of residual monomer released from orthodontic acrylic materials. Angle Orthod 84(5):862–867. https://doi.org/10.2319/060713-435.1
Bayraktar G, Guvener B, Bural C, Uresin Y (2006) Influence of polymerization method, curing process, and length of time of storage in water on the residual methyl methacrylate content in dental acrylic resins. J Biomed Mater Res B Appl Biomater 76(2):340–345. https://doi.org/10.1002/jbm.b.30377
Ayman AD (2017) The residual monomer content and mechanical properties of CAD\CAM resins used in the fabrication of complete dentures as compared to heat cured resins. Electron Physician 9(7):4766–4772. https://doi.org/10.19082/4766
Gautam R, Singh RD, Sharma VP, Siddhartha R, Chand P, Kumar R (2012) Biocompatibility of polymethylmethacrylate resins used in dentistry. J Biomed Mater Res B Appl Biomater 100(5):1444–1450. https://doi.org/10.1002/jbm.b.32673
Urban VM, Machado AL, Oliveira RV, Vergani CE, Pavarina AC, Cass QB (2007) Residual monomer of reline acrylic resins: effect of water-bath and microwave post-polymerization treatments. Dent Mater 23(3):363–368. https://doi.org/10.1016/j.dental.2006.01.021
Urban VM, Machado AL, Vergani CE, Giampaolo ET, Pavarina AC, de Almeida FG, Cass QB (2009) Effect of water-bath post-polymerization on the mechanical properties, degree of conversion, and leaching of residual compounds of hard chairside reline resins. Dent Mater 25(5):662–671. https://doi.org/10.1016/j.dental.2008.10.017
Bettencourt AF, Neves CB, de Almeida MS, Pinheiro LM, Oliveira SA, Lopes LP, Castro MF (2010) Biodegradation of acrylic based resins: a review. Dent Mater 26(5):e171–e180. https://doi.org/10.1016/j.dental.2010.01.006
Goodacre CJ, Garbacea A, Naylor WP, Daher T, Marchack CB, Lowry J (2012) CAD/CAM fabricated complete dentures: concepts and clinical methods of obtaining required morphological data. J Prosthet Dent 107(1):34–46. https://doi.org/10.1016/S0022-3913(12)60015-8
Ayaz EA, Durkan R, Koroglu A, Bagis B (2014) Comparative effect of different polymerization techniques on residual monomer and hardness properties of PMMA-based denture resins. J Appl Biomater Funct Mater 12(3):228–233. https://doi.org/10.5301/jabfm.5000199
Vallittu PK, Miettinen V, Alakuijala P (1995) Residual monomer content and its release into water from denture base materials. Dent Mater 11(5):338–342. https://doi.org/10.1016/0109-5641(95)80031-X
Shim JS, Watts DC (1999) Residual monomer concentrations in denture-base acrylic resin after an additional, soft-liner, heat-cure cycle. Dent Mater 15(4):296–300. https://doi.org/10.1016/S0109-5641(99)00048-2
Zissis A, Yannikakis S, Polyzois G, Harrison A (2008) A long term study on residual monomer release from denture materials. Eur J Prosthodont Restor Dent 16(2):81–84
Nik TH, Shahroudi AS, Eraghihzadeh Z, Aghajani F (2014) Comparison of residual monomer loss from cold-cure orthodontic acrylic resins processed by different polymerization techniques. J Orthod 41(1):30–37. https://doi.org/10.1179/1465313313y.0000000078
Steinmassl PA, Wiedemair V, Huck C, Klaunzer F, Steinmassl O, Grunert I, Dumfahrt H (2017) Do CAD/CAM dentures really release less monomer than conventional dentures? Clin Oral Investig 21(5):1697–1705. https://doi.org/10.1007/s00784-016-1961-6
DIN EN ISO 20795-1:2013-06: Dentistry - base polymers - Part 1: denture base polymers (ISO 20795-1:2013); German version EN ISO 20795-1:2013. doi:
Urban VM, Cass QB, Oliveira RV, Giampaolo ET, Machado AL (2006) Development and application of methods for determination of residual monomer in dental acrylic resins using high performance liquid chromatography. Biomed Chromatogr 20(4):369–376. https://doi.org/10.1002/bmc.575
Denis AB, Diagone CA, Plepis AM, Viana RB (2015) The effect of the polymerization initiator and light source on the elution of residual Bis-GMA and TEGDMA monomers: a study using liquid chromatography with UV detection. Spectrochim Acta A Mol Biomol Spectrosc 151:908–915. https://doi.org/10.1016/j.saa.2015.07.040
Tuna EB, Rohlig BG, Sancakli E, Evlioglu G, Gencay K (2013) Influence of acrylic resin polymerization methods on residual monomer release. J Contemp Dent Pract 14(2):259–264
Lamb DJ, Ellis B, Priestley D (1982) Loss into water of residual monomer from autopolymerizing dental acrylic resin. Biomaterials 3(3):155–159
Stafford GD, Brooks SC (1985) The loss of residual monomer from acrylic orthodontic resins. Dent Mater 1(4):135–138
Jorge JH, Giampaolo ET, Machado AL, Vergani CE (2003) Cytotoxicity of denture base acrylic resins: a literature review. J Prosthet Dent 90(2):190–193. https://doi.org/10.1016/S0022391303003494
Vallittu PK, Ruyter IE, Buykuilmaz S (1998) Effect of polymerization temperature and time on the residual monomer content of denture base polymers. Eur J Oral Sci 106(1):588–593
Lamb DJ, Ellis B, Priestley D (1983) The effects of process variables on levels of residual monomer in autopolymerizing dental acrylic resin. J Dent 11(1):80–88
Kedjarune U, Charoenworaluk N, Koontongkaew S (1999) Release of methyl methacrylate from heat-cured and autopolymerized resins: cytotoxicity testing related to residual monomer. Aust Dent J 44(1):25–30
Gauthier MA, Stangel I, Ellis TH, Zhu XX (2005) Oxygen inhibition in dental resins. J Dent Res 84(8):725–729. https://doi.org/10.1177/154405910508400808
Baker S, Brooks SC, Walker DM (1988) The release of residual monomeric methyl methacrylate from acrylic appliances in the human mouth: an assay for monomer in saliva. J Dent Res 67(10):1295–1299. https://doi.org/10.1177/00220345880670101001
Blanchet LJ, Bowman DC, McReynolds HD (1982) Effects of methyl methacrylate monomer vapors on respiration and circulation in unanesthetized rats. J Prosthet Dent 48(3):344–348
Oysaed H, Ruyter IE, Sjovik Kleven IJ (1988) Release of formaldehyde from dental composites. J Dent Res 67(10):1289–1294. https://doi.org/10.1177/00220345880670100901
Vallittu PK (1996) The effect of surface treatment of denture acrylic resin on the residual monomer content and its release into water. Acta Odontol Scand 54(3):188–192
Goiato, M.C., E. Freitas, D. dos Santos, R. de Medeiros, and M. Sonego (2015) Acrylic Resin Cytotoxicity for Denture Base--Literature Review. Adv Clin Exp Med 24(4):679-686. doi: 10.17219/acem/33009
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keul, C., Seidl, J., Güth, JF. et al. Impact of fabrication procedures on residual monomer elution of conventional polymethyl methacrylate (PMMA)—a measurement approach by UV/Vis spectrophotometry. Clin Oral Invest 24, 4519–4530 (2020). https://doi.org/10.1007/s00784-020-03317-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03317-1