Abstract
Objective
To evaluate the changes in alveolar contour after guided bone regeneration (GBR) with two different combinations of biomaterials in dehiscence defects around implants.
Material and methods
Chronic alveolar ridge defects were created bilaterally in the mandible of eight Beagle dogs. Once implants were placed, three treatment groups were randomly allocated to each peri-implant dehiscence defect: (i) test group received a bone substitute composed of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) covered by a cross-linked collagen membrane, (ii) positive control group with placement of deproteinized bovine bone mineral (DBBM) plus a porcine natural collagen membrane, and (iii) a negative control with no treatment. Two healing periods (8 and 16 weeks) were evaluated. Dental casts were optically scanned, the obtained files were uploaded into an image analysis software and superimposed to evaluate the linear changes.
Results
In both healing periods, the gains in linear contours were higher in the test group and at the intermediate level (3 mm below the gingival margin). While at 8 weeks, no significant differences were found between the groups; at 16 weeks, the test and positive control groups demonstrated significant gains in contour compared with negative control.
Conclusions
GBR using different biomaterials significantly increased the buccal contours of the alveolar crest when used at dehiscence defects around dental implants.
Clinical relevance
Particulate highly porous synthetic bone substitute and a cross-linked collagen membrane demonstrated similar outcomes in terms of contour augmentation when compared to bovine xenograft (DBBM) and a collagen membrane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well established that irrespective of its cause, tooth loss will result in significant alterations in the alveolar process, in both the horizontal and vertical dimensions and hence impacting the hard and soft tissue contours [1, 2]. Recent systematic reviews have reported that the mean vertical loss at the buccal bone wall was 1.67 mm and the horizontal loss was 3.85 mm [3], with percentages of vertical and horizontal crestal bone resorption ranging between 11–22% and 29–63%, respectively. This high variability will be dependent on the cause of tooth loss. The main consequence of these changes is the compromise in bone availability for implant therapy and the direct impact on aesthetic contours of the maxillary profile.
This alveolar bone resorption can be compensated by different bone regenerative interventions, which have demonstrated efficacy for providing enough bone to allow ideal implant placement and for attaining aesthetic and functional implant supported restorations [4]. Among the different bone regenerative procedures, guided bone regeneration (GBR) using a bone replacement graft covered by a barrier membrane is currently the regenerative approach most widely used and documented in literature [5, 6].
Different biomaterials have been used as bone replacement grafts, such as autologous, allogenic, xenogeneic, and alloplastic materials [7, 8]. Although autologous grafts have been considered the standard of care for many years due to their osteogenic and osteoinductive properties, their use has important shortcomings, such as their fast resorption rate and the increased patient morbidity associated with its harvesting. On the other hand, xenografts composed of deproteinized bovine bone mineral (DBBM) exhibit excellent mechanical properties, high osteoconductivity, and slow bio-absorbability [9, 10].
Synthetic biomaterials, mainly ceramics, have also been widely used as bone replacement grafts. These are usually composed of biphasic calcium phosphates with different percentages of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP). While β-TCP has a high turnover rate and rapid bio-absorbability, sintered HA may slow this process and allow for the needed of scaffolding effect and sustained space maintenance [8]. These synthetic biomaterials have shown promising results in experimental investigations [11, 12], although their predictive and clinical efficacy has not yet been demonstrated [10]. Recently, a new bone replacement graft made of biphasic calcium phosphate and hydroxyapatite (60% HA and 40% β-TCP) has reported enhanced wetting, high porosity, and excellent osteoconductivity [13].
Similar to bone replacement grafts, non bio-absorbable membranes were the state of the art when this regenerative concept was developed [14]. However, the frequent occurrence of exposures and its associated high morbidity have converted bio-absorbable membranes the barrier of choice for lateral bone augmentation [10]. These bio-absorbable membranes may undergo resorption either by enzymatic degradation (collagen membranes) or by hydrolysis (synthetic polymeric membranes), hence improving their tissue tolerance during wound healing, although these barrier membranes do not have the intrinsic capability for space maintenance and they always need to be used with a bone replacement graft to provide the needed scaffolding effect. This effect is dependent of the membrane bio-absorbability rate, which depends on its composition and the local environment conditions during healing (pH, temperature, etc.). Experimental investigations have reported that degradation of natural collagen membranes may start within 4 days to 4 weeks after membrane placement [15, 16]. This process may be extended by cross-linking the collagen composition of the membranes [17], although this usually requires chemical methods that may modify the collagen structure and cause undesirable local effects [18]. There is, however, no clear evidence on which is the ideal time for membrane degradation in order to maintain the barrier effect that attains optimal bone regeneration [8].
