Abstract
Objectives
To assess the clinical and radiographic outcomes of implants treated by means of non-surgical debridement with systemic antibiotic therapy.
Materials and methods
A prospective case series study evaluating the 12-month clinical and radiographic outcomes of peri-implantitis lesions treated with ultrasonic scaler debridement, a glycine air abrasive, and metronidazole followed by supportive maintenance. Clinical and radiographic variables and success criteria were defined a priori.
Results
Overall, 21 patients were included. One implant failed during the study period (implant survival rate 95.24%). Substantial changes occurred at 12 months in all the clinical and radiographic variables, reaching strong statistical significance in the majority of them. According to the success criteria applied, 40.90% of the peri-implantitis were arrested and resolved, while 59.1% presented with at least one probed site with bleeding on probing (BoP). Moreover, 95.45% exhibited peri-implant pocket depth (PPD) < 5 mm at the end of the study. None of the implants presented with progressive bone loss.
Conclusion
Non-surgical therapy of peri-implantitis is effective to arrest progressive bone loss, reduce PPD and suppuration, and achieve radiographic bone fill in the majority of cases. Nevertheless, it failed to be completely efficacious in the achievement of successful therapeutic outcomes as BoP remained frequently present.
Clinical relevance
Non-surgical therapy achieved significant clinical and radiological improvements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The non-linear accelerative progressive pattern of bone loss in peri-implantitis leads to implant failure if the given infection is not proficiently arrested [1]. A variety of different interventions have been proposed for the treatment of peri-implantitis. Namely, non-surgical or surgical management by means of access flap debridement with numerous variants such as the use of lasers to detoxify the implant surface, implantoplasty to smooth the surface, resective procedures, and regenerative approaches [2,3,4]. The predictability of these interventions regarding clinical (i.e., pocket depth reduction and resolution of inflammation) and radiographic (i.e., bone fill) outcomes still remains controversial [2, 3, 5]. In fact, the therapy of peri-implantitis has been regarded as challenging and unsustainable in the long term [6,7,8,9]. Nevertheless, the treatment of peri-implantitis is essential to upgrade the implant prognosis [10, 11].
Consequently, the primary goal of peri-implantitis treatment must be the resolution of peri-implant soft tissue inflammation (i.e., no bleeding on probing, no suppuration) and the maintenance/stability of the supporting bone [9]. This desirable environment should be populated by bacteria compatible with peri-implant health [12]. This, in combination with the adherence of adequate personal-/professional-administered oral hygiene measures to eliminate biofilm deposits, should be conducive to the long-term stability of the peri-implant tissues [13].
While the non-surgical therapy for mucositis has demonstrated to be successful, predictable, and suitable for the patients due to the low morbidity and limited cost, conflicting outcomes with limited predictability have been exhibited for the non-surgical therapy of peri-implantitis [14]. The idea that peri-implantitis is a plaque-associated chronic inflammatory entity—like periodontitis—could make the clinicians contemplate the effectiveness of non-surgical therapy for its management. As a matter of fact, recent evidence is exhibiting promising non-surgical interventions for the management of peri-implantitis [11, 15]. Mettraux et al. showed, in a 2-year clinical study, the significant reduction in bleeding on probing and suppuration from 100 to 43% and from 87 to 0%, respectively, treated with carbon fiber and metal curettes followed by repeated application of a diode laser 3×for 30 s at days 0, 7, and 14 [11]. Alike, Bassetti et al. showed, in a 1-year clinical study, the statistical significant reduction for probing depth, bleeding on probing, bacterial counts, and IL-1B regardless of the use of photodynamic therapy for the non-surgical treatment of peri-implantitis [15]. Nevertheless, one of the major limitations is on the gain of the radiographic bone level [10], thus failing to achieve the criteria to consider successful therapeutic outcome [8].
The use of adjunctive use of antibiotics in the non-surgical treatment of periodontal disease has shown to be beneficial under certain conditions (i.e., aggressive periodontitis or moderate/severe chronic periodontal disease) [16]. Based upon the current understanding that peri-implant infections share the etiology with periodontal diseases, the use of systemic antibiotics for the therapy of peri-implantitis has been advocated [7]. Recently, Stein et al. yielded favorable results by means of clinical outcomes 1 year after ultrasonic decontamination, soft tissue curettage, and submucosal air polishing [17]. Notwithstanding, the reduction of implant sites with probing depth > 4 mm and bleeding on probing was significantly higher in patients taking amoxicillin + metronidazole in a post-operative regimen. However, this favorable finding is not consistent across the literature [7,8,9, 15].
