Abstract
Objectives
This study is aimed at evaluating the effect of a new glass ionomer cement (GIC) containing fluoro-zinc-silicate fillers on biofilm formation and ion incorporation.
Materials and methods
Streptococcus mutans biofilms were developed on two GIC materials: Caredyne Restore (CD) and Fuji VII (FJ); and hydroxyapatite (HA) for 24 h at 37 °C using a flow cell system. The morphological structure and bacterial viability were analyzed using a confocal laser scanning microscopy. Bacterial adhesion during the initial 2 h was also assessed by viable cell counting. To study the ion incorporation, restored cavities prepared on the root surfaces of human incisors were subjected to the elemental mapping of the zinc and fluoride ions in the GIC-dentin interface using a wavelength-dispersive X-ray spectroscopy electron probe microanalyzer.
Results
Morphological observations revealed that biofilm formation in the CD group was remarkably inhibited compared with the HA and FJ groups, exhibiting sparse, thinner biofilm clusters. The microorganisms adhering to the CD group were significantly inhibited, revealing 2.9 ± 0.4 for CD, 4.9 ± 0.2 for FJ, and 5.4 ± 0.4 log colony-forming units (CFU) for HA. The CD zinc ion incorporation depth was 72.2 ± 8.0 μm. The fluoride penetration of CD was three times deeper than that of FJ; this difference was statistically significant (p < 0.05).
Conclusions
Enhanced by the incorporation of zinc and fluoride ions, the new GIC inhibited biofilm formation by interfering with bacterial adhesion.
Clinical relevance
A novel GIC comprised of fluoro-zinc-silicate fillers may improve clinical outcomes, such as root caries and minimally invasive dentistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glass ionomer cements (GICs) have been widely used in dentistry for 40 years due to their fluoride release and uptake, low thermal expansion coefficient, good biocompatibility with dental pulp tissue, and adhesion to the surface of the tooth [1,2,3]. GICs have been particularly revolutionary for minimally invasive dentistry, such as atraumatic restorative treatment (ART) [4, 5]. ART is useful for preventative care in underserved areas of the world that lack facilities and resources, such as electricity or rotating and cutting equipment. GIC is also recommended when caries have progressed to the subgingival area or when moisture control is difficult [6].
In addition, fluoride is also known to inhibit bacterial growth and the acid production of oral biofilm, which results in demineralization [7,8,9]. It does so by interfering with bacterial metabolism and dental plaque acidogenicity, inhibiting the glycolytic enzyme enolase and the proton-extruding ATPase, and preventing bacterial colonization and competition [8]. However, some studies have demonstrated that viable bacterial biomasses can develop on GICs, although the biofilm formation on GICs was less than on enamel and resin composites [10,11,12,13]. As the GICs cannot completely prevent the bacteria from biofilm formation, cariogenic bacteria in biofilm can invade the GIC-dentin interfaces via microleakage to induce the occurrence of secondary caries.
Recent studies have sought to improve the antimicrobial effect and the mechanical properties of GICs [14]. These studies examined GICs containing an antimicrobial compound or nanoparticle, such as casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) [15], chitosan [16], chlorhexidine [17], chlorhexidine hexametaphosphate [18], epigallocatechin-3-gallate [19], propolis [20], or titanium dioxide [21].
The purpose of this study was to estimate the antibiofilm effect and ion penetration of a newly launched GIC known as Caredyne Restore (CD; GC Corporation, Tokyo, Japan) formulated using a fluoro-zinc-silicate glass (BioUnion™) filler in addition to fluoro-alumino-silicate glass [22]. It has been reported that zinc ions inhibit acid production by interfering with bacterial glycolysis and bacterial growth [23]. Zinc and fluoride, alone or in combination, inhibited production-secretion of glucosyltransferases by Streptococcus mutans [24]. The null hypothesis was that antibiofilm effect is not different between a GIC containing fluoro-zinc-silicate glasses in addition to fluoro-alumino-silicate and a GIC containing fluoro-alumino-silicate glasses alone. We also analyzed the incorporations of the zinc and fluoride ions into dentin using a wavelength-dispersive X-ray spectroscopy electron probe microanalyzer (EPMA).