One controversial issue when assessing the efficacy of bone regenerative interventions, such as GBR, is how to evaluate the outcome, since the ideal histological results are restricted to experimental studies. While radiographic methods may seem ideal in light of the current 3D techniques such as cone beam computed tomography (CBCT) [19], the need of repeated examinations limits their use for obvious radiation protection measures. Direct bone measurements have been the most widely used [19]. These measurements require a secondary surgical intervention, which may coincide with the surgical intervention to place the implants; however, when bone augmentation is made in conjunction with implant placement, this second intervention is usually not needed. The advent of optical digital scanning has provided the potential to acquire precise and less invasive 3D stereolithographic (STL) images, which enables the superimposition of soft tissue contours and the comparison of both linear and volumetric changes, at both aesthetic and posterior zones [20]. The study of dimensional changes in alveolar ridges by means of STL image superimposition has been evaluated in both preclinical and clinical investigations [21,22,23,24,25]. It was therefore the objective of this experimental investigation to evaluate, by STL image superimposition, the efficacy of a lateral bone augmentation techniques based on the GBR principles, comparing a synthetic biphasic bone replacement graft plus a cross-linked collagen membrane with a positive control (DBBM plus a natural collagen membrane) and a negative control (no GBR).
Material and methods
Study design
This pre-clinical in vivo investigation was designed according to the modified ARRIVE guidelines [26] as a randomized controlled trial on large experimental animals (beagle dogs). The study was carried out at the Experimental Surgical Department of the Minimally Invasive Surgery Centre, Cáceres, Spain, after receiving approval from the Regional Ethics Committee for Animal Research. The digital analysis was performed in the Department of Periodontology, Faculty of Odontology of the University Complutense of Madrid, Spain.
Study population
Eight adult beagle dogs (6–7 years old) weighting between 10 and 20 kg were used for this investigation (four males and four females). The animals received a unique identification code through a subcutaneous chip (RFID). The research project was approved by the local ethics committee (CCMIJU Ref: 011/15). Animals were installed in individual kennels in a light/darkness cycle of 12:12 with a temperature of 21–22°. Food was based on hard animal food specific for this species and with free access to water. Animals were kept in groups in an area with natural light, fresh air, and regulated temperature. All animals were observed 2 weeks prior to the experiment to assess their general health status.
Surgical interventions
The surgical procedures have been previously described in an independent publication reporting the histological outcomes [13]. In brief, animals were sedated using propofol (2 mg/kg/i.v., Propovet, Abbott Laboratories, Kent, UK) and placed under general anesthesia with 2.5–4% of isoflurane (Isoba-vet, Schering-Plough, Madrid Spain), using a mechanical respirator during the entire surgery. Lidocaine 2% with epinephrine 1:100,000 (2% Xylocaine Dental, Dentsply, York, PA, USA) was further infiltrated locally.
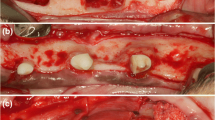
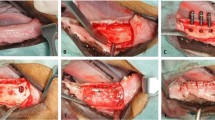
The first surgery consisted on the extractions of P2, distal root of P3, mesial root of P4, and mesial root of M1 (Fig. 1a) and surgical creation of standardized osseous defects (10 × 10 × 5 mm) (Fig. 1b). The second surgery was carried out after 8 weeks of healing when these defects were chronified leading to a three wall knife edge alveolar crest. Once these defects were isolated after raising muco-periosteal flaps, two customized implants of 2.5 mm in diameter and 7–9 mm in length (Dentium® NR; Suwon, Korea) were placed on each of the three defect sites in each hemi-mandible. Implants were placed resulting in buccal dehiscence (Fig. 1c, d), which was measured with a periodontal probe UNC15 (Hu Friedy, Chicago, IL, USA) (Fig. 1e). These dehiscence defects were randomly treated in both the test and positive control groups with GBR or left untreated as the negative control group (Fig. 2a, b).
Picture of the experimental surgeries. a Hemisection of P2, P3, P4, and M1 and extractions of P2, distal root of P3, mesial root of P4, and mesial root of M1. b Creation of bony defects after tooth extractions. c Implant placement with vestibular dehiscence in one defect. d Implant placement with vestibular dehiscence in the entire hemi-mandible. e Measurements of the dehiscence by periodontal probe
The same protocol of extractions was carried out in the contralateral hemi-mandible
The test GBR intervention consisted on a synthetic bone replacement graft composed of 60% HA + 40% β-TCP (Osteon III®, Dentium, Suwon, Korea) and a crossed-linked collagen membrane (GENOSS® (Dentium, Suwon, Korea).
The positive control GBR intervention consisted on deproteinized bovine bone mineral (DBBM) (BioOss® Geistlich, Wolhusen, Switzerland) and a natural porcine collagen membrane (BioGide® Geistlich, Wolhusen, Switzerland).
The third surgery carried out after a healing period of 8 weeks consisted on the same intervention described on the second surgery on the site where extractions were performed at surgery 2, thus allowing for two healing periods (2 and 4 months). After 8 weeks of healing from surgery 3, experimental animals were euthanized using a lethal dose of sodium Pentothal® (40–60 mg/kg/i.v., Dolethal, Vetoquinol, France), and mandibular specimens were retrieved for histologic analysis.
Stereolithography (STL) image acquisition and matching
Before the first surgical intervention, individual impression trays were fabricated for each dog, and before tooth extraction, mandibular impressions were obtained with a light/heavy silicon (Elite HD +, Zhermack spa, RO, Italy). The same procedure was repeated before each surgical intervention. From these impressions, a total of 24 cast models were poured in dental stone (Fujirock type 4, GC. Corp, Tokyo, Japan), which allowed for comparisons between baseline, 2 and 4 months of healing after the GBR intervention. Casts were digitized using a desktop 3D scanner (Zfx Evolution Scanner, Zimmer Dental, Bolzano, Italy) and STL files were obtained. Baseline, 8-week, and 16-week STL files were uploaded to a dedicated software® (SMOP, Swissmeda Software, Swissmeda AG, Zurich, Switzerland) for the process of matching (Fig. 3a). First matching was carried out using three clear and visible common references in both STLs, the baseline and follow-up casts, thus achieving a rough fit. Once this process was completed, further common reference points (no fewer than 10) were selected to achieve a “fine fit” where the software automatically superimposed the models using a series of mathematical algorithms [22].
Dimensional change measurements
Once the STL files were fully matched, a longitudinal slice that divided the ridge mesio-distally into two equal parts was selected. After that, a line coinciding with the axis of the tooth prior to its extraction was drawn in the middle of each cross-sectional image (vertical line). Then, perpendicular lines were drawn at three different levels, at 1, 3, and 5 mm from the most coronal aspect of the ridge, corresponding to the coronal, intermediate, and apical part of the defect. Linear measurements from the vertical line to the baseline and follow-up contours were calculated at the previously specified heights. To assess the changes in the ridge contour, the distance from the vertical line to the follow-up contour was subtracted to the distance from the vertical line to the baseline contour (Fig. 3b). All measurements were performed by a calibrated investigator (RDR).
Data analysis
Randomization of the interventions was performed using a computer-generated list that considers the side and position in the jaw (IBM SPSS Statistics® V20 JM.Domenech). Linear contour changes were calculated for each period (baseline–8 weeks/baseline–16 weeks) and expressed as means, standard deviation (SD), confidence intervals, and frequency distributions.
Shapiro-Wilk normality tests were performed to assess the data distribution. A general linear model was used to assess for multiple comparisons between absolute measurements of the primary outcome variable (“contour change”), considering the treatment group and healing time. ANOVA tests were used for the intergroup comparisons, as well as for the differences depending on the height of the measurement (crestal, intermediate, and apical measurements at 1, 3, and 5 mm from the rim of the crest) and the position of the defect (mesial, central, or distal). Bonferroni corrections were performed for multiple comparisons. The alpha error was set at 0.05.
Intra-group comparisons were also performed to compare the contour changes within each treatment group between baseline-8 and baseline-16 weeks.
Results
Healing after the surgical interventions occurred uneventfully. One dog could not undergo final surgery due to an acute infection and the need of a hysterectomy. Another dog hemi-mandible could not be analyzed due to poor quality of the stone cast. Finally, 42 defects where analyzed, 14 for test group, 14 for positive control, 14 for negative control. Twenty-four defects were evaluated at 16 weeks of healing (8 test, 8 positive control and 8 negative control), while the remaining 18 defects were evaluated at 8 weeks of healing (6 test, 6 positive control and 6 negative control).