Materials and methods
Biofilm development on glass ionomer cement
Material disc preparation
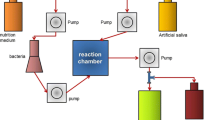
Two GIC materials: CD and Fuji VII (FJ) (GC Corporation, Tokyo, Japan); and hydroxyapatite (HA) (Olympus, Tokyo, Japan) were used in this study (Table 1). The GIC materials were mixed by hand according to the manufacturer’s instructions, poured into 8 mm (diameter) × 1 mm (height) acrylic molds, covered with a polyethylene terephthalate sheet, and stored for 1 h at 37 °C in a relative humidity of 100% [25]. The HA discs measuring 6 mm in diameter were used as a control material. The specimens were polished using 4000 grit SiC paper (Marumoto Struers KK, Tokyo, Japan). The specimens were washed with distilled water (pH 7.2), air dried, and then mounted in a flow cell chamber (Convertible Flow Cell® CFCAS0003, IBI Scientific, USA). Two specimens were placed at either end so that the fluid flow did not interfere (Fig. S1). A total of 10 discs for each material type (i.e., five chambers per experimental group) were prepared. The chamber was sterilized using ethylene oxide gas for 4 h. The flow cell system consisted of a medium reservoir, a peristaltic pump, and a carboy for waste. Silicone tubing connected these components (Fig. S1).
Bacteria
Streptococcus mutans MT 8148 was cultured according to a previously published protocol [26]. S. mutans was grown overnight from frozen stock in a brain heart infusion (BHI) broth (Difco Laboratories, Detroit, MI, USA) at 37 °C under anaerobic conditions. The starter culture was transferred into fresh BHI broth and cultured for 6 h at 37 °C under the same conditions. The optical density of the bacterial suspension was adjusted to 0.025 at 600 nm prior to inoculation.
Biofilm formation
An adjusted saliva solution (10 ml) was pumped into a chamber at a flowrate of 2 ml/min and kept static for 1 h at 37 °C to allow salivary pellicle formation. The saliva was prepared in accordance with the protocol by Takenaka et al. [26]. The same volume of S. mutans was then pumped into the chamber and kept static for 30 min to acquire initial adhesion [26].
After 1 h, medium flow was initiated at a flowrate of 2 ml/min. The medium was 1/10 strength BHI broth supplemented with 0.05% sucrose (pH = 7.4). Biofilms were allowed to develop for 24 h under a continuous flow condition and with incubation at 37 °C under anaerobic conditions.
Following this, the specimens were collected from the chamber, washed twice with phosphate-buffered saline (PBS; pH = 7.0), and subjected to morphological observations using either confocal laser scanning microscopy (CLSM) or scanning electron microscopy (SEM).
CLSM analysis
Samples were stained with a fluorescent bacterial viability kit (LIVE/DEAD® BacLight™ Bacterial Viability Kit, Thermo Fisher Scientific, Waltham, USA) for 30 min at room temperature in the dark [26]. The biofilm imaging was performed using CLSM (FluoView™ 300, Olympus, Tokyo, Japan) with Ar 488 nm and He-Ne 543 nm lasers. Filters of 510–530 nm and ≥ 610 nm were used to detect SYTO 9 and propidium iodide (PI). A water immersion objective lens (× 60) was used. To determine viability and thickness, five randomly selected fields representing the four quadrants and center segment of each sample were evaluated [27]. Stacks of the horizontal images (235 × 235 μm) were acquired every 0.45 nm. The total number of red and green pixels was enumerated using the MetaMorph® software (Molecular Devies, Sunnyvale, USA) [28]. Three-dimensional reconstructed images were created using the Imaris® software (Bitplane AG, Zurich, Switzerland), and the maximum thickness of the biofilm was measured [28]. This assay was performed for a total of five replicates per material.