Intra-group comparisons
At 8 weeks, when comparing the width of the buccal contour changes in the three groups, a statistically significant increase was found only for the GBR procedures at 1 and 3 mm below the rim. Hence, at 1 mm below the rim of the crest, the mean lateral bone augmentation at test, positive control and negative control sites were of 0.77 mm (SD = 0.42) (p = 0.002), 0.84 mm (SD = 0.50) (p = 0.001), and 0.39 mm (SD = 0.55), respectively. At 3 mm below the crest, mean lateral bone augmentation at test, positive control and negative control sites were of 1.30 mm (SD = 0.76) (p = 0.004), 0.89 mm (SD = 0.58) (p = 0.035), and 0.66 mm (SD = 1.31), respectively. Finally, at 5 mm, mean lateral bone augmentation at test, positive control and negative control sites were of 0.84 mm (SD = 0.81), 1.06 mm (SD = 1.03), and 0.48 mm (SD = 1.85), respectively. None of these lasts three measures demonstrated statistically significant differences.
At 16 weeks, only GBR procedures (test and positive control groups) obtained a statistically significant increase at the three different heights of the crest. At 1 mm below the crest, the mean lateral bone augmentation at test, positive control, and negative control sites were, respectively, 1.02 mm (SD = 0.74), 0.66 mm (SD = 0.90), − 0.07 mm (SD = 0.49). At 3 mm below the crest the mean lateral bone augmentation at test, positive control and negative control sites were 1.69 mm (SD = 0.62), 1.19 mm (SD = 0.62), and 0.40 mm (SD = 0.56), respectively. At 5 mm below the crest, the mean lateral bone augmentation at test, positive control, and negative control sites were 1.76 mm (SD = 0.93), 0.83 mm (SD = 0.52), and 0.22 mm (SD = 0.67), respectively (Table 1).
Inter-group comparisons
At 8 weeks, both GBR interventions achieved increased contour gains at the three different levels of the crest (1, 3, and 5 mm) compared to the negative control. One millimeter below the crest, the increase between the test and negative control groups was 0.44 mm (95% C.I. = − 0.36; 1.25), while the increase between the positive control and negative control groups was 0.51 mm (95% C.I. = − 0.29; 1.32). At 3 mm below the crest, the increase between the test and positive control groups versus the negative control was 0.64 mm (95% C.I. = − 0.82; 2.11) and 0.22 mm (95% C.I. = − 1.24; 1.69), respectively. Contour changes between the test and positive control, versus the negative control groups 5 mm below the crest, were 0.36 mm (95% C.I. = − 1.71; 2.45) and 0.58 mm (95% C.I. = − 1.60; 2.77), respectively. No one of these differences was statistically significant.
At 16 weeks, contour changes reached statistical significance when compared with the negative control group, although differences between the GBR groups were not statistically significant. One millimeter below the crest, contour changes between the test and negative control groups were 1.02 mm (95% C.I. = 0.07; 1.98) (p = 0.032) while the contour changes between the positive control and negative control groups were 0.66 mm (95% C.I. = − 0.28; 1.62) (p = 0.247). At 3 mm below the crest, contour changes between the test and positive control groups versus the negative control were 1.29 mm (95% C.I. = 0.51; 2.07) (p = 0.001) and 0.79 mm (95% C.I. = 0.01; 1.57) (p = 0.044), respectively. Contour changes between the test and positive control, versus the negative control groups 5 mm below the crest, were 1.54 mm (95% C.I. = 0.45; 2.63) (p = 0.005) and 0.61 mm (95% C.I. = − 0.47; 1.70) (p = 0.462), respectively (Table 2).
Contour changes from baseline to both healing periods (8 and 16 weeks) depending on the defect position
Although the contour changes were higher for the mesial defects at 1 and 3 mm below the crest, differences in contour changes among the defects of the different treatment groups were not statistically significant (Table 3).
Contour changes were also assessed depending on the apico-coronal level from the rim of the crest: the most coronal level (1 mm from the rim), intermediate (3 mm), and apical (5 mm). Considering all the sample together, mean changes were 0.59 mm (SD = 0.71), 1.03 mm (SD = 0.84), and 0.88 mm (SD = 1.09), respectively. These differences were not statistically significant.
However, when the healing periods were analyzed separately, the mean changes in crest profile after 8 weeks at the intermediate, apical, and coronal level were 0.95 mm (SD = 0.92), 0.78 mm (SD = 1.26), and 0.65 mm (SD = 0.54), respectively (Fig. 4a). After 16 weeks, these changes at the intermediate, apical, and coronal level were 1.09 mm (SD = 0.78), 0.97 mm (SD = 0.95), and 0.55 mm (SD = 0.82), respectively (Fig. 4b).
Discussion
This experimental study evaluated the contour changes when comparing two lateral bone augmentation interventions based on the principles of guided bone regeneration in conjunction with implant placement. The treatment groups consisted of a test group using a synthetic bone replacement graft composed of 60% HA + 40% β-TCP together with a cross-linked collagen membrane, a positive control group using as xenogeneic bone replacement graft (DBBM) and a native collagen barrier membrane and finally a negative control group without any regenerative materials. At 8 weeks, there were no significant differences among the tested interventions. After 16 weeks of healing, significantly greater gain in ridge contours was found in both the test and positive control groups when compared to the negative control. Although the gains in buccal crestal contours were superior in the test group when compared with the positive control group, these differences were not statistically significant.
These results may be explained by the different behavior of the biomaterials and membranes used due to their inherent biologic properties. When used as bone replacement grafts, histological studies have demonstrated the different resorption rates of HA and β-TCP, with HA demonstrating slower resorption and hence, greater scaffolding effect [27, 28]. These findings were also corroborated in a preclinical study in which GBR procedures were performed with these two bone substitutes. It was demonstrated that after 3 months, there was significant resorption of β-TCP and complete substitution with new bone after 24 months, while DBBM particles remained unresorbed throughout 24 months [29]. Moreover, in a recent clinical study, it was reported that 11 years after sinus floor augmentation, DBBM particles were identified integrated with the regenerated bone [30].
In an experimental study in minipigs, healing dynamic and histometric differences were assessed between β-TCP, DBBM and an autograft when used in GBR procedures with a non-resorbable e-PTFE membrane. Authors concluded that at the initial healing stages, newly formed bone was present in higher amounts in the autograft when compared to the β-TCP and DBBM. Nevertheless, after 8 weeks of healing, the percentage of newly formed bone was comparable between β-TCP and the autograft, being statistically higher than DBBM. At the conclusion of the study autograft and β-TCP were almost totally substituted by newly formed bone, whereas DBBM remained stable [31]. In the present study, no superiority was observed by DBBM which may be explained by the use of a different barrier membrane which may have affected the behavior of the biomaterial.
In this study, the rate of HA/β-TCP was 60/40 what may have decreased the biomaterial resorption but maintaining the high porosity and osteoconductivity demonstrated by β-TCP. These properties have been demonstrated when DBBM is used as a bone replacement graft [32]. In fact, in this investigation, the use of both bone replacement grafts demonstrated a similar performance in regard to the hard tissue gains when the histological outcomes were reported [13]. Randomized clinical trials have also failed to find differences when comparing DBBM and β-TCP for the treatment of peri-implant dehiscence defects [7]. However, when evaluating the augmented bone thickness at 0, 1, and 2 mm apical to the implant shoulder, the histological results reported significantly greater gains for HA/β-TCP+ a cross-linked collagen membrane when compared to DBBM+ a natural collagen membrane [13]. These findings were attributed to the utilization of a cross-linked collagen membrane in the test group which caused the formation of a band of periosteum-like tissue. The contour changes in this study corroborate these histological outcomes, which may be more attributable to the different membranes used, rather than the bone replacement graft. The study of the behavior of cross-linked collagen membranes has shown that they may retain their structure during a period of 16 weeks [33], while native collagen membrane has faster resorption rates (approximately 8 weeks) [18, 34]. This prolonged barrier function may have provided with better space maintenance, which may explain the greater contour increase in the test group at 16 weeks, whereas at 8 weeks when both membranes were not completely biodegraded, there were no differences. Clinically, it appears that the method of cross linking determines the behavior of the membrane since there are reports of improved clinical outcomes when using ribose cross-linked collagen membranes compared against native collagen membranes [35], while other studies have reported soft tissue complications when using cross-linked collagen membrane and inferior outcomes [18, 36, 37].