SEM analysis
A total of five discs per each experimental group were subjected to SEM observation. The specimens were fixed with 10% paraformaldehyde and 2.5% glutaraldehyde in a 0.2 M cacodylate buffer overnight at 4 °C. The discs were washed twice with PBS, dehydrated in an ascending series of ethanol, dried to a critical point, and subsequently sputtered with gold palladium [26]. The biofilms were observed using an electron probe microanalyzer (EPMA; EPMA-1610®; Shimazu, Kyoto, Japan) at × 300 and × 1000 magnifications [26].
Bacterial adhesion assay
The discs and a flow cell system were prepared as previously described. A total of six discs per experimental group were analyzed. Following the salivary pellicle formation, the bacterial suspension was adjusted to 0.025 at 600 nm in a BHI broth supplemented with 0.5% sucrose. This broth was pumped into a chamber at a rate of 2 ml/min for 2 h at 37 °C and under anaerobic conditions [26]. During the operation time, fresh media was constantly delivered to the bacterial suspension to maintain optimal density (Fig. S2). The discs were washed twice with PBS, and the biofilm was detached by vigorous shaking for 1 min, ultrasonication for 5 min, and shaking again for 1 min. The bacterial suspension was homogenized, serially diluted, and plated on the BHI. The plates were incubated anaerobically for 2 days at 37 °C, and all of the colonies that formed were enumerated.
Ion incorporation in human dentin adjacent to glass ionomer cement
Cavity preparation and restoration
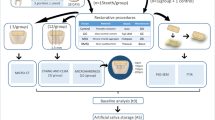
Five extracted, caries-free, human maxillary incisors within 1 year were used. The teeth were stored in 0.5% chloramine solution at 4 °C until use [29]. The Niigata University Ethics Committee approved all of the experimental protocols (27-R20-11-17). Cavity preparation and restoration were performed as described by Hotta et al. [30], with a slight modification. Two cylindrical butt-joint cavities (2.0 mm in diameter and 1.0 mm in depth) were prepared centrally on the buccal root surface using a cylinder diamond bar (Fig. 1A). Two cavities were assigned to either the CD or FJ group (Fig. 1B). A cavity was also prepared on the labial root surface and served as a control. All materials were handled according to the manufacturer’s instructions. After filling, the GIC surface was covered with Fuji varnish (GC Int., Tokyo, Japan) and stored for 6 min at 37 °C. After storing the teeth for 1 h in a 100% humidity environment, the samples were soaked in 20 ml of distilled water for 7 days (Fig. 1C) [30, 31]. The specimens were placed in fresh media every 24 h.
Schematic descriptions of sample preparation for EPMA analysis. a Preparation of cervical cavities. b All cavities were restored with the allocated materials. c Storing in distilled water for 7 days. d Cutting of the crown and apical area. e Longitudinally sectioning along the long axis of the tooth through the center of the cavities. f Observation of GIC-dentin interface
Electron probe microanalyzer analysis
Each sample was sectioned using a diamond disc 2 mm below the cement enamel junction and 5 mm above the root apex (Fig. 1D). The specimen was sectioned along the long axis of the tooth through the center of the cavity with a diamond saw (Fig. 1F) and embedded in a chemically polymerizing resin (Technovit 4071, Kulzer, Wehrheim, Germany). The cut surface was serially polished with 2400-grit and 4000-grit SiC paper (Marumoto Struers KK, Tokyo, Japan; Fig. 1E). The specimen was sputter coated with a 300-Å gold layer using an ion coater (IC-50, Shimazu, Kyoto, Japan) and analyzed using EPMA (EPMA1610, Shimazu, Kyoto, Japan) with an image observation function [32].
Element mappings of fluoride and zinc ions at the interface of the filling were conducted. The maximum penetration depth of each element in five randomly selected fields was measured by element line scanning. The difference between CD and FJ was compared.
Statistical analyses
Statistical analyses were performed using SPSS® 11.0 (SPSS Inc., Chicago, IL, USA) and Excel Statistics 7.0 (Esumi Co., Ltd., Tokyo, Japan). Where applicable, the data are presented as the mean ± standard deviation (SD). Significance was determined using the Kruskal-Wallis test with a post hoc Steel-Dwass test or a post hoc Dunnett test. A paired Student t test was used to compare the EPMA analyses.
Results
Effect on biofilm formation
Three-dimensional reconstructed images revealed that S. mutans biofilms successfully developed on all materials after a 24-h incubation (Fig. 2). However, the biovolume on the CD group was smaller than that on the HA and FJ groups, and the maximum thickness of the biofilm as determined by CLSM was 31.5 ± 2.1 μm for HA, 28.5 ± 1.7 μm for FJ, and 8.7 ± 1.1 μm for CD (p < 0.05).
Representative images of S. mutans biofilm formed on each specimen (upper). Vertical section (X–Z) images of S. mutans biofilm (lower). Scale bar = 30 μm. Hydroxyapatite (HA), Fuji VII (FJ), and Caredyne Restore (CD). The biovolume on the CD group was smaller than that on the HA and FJ groups, whereas the microorganisms in the biofilm were mostly alive
The calculated viable ratios determined from the fluorescent stacks were 95.3 ± 4.2 for HA, 96.1 ± 1.6 for FJ, and 89.8 ± 6.2 for CD. There were no significant differences among these groups (p > 0.05). The SEM images revealed that a large amount of biofilm clusters formed on the HA and FJ groups throughout the field of view, whereas an extremely small amount of clusters was observed on the CD group (Fig. 3).
Representative SEM images of S. mutans biofilms formed on each specimen after 24 h. A large amount of biofilm clusters formed on the HA and FJ groups throughout the field of view, whereas an extremely small amount of clusters was observed on the CD group. Hydroxyapatite (HA), Fuji VII (FJ), and Caredyne Restore (CD). Scale bars = 100 μm (upper) 20 μm (lower)
Preventing the effect of bacterial adhesion
The number of microorganisms adhered to each material as determined by the bacterial adhesion assay is shown in Fig. 4. The values are expressed as colony-forming units (CFU) per square millimeter. The CD group showed a significant decrease compared with HA and FJ, and the difference between CD and FJ was 2 log CFU per square millimeter (p < 0.05). There was no significant difference between the HA and FJ groups.
Ion incorporation in human dentin
The fluoride and zinc incorporations into dentin after 7 days following the restoration are shown in Fig. 5. The FJ and CD groups exhibited F uptake from the interface (black arrow) to the dentin. The CD fluoride incorporation depth (120.1 ± 24.1 μm) was three times deeper than that of FJ (44.1 ± 15.4 μm), and there was a significant difference between the two materials (p < 0.05). The CD zinc incorporation showed a concentration gradient along the inside, exhibiting the richest layer at the interface. The zinc incorporation depth was 72.2 ± 8.0 μm.
Fluoride (F; upper) and zinc (Zn; lower) distribution profiles on the cross section of the axial wall area following soaking for 7 days after restoring with the test materials. The fluoride incorporation depth of the Caredyne Restore (CD) group was three times deeper than that of the Fuji VII (FJ) group. The CD zinc incorporation showed a concentration gradient along the inside. Glass ionomer cement (GIC), dentin (D), and interface (black arrow)
Discussion
The incorporation of nanoparticles with antimicrobial activity into GICs has been investigated to improve their ability to prevent recurrent dental caries [15,16,17,18,19,20,21]. In this study, the effects of a newly developed GIC formulated with fluoro-zinc-silicate glass were compared with a conventional GIC for two effects: biofilm formation and ion exchange in the dentin. The findings rejected the null hypothesis that antibiofilm effect is not different between a GIC containing fluoro-zinc-silicate glasses in addition to fluoro-alumino-silicate and a GIC containing fluoro-alumino-silicate glasses alone.