The methodology used in this preclinical investigation allowed the evaluation of the changes in ridge contours in a non-invasive manner granting for multiple comparisons over time [25, 38,39,40,41]. The use of digital STL analysis allows to study not only the possible hard tissue gains, but also the soft tissue changes after the use of bone augmentation procedures. Recently, an experimental investigation using a similar methodology reported that the combination of a bone replacement graft plus a collagen membrane led to a greater buccal volume gain when compared to membrane and biomaterial alone, in staged augmentation procedures, although none of the regenerative interventions was able to recover the volume lost after defect creation [23]. In spite of the differences in study design (staged versus simultaneous augmentation), these results are in line with the findings of the present investigation in which after both healing periods (8 and 16 weeks), crestal contours were greater in the regenerative groups when compared to the negative control.
Interestingly, when the contour changes in the most apical levels were evaluated in the present investigation, no significant differences were observed at the 8-week healing period; however, statistical significant differences were observed in the apical level of the crest at the 16 healing week period. These findings are challenging to explain taking the lack of interventions in the period where changes occurred. The apical portions of the crest are the most sensitive areas to register taking that alveolar mucosa is frequently encountered which can vary according to pressure, and therefore, inaccurate readings may have been introduced. It is thus possible that an apical displacement of the biomaterial occurred throughout the last 8 weeks of the healing and influenced the contour changes.
The present data should be interpreted with caution due to the experimental in vivo nature of this investigation which used different membranes that prevented a clear comparison of the effect of the bone substitute material. Moreover, the present investigation only reported the changes in tissue contour which provides insufficient information to clearly understand the tissue dynamics since the hard tissue information is lacking. Nevertheless, the present investigation provided with information on the effect of different regenerative strategies on the soft tissue contours allowing to establish clear relationships with the observed hard tissue changes.
Conclusions
Within the limitations of the present experimental investigation, it can be concluded that test (HA + β-TCP) and positive control group (HA) obtained statistically significant more volume gain after lateral bone augmentation compared with negative control after 16 weeks with no significant differences between two regenerative approaches.
References
Schropp L, Kostopoulos L, Wenzel A, Isidor F (2005) Clinical and radiographic performance of delayed-immediate single-tooth implant placement associated with peri-implant bone defects. A 2-year prospective, controlled, randomized follow-up report. J Clin Periodontol 32(Suppl. 5):480–487
Fickl S, Zuhr O, Wachtel H, Stappert CF, Stein JM, Hurzeler MB (2008) Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J Clin Periodontol 35(Suppl. 10):906–913
Van der Weijden F, Dell’Acqua F, Slot DE (2009) Alveolar bone dimensional changes of post-extraction sockets in humans: a systematic review. J Clin Periodontol 36(Suppl. 12):1048–1058
Benic GI, Hammerle CH (2014) Horizontal bone augmentation by means of guided bone regeneration. Periodontol 2000 66(Suppl 1):13–40
Hammerle CH, Jung RE (2003) Bone augmentation by means of barrier membranes. Periodontol 2000(33):36–53
Von Arx T, Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D (2001) Lateral ridge augmentation and implant placement: an experimental study evaluating implant osseointegration in different augmentation materials in the canine mandible. Int J Oral Maxillofac Implants 16:3
Van Assche N, Michels S, Naert I, Quirynen M (2013) Randomized controlled trial to compare two bone substitutes in the treatment of bony dehiscences. Clin Implant Dent Relat Res 15(Suppl 4):558–568
Sanz M, Vignoletti F (2016) Key aspects on the use of bone substitutes for bone regeneration of edentulous ridges. Dent Mater 31(Suppl 6):640–647
Araujo M, Linder E, Lindhe J (2009) Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog. Clin Oral Implants Res 20(1):1–6