The effect on biofilm formation was estimated by SEM and CLSM analyses of S. mutans biofilms following development on each GIC surface for 24 h using a flow cell system. Morphological observation revealed that the biofilm formation on the CD group was remarkably inhibited in comparison with the HA and FJ groups, exhibiting sparse and thinner biofilm clusters (Fig. 2). The CD group included the fluoro-zinc-silicate glass in addition to the fluoro-alumino-silicate glass in powder (Table 1), and the cumulative fluoride release from the CD group into deionized water after 24 h was double that of FJ (data not shown). Thus, the eminent antibiofilm property of CD could be due to the zinc ions alone or to the combination effect with the fluoride ions. At the same time, no materials used in this study affected the viability of the biofilm that developed on the surface. This fact suggests that the antibiofilm effect of CD may be due to preventing bacterial adhesion.
To elucidate this hypothesis, a bacterial adhesion assay was performed. The total amount of bacteria adhered on the CD group during the test period of 2 h decreased to one hundredth that of the FJ group. This result suggests that possible interference from released ions prevents the floating bacteria from initial adhesion. However, if adhesion is achieved, biofilm formation may no longer be prevented.
The results obtained in this study were partially consistent with previous reports in which the GIC surface became completely covered with a mature biofilm of considerable biovolume and thickness [10,11,12, 25, 33]. Padovani et al. [33] evaluated in situ the influence against biofilm accumulation of different restorative materials, such as ceramic, resin composite, GIC, and amalgam. The quantitative analysis, including biovolume, thickness, and roughness coefficient, showed no statistical difference regarding the different materials. This result suggests that the microorganisms accomplished bacterial adhesion on a GIC might survive and initiate biofilm formation.
However, contrary to our results, some reports have shown that large amounts of dead bacteria were observed on the surface of GICs using CLSM [10, 12, 33]. One explanation for this discrepancy can be the differences in the experimental conditions applied. The flow cell system used in this study maintained the pH in the chamber at 6.8 even after 24 h, meaning that the continuous flow of fresh media prevented the bacteria-induced pH from falling to the bacteria-material interface and washed out the released ions. Research has demonstrated that the rate of fluoride release under a neutral pH condition is significantly slower than at 4 pH [34]. Since the antibiofilm activity of GICs is closely correlated with their fluoride release rate during biofilm formation, a static culture may result in overestimation. In fact, when we examined the bacterial viability of the S. mutans biofilm using CLSM following the 24 h of static conditions, the biofilms on FJ and CD consisted almost entirely of dead microorganisms (data not shown).
We also investigated the fluoride and zinc ion uptakes into dentin by EPMA analysis. Fluoride incorporation extended to approximately 120 μm for the CD group, which was three times deeper than that of FJ. CD zinc ions also reached approximately 70 μm. Although the ion concentration on the forefront was clear, this may be expected for the inhibition of demineralization since only small amounts of fluoride, approximately 0.03–0.08 ppm, are necessary to shift the equilibrium from demineralization to remineralization [8]. The incorporated distance from the GIC-dentin interface varied according to the underlying residual carious, morphological structure, and duration. Hotta et al. [30] used EPMA analysis to assess fluoride uptake after restoring an experimentally made cavity with conventional GIC using bovine dentin, and the results showed that the width of the fluoride zone after 30 days was no more than 21.3 μm. Ngo et al. [35] evaluated the remineralization of carious dentin following the restoration of an extensive lesion in a permanent molar using a high strength glass ionomer cement, and the EPMA demonstrated that fluoride had penetrated deep into the underlying demineralized dentin, showing over 1000 μm in some cases [35].
Another product that includes zinc content as part of its glass particles is ChemFil Rock (Dentsply DeTrey, Konstanz, Germany). This GIC, which consists of calcium-aluminum-zinc-fluoro-phosphor-silicate glass, showed higher mechanical properties, such as compressive strength and longevity, than conventional GIC containing alumina silicate glass [36, 37]. However, the antibiofilm and remineralization properties remain unclear because no in vitro study has yet been reported.
Several in vitro studies have demonstrated that zinc may contribute to enhanced tooth remineralization and antibiofilm efficacy. Lynch et al. [38] investigated the effects of zinc and fluoride on remineralization using artificial carious lesions. The results showed that the treatment with both zinc and fluoride resulted in significantly greater remineralization than just fluoride treatment. Similarly, Lippert [39] investigated the dose-response effects of zinc and fluoride on carious lesion remineralization and subsequent protection from demineralization. The lesion remineralization was enhanced in the presence of zinc, exhibiting a synergistic, dose-response effect. As for the inhibitory effects of zinc on biofilm formation, a few reports, including in vitro studies, have evaluated this. Gu et al. evaluated the effects of zinc chloride on the development and vitality pattern of dental biofilm in situ [40]. The subjects wore acrylic appliance-mounted glass discs and rinsed twice daily for 2 min with zinc chloride for up to 48 h. The volume and bacterial vitality of the biofilms treated with various concentrations of zinc chloride were significantly reduced, demonstrating its antibiofilm property.
Within the limitations of this in vitro study, GIC comprised of fluoro-zinc-silicate fillers demonstrated the possibility of improved tooth remineralization and antibiofilm effects against S. mutans. Therefore, further clinical studies are needed to evaluate the impact of fluoride-zinc-releasing restoratives on secondary caries development as well as their remineralization properties.
Conclusion
A new GIC containing both fluoro-zinc-silicate fillers and fluoro-alumino-silicate exhibited effective antibiofilm properties through reduced bacterial adhesion in comparison with a GIC containing fluoro-alumino-silicate glasses alone. The new GIC also showed the extensive penetration of fluoride and the newly formulated zinc ion into dentin. These improved properties may be responsible for better clinical outcomes, such as for root caries and minimally invasive dentistry. Further investigations are needed to determine whether these antibacterial properties clinically contribute to the antibiofilm effect.
References
Oliva A, Della Ragione F, Salerno A, Riccio V, Tartaro G, Cozzolino A, D’Amato S, Pontoni G, Zappia V (1996) Biocompatibility studies on glass ionomer cements by primary cultures of human osteoblasts. Biomaterials 17:1351–1356
Forsten L (1998) Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials 19:503–508
Sidhu SK, Nicholson JW (2016) A review of glass-ionomer cements for clinical dentistry. J Funct Biomater 7:16. https://doi.org/10.3390/jfb7030016
Frencken JE, Leal SC, Navarro MF (2012) Twenty-five-year atraumatic restorative treatment (ART) approach: a comprehensive overview. Clin Oral Investig 16:1337–1346
Frencken JE (2017) Atraumatic restorative treatment and minimal intervention dentistry. Br Dent J 223:183–189
Momoi Y, Hayashi M, Fujitani M, Fukushima M, Imazato S, Kubo S, Nikaido T, Shimizu A, Unemori M, Yamaki C (2012) Clinical guidelines for treating caries in adults following a minimal intervention policy—evidence and consensus based report. J Dent 40:95–105
Liao Y, Brandt BW, Li J, Crielaard W, Van Loveren C, Deng DM (2017) Fluoride resistance in Streptococcus mutans: a mini review. J Oral Microbiol 9:1344509