Sanz-Sanchez I, Ortiz-Vigon A, Sanz-Martin I, Figuero E, Sanz M (2015) Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review and Meta-analysis. J Dent Res 94(Suppl 9):128S–142S
Tanuma Y, Matsui K, Kawai T, Matsui A, Suzuki O, Kamakura S (2013) Comparison of bone regeneration between octacalcium phosphate/collagen composite and beta-tricalcium phosphate in canine calvarial defect. Oral Surg Oral Med Oral Pathol Oral Radiol 115(Suppl 1):9–17
Trisi P, Rao W, Rebaudi A, Fiore P (2003) Histologic effect of pure-phase beta-tricalcium phosphate on bone regeneration in human artificial jawbone defects. Int J Periodontics Restorative Dent 23(Suppl 1):69–77
Jung UW, Cha JK, Vignoletti F, Nuñez J, Sanz-Esporrin J, Sanz M (2017) Simultaneous lateral bone augmentation and implant placement using a particular synthetic bone substitute around chronic peri-implant dehiscence defects in dogs. J Clin Periodontol 44(11):1172–1180
Dahlin C, Linde A, Gottlow J, Nyman S (1988) Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg 81:672–676
Zhao S, Pinholt EM, Madsen JE, Donath K (2000) Histological evaluation of different biode- gradable and nonbiodegradable membranes im- planted sub-cutaneously in rats. J Craniomaxillofac Surg 28:116–122
Owens KW, Yukna RA (2001) Collagen membrane resorption in dogs: a comparative study. Implant Dent 10:49–56
Paul BF, Mellonig JT, Towle HJ III, Gray JL (1992) Use of a collagen barrier to enhance healing in human periodontal furcation defects. Int J Periodontics Restorative Dent 12:123–131
Schwarz F, Rothamel D, Herten M, Wustefeld M, Sager M, Ferrari D (2008) Immunohistochemical characterization of guided bone regeneration at a dehiscence-type defect using different barrier membranes: an experimental study in dogs. Clin Oral Implants Res 19(Suppl 4):402–415
Chen LC, Lundgren T, Hallstrom H, Cherel F (2008) Comparison of different methods of assessing alveolar ridge dimensions prior to dental implant placement. J Periodontol 79(Suppl 3):401–405
Hammerle CHF, Cordaro L, Van Assche N, Benic GI, Bornstein M, Gamper F, Gotfredsen K, Harris D, Hurzeler M, Jacobs R, Kapos T, Kohal RJ, Patzelt SBM, Sailer I, Tahmaseb A, Vercruyssen M, Wismeijer D (2015) Digital technologies to support planning, treatment, and fabrication processes and outcome assessments in implant dentistry. Summary and consensus statements. The 4th EAO consensus conference 2015. Clin Oral Implant Res 26(Suppl 11):97–101
Fickl S, Schneider D, Zuhr O, Hinze M, Ender A, Jung RE (2009) Dimensional changes of the ridge contour after socket preservation and buccal overbuilding: an animal study. J Clin Periodontol 36(Suppl 5):442–448
Sanz Martin I, Vignoletti F, Nuñez J, Permuy M, Muñoz F, Sanz-Esporrin J, Fierravanti L, Shapira L, Sanz M (2017) Hard and soft tissue integration of immediate and delayed implants with a modified coronal macro design: Histological, micro CT and volumetric soft tissue changes from a pre-clinical in vivo study. J Clin Periodontol 44(8):842–853
Sanz Martin I, Ferrantino L, Vignoletti F, Nuñez J, Baldini N, Duvina M, Alcaraz J, Sanz M (2018) Contour changes after guided bone regeneration of large non-contained mandibular buccal bone defects using deproteinized bovine bone mineral and a porcine-derived collagen membrane: an experimental in vivo investigation. Clin Oral Invest 22(3):1273–1283
Gonzalez-Martin O, Veltri M, Moraguez O, Belser UC (2014) Quantitative three-dimensional methodology to assess volumetric and profilometric outcome of subepithelial connective tissue grafting at pontic sites: a prospective pilot study. Int J Periodontics Restorative Dent 34(Suppl 5):673–679
Sanz Martin I, Benic GI, Hammerle CH, Thoma DS (2016) Prospective randomized controlled clinical study comparing two dental implant types: volumetric soft tissue changes at 1 year of loading. Clin Oral Implants Res 27(4):406–411
Vignoletti F, Abrahamsson I (2012) Quality of reporting of experimental research in implant dentistry. Critical aspects in design, outcome assessment and model validation. J Clin Periodontol 39(Suppl. 12):6–27
Schwarz F, Herten M, Ferrari D, Wieland M, Schmitz L, Engelhardt E (2007) Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite + beta tricalcium phosphate (Bone Ceramic) or a collagen-coated natural bone mineral (BioOss Collagen): an immunohistochemical study in dogs. Int J Oral Maxillofac Surg 36(Suppl 12):1198–1206
Lee IK, Lim HC, Lee JS, Hong JY, Choi SH, Jung UW (2016) Layered approach with autogenous bone and bone substitute for ridge augmentation on implant dehiscence defects in dogs. Clin Oral Implants Res 27(Suppl 5):622–628