Wiegand A, Buchalla W, Attin T (2007) Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater 23:343–362
Marquis RE (1995) Antimicrobial actions of fluoride for oral bacteria. Can J Microbiol 41:955–964
Hahnel S, Ionescu AC, Cazzaniga G, Ottobelli M, Brambilla E (2017) Biofilm formation and release of fluoride from dental restorative materials in relation to their surface properties. J Dent 60:14–24
Chau NP, Pandit S, Cai JN, Lee MH, Jeon JG (2015) Relationship between fluoride release rate and anti-cariogenic biofilm activity of glass ionomer cements. Dent Mater 31:e100–e108
De Fúcio SB, Puppin-Rontani RM, de Carvalho FG, Mattos-Graner Rde O, Correr-Sobrinho L, Garcia-Godoy F (2009) Analyses of biofilms accumulated on dental restorative materials. Am J Dent 22:131–136
Sousa RP, Zanin LC, Lima JP, Vasconcelos SM, Melo MA, Beltrão HC, Rodrigues LK (2009) In situ effects of restorative materials on dental biofilm and enamel demineralization. J Dent 37:44–51
Najeeb S, Khurshid Z, Zafar MS, Khan AS, Zohaib S, Martí JM, Sauro S, Matinlinna JP, Rehman IU (2016) Modifications in glass ionomer cements: nano-sized fillers and bioactive nanoceramics. Int J Mol Sci 17:1134
Dashper SG, Catmull DV, Liu SW, Myroforidis H, Zalizniak I, Palamara JE, Huq NL, Reynolds EC (2016) Casein phosphopeptide-amorphous calcium phosphate reduces Streptococcus mutans biofilms development on glass ionomer cement and disrupts established biofilms. PLoS One 11:e0162322
Ibrahim MA, Neo J, Esguerra RL, Fawzy AS (2015) Characterization of antibacterial and adhesion properties of chitosan-modified glass ionomer cement. J Biomater Appl 30:409–419
Yan H, Yang H, Li K, Yu J, Huang C (2017) Effects of chlorhexidine-encapsuled mesoporous silica nanoparticles on the anti-biofilm and mechanical properties of glass ionomer cement. Molecules 22:1225
Hook ER, Owen OJ, Bellis CA, Holder JA, O’Sullivan DJ, Barbour ME (2014) Development of a novel antimicrobial-releasing glass ionomer cement functionalized with chlorhexidine hexametaphosphate nanoparticles. J Nanobiotechnol 12:3
Hu J, Du X, Huang C, Fu D, Ouyang X, Wang Y (2013) Antibacterial and physical properties of EGCG-containing glass ionomer cements. J Dent 41:927–934
Topcuoglu N, Ozan F, Ozyurt M, Kulekci G (2012) In vitro antibacterial effects of glass-ionomer cement containing ethanolic extract of propolis on Streptococcus mutans. Eur J Dent 6:428–433
Elsaka SE, Hamouda IM, Swain MV (2011) Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: influence on physical and antibacterial properties. J Dent 39:589–598
Publication number: WO/2017/168836, WO/2017/168837
He G, Pearce EI, Sissons CH (2002) Inhibitory effect on ZnCl(2) on glycolysis in human oral microbes. Arch Oral Biol 47:117–129
Koo H, Sheng J, Nguyen PT, Marquis RE (2006) Co-operative inhibition by fluoride and zinc of glucosyl transferase production and polysacharide synthesis by mutans streptococci in suspension cultures and biofilms. FEMS Microbiol Lett 254:134–140
Hengtrakool C, Pearson GJ, Wilson M (2006) Interaction between GIC and S. sanguis biofilms: antibacterial properties and changes of surface hardness. J Dent 34:588–597
Takenaka S, Oda M, Domon H, Ohsumi T, Suzuki Y, Ohshima H, Yamamoto H, Terao Y, Noiri Y (2016) Vizantin inhibits bacterial adhesion without affecting bacterial growth and causes Streptococcus mutans biofilm to detach by altering its internal architecture. Biochem Biophys Res Commun 480:173–179
Wakamatsu R, Takenaka S, Ohsumi T, Terao Y, Ohshima H, Okiji T (2014) Penetration kinetics of four mouthrinses into Streptococcus mutans biofilms analyzed by direct time-lapse visualization. Clin Oral Investig 18:625–634
Ohsumi T, Takenaka S, Wakamatsu R, Sakaue Y, Narisawa N, Senpuku H, Ohshima H, Terao Y, Okiji T (2015) Residual structure of Streptococcus mutans biofilm following complete disinfection favors secondary bacterial adhesion and biofilm re-development. PLoS One 10:e0116647
Ngo HC, Mount G, Mclntyre J, Do L (2011) An in vitro model for the study of chemical exchange between glass ionomer restorations and partially demineralized dentin using a minimally invasive restorative technique. J Dent 39(Suppl 2):S20–S26
Hotta M, Li Y, Sekine I (2001) Mineralization in bovine dentin adjacent to glass-ionomer restorations. J Dent 29:211–215
Han L, Okiji T (2011) Evaluation of the ions release / incorporation of the prototype S-PRG filler-containing endodontic sealer. Dent Mater J 30:898–903
Sakaue Y, Takenaka S, Ohsumi T, Domon H, Terao Y, Noiri Y (2018) The effect of chlorhexidine on dental calculus formation: an in vitro study. BMC Oral Health 18:52
Padovani GC, Fúcio SB, Ambrosano GM, Correr-Sobrinho L, Puppin-Rontani RM (2015) In situ bacterial accumulation on dental restorative materials. CLSM/COMSTAT analysis. Am J Dent 28:3–8
Carey CM, Spencer M, Gove RJ, Eichmiller FC (2003) Fluoride release from a resin-modified glass-ionomer cement in a continuous-flow system. Effect of pH. J Dent Res 82:829–832
Ngo HC, Mount G, Mc Intyre J, Tuisuva J, Von Doussa RJ (2006) Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: an in vivo study. J Dent 34:608–613
Zoergiebel J, llie L (2013) Evaluation of a conventional glass ionomer cement with new zinc formulation: effect of coating, aging and storage agents. Clin Oral Investig 17:619–626
Molina GF, Cabral RJ, Mazzola I, Lascano LB, Frencken JE (2013) Mechanical performance of encapsulated restorative glass-ionomer cements for use with atraumatic restorative treatment (ART). J Appl Oral Sci 21:243–249
Lynch RJ, Churchley D, Butler A, Keams S, Thomas GV, Badrock TC, Cooper L, Higham SM (2011) Effects of zinc and fluoride on the remineralization of artificial carious lesions under simulated plaque fluid conditions. Caries Res 45:313–322
Lippert F (2012) Dose-response effects of zinc and fluoride on caries lesion remineralization. Caries Res 46:62–68
Gu H, Fan D, Gao J, Zou W, Peng Z, Zhao Z, Ling J, LeGeros RZ (2012) Effect of ZnCl2 on plaque growth and biofilm vitality. Arch Oral Biol 57:369–375
Funding
This work was supported, in part, by Grant-in-Aid for Scientific Research (grant no. 15H05021) from the Japan Society for the Promotion of Science. This work was also partially supported by the Mitsubishi Foundation and GC Corporation, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research is partially supported by GC Corporation (supplies expenses and publication fee).
Ethical approval
All of the experimental protocols involving the donation of an extracted tooth were approved by the Niigata University Ethics Committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclaimer
The sponsor had no control for the interpretation, writing, or publication of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig S1
Flow cell system for biofilm formation. The flow cell system consisted of a medium reservoir, a peristaltic pump, and a carboy for waste. Two specimens were placed at either end so that the fluid flow did not interfere. (JPG 587 kb)
Supplementary Fig S2
Flow cell system for bacterial adhesion test. During the operation time, fresh media was constantly delivered to the bacterial suspension to maintain optimal density. (JPG 311 kb)
Rights and permissions
About this article
Cite this article
Hasegawa, T., Takenaka, S., Ohsumi, T. et al. Effect of a novel glass ionomer cement containing fluoro-zinc-silicate fillers on biofilm formation and dentin ion incorporation. Clin Oral Invest 24, 963–970 (2020). https://doi.org/10.1007/s00784-019-02991-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02991-0