Artzi Z, Weinreb M, Givol N, Rohrer MD, Nemcovsky CE, Prasad HS, Tal H (2004) Biomaterial Resorption Rate and Healing Site Morphology of Inorganic Bovine Bone and β-Tricalcium Phosphate in the Canine: A 24-month Longitudinal Histologic Study and Morphometric Analysis. Int J Oral Maxillofac Implants 19(Suppl 3):357–368
Mordenfeld A, Hallman M, Johansson CB, Albrektsson T (2010) Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin Oral Implants Res 21(Suppl 9):961–970
Jensen SS, Broggini N, Hjørting-Hansen E, Schenk R, Buser D (2006) Bone healing and graft resorption of autograft, anorganic bovine bone and β-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res 17(Suppl 3):237–243
Sanz M, Ferrantino L, Vignoletti F, De Sanctis M, Berglundh T (2017) Guided bone regeneration of non-contained mandibular buccal bone defects using deproteinized bovine bone mineral and a collagen membrane: an experimental in vivo investigation. Clin Oral Implant Res 28(11):1466–1476
Cha JK, Joo MJ, Yoon S, Lee JS, Choi SH, Jung UW (2017) Sequential healing of only bone grafts using combining biomaterials with cross-linked collagen in dogs. Clin Oral Implants Res 28:76–85
Schwarz F, Rothamel D, Herten M, Sager M, Becker J (2006) Angiogenesis pattern of native and cross-linked collagen membranes: an immunohistochemical study in the rat. Clin Oral Implants Res 17(Suppl 4):403–409
Friedman A, Gissel K, Soudan M, Kleber BM, Pitaru S, Dietrich T (2011) Randomized controlled trial on lateral augmentation using two collagen membranes: morphometric results on mineralized tissue compound. J Clin Periodontol 38(7):677–685
Bornstein MM, Bosshardt D, Buser D (2007) Effect of two different bioabsorbable collagen membranes on guided bone regeneration: a comparative histomorphometric study in the dog mandible. J Periodontol 78(Suppl 10):1943–1953
Becker J, Al-Nawas B, Klein MO, Schliephake H, Terheyden H, Schwarz F (2009) Use of a new cross-linked collagen membrane for the treatment of dehiscence-type defects at titanium implants: a prospective, randomized-controlled double-blinded clinical multicenter study. Clin Oral Implants Res 20(Suppl 7):742–749
Jemt T, Lekholm U (2003) Measurements of buccal tissue volumes at single-implant restorations after local bone grafting in maxillas: a 3-year clinical prospective study case series. Clin Implant Dent Relat Res 5(Suppl 2):63–70
Jemt T, Lekholm U (2005) Single implants and buccal bone grafts in the anterior maxilla: measurements of buccal csrystal contours in a 6 year prospective clinical study. Clin Implant Dent Relat Res 7(3):127–135
Henriksson K, Jemt T (2004) Measurements of soft tissue volume in association with single implant restoration: a 1 year comparative study after abutment connection surgery. Clin Implant Dent Relat Res 6(4):181–189
Schneider D, Gründer U, Ender A, Hammerle CH, Jung RE (2011) Volume gain and stability of peri-implant tissue following bone and soft tissue augmentation: 1 year results from a prospective cohort study. Clin Oral Implant Res 22(1):28–37
Acknowledgments
The authors acknowledge professor Ui-Won Jung for the active involvement in the surgical procedure. We also thank the veterinary doctors, Maria Carmen Calles-Vázquez and Elena Abellán, as well as the staff from the Minimally Invasive Surgery Centre, Cáceres, Spain, who so effectively took care of the experimental animals used in this investigation.
Funding
This work was partially supported through a research contract between the University Complutense of Madrid and Dentium Implants, Suwon (Korea). Support was also obtained from the ETEP (Etiology and therapeutics in Periodontal Diseases) Research Group at the Faculty of Odontology, University Complutense of Madrid (Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article contains data from an experimental study with animals performed at the Experimental Surgical Department of the Minimally Invasive Surgery Centre in Cáceres (Spain) after receiving approval from the Regional Ethics Committee for Animal Research (CCMIJU Reference 011/15). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Di Raimondo, R., Sanz-Esporrín, J., Plá, R. et al. Alveolar crest contour changes after guided bone regeneration using different biomaterials: an experimental in vivo investigation. Clin Oral Invest 24, 2351–2361 (2020). https://doi.org/10.1007/s00784-019-03092-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03092-8
Keywords
- Guided bone regeneration
- Synthetic bone graft
- Collagen membrane
- Dental implant
- Animal model
- Prophilometric changes