Hence, the purpose of this study was to assess the clinical and radiographic outcomes of implants treated by means of non-surgical mechanical debridement combined with systemic antibiotic therapy followed by a regular peri-implant maintenance therapy.
Material and methods
Patient population
This prospective clinical and radiographic case series study was performed in one single private practice from November 2014 until January 2018. Consecutive patients diagnosed with peri-implantitis according to the following case definition were included in the present study [18]:
Progressive peri-implant marginal bone loss > 2 mm from a baseline X-ray recorded at the time of prosthesis delivery.
Bleeding on gentle probing (BoP) + and erythema +
Suppuration +/−
Increase in probing depth from baseline (i.e., ≥ 5 mm)
Furthermore, the following inclusion criteria were considered: (1) age ≥ 18 years, (2) treated chronic/aggressive periodontitis, (3) full-mouth plaque score (FMPS) < 25%, (4) full-mouth bleeding score (FMBS) < 25%, (5) cemented or screw-retained single-unit crowns and partial dental prosthesis that allowed correct access for brushing, and, if not, (6) prostheses that could be modified. Patients were excluded on the basis of (1) clinical implant mobility; (2) radiographic peri-implant bone loss > 50%; (3) pregnancy or lactating females; (4) any medical condition which contraindicated non-surgical peri-implant therapy; (5) previous surgical and non-surgical treatment of the affected implants at least 12 months before; (6) systemic diseases, medications, or conditions that may compromise wound healing influencing the outcome of the therapy; (7) known allergy or intolerance to metronidazole; (8) use of systemic antibiotics during the previous 3 months; (9) use of systemic antibiotics for endocarditis prophylaxis; (10) smoking more than 10 cigarettes/day; and (11) horizontal component of the peri-implant defects.
This case series study was performed in accordance with the Universitat Internacional de Catalunya Ethical Committee (PERECL201802) and Helsinki Declaration. All patients read and signed an appropriate informed consent document prior to participation in the study.
Non-surgical peri-implant therapy
Patients received a supragingival prophylaxis with oral hygiene instructions 1 week before the subgingival instrumentation, which was standardized as follows: after local anesthesia (articaine 4% and adrenaline 1:100,000), the implant surfaces were treated with ultrasonic devices (Newtron P5, Satelec Acteon; Olliergues, France) with the steel alloy H3 dental ultrasonic scaler tip (H3, Satelec Acteon; Olliergues, France), curettage (SyG 7/89 Everdge, Hu-Friedy; Chicago, IL, USA) of the bone defect was performed, and glycine air powder applied subgingivally (Air-flow® powder sub + supragingival PERIO, EMS; Nyon, France) with an air-flow piezon device (Air-flow master piezon®, EMS, Nyon; France). Finally, the implants’ prosthesis were modified by making them cleansable [understanding cleansable prosthesis as those that allowed the correct access of the interproximal brush (Interprox®; Barcelona, Spain)] with burs (preparation and finishing drills kit, Sweden&Martina; Padova, Italy) and porcelain polishing and contouring discs (rotatory grinding and polishing instruments, EVE Ernst Vetter GmbH; Keltern, Germany). Oral hygiene instructions around the modified implant prosthesis were given immediately after. All procedures were performed by an experienced periodontist (JN).
After mechanical treatment, the antibiotic regimen consisting on Metronidazole 500 mg every 8 h for 7 days was prescribed for all patients.
Supportive peri-implant maintenance therapy
All the patients were enrolled in a peri-implant maintenance therapy (PIMT) program every 3–6 months according to the patient’s risk profiling [19]. At each PIMT visit, a reinforcement of oral hygiene instructions, supragingival and, if needed, subgingival debridement [BoP and/or peri-implant pocket depth (PPD) ≥ 5 mm] as well as tooth polishing were performed.
Demographic data
At the beginning of the study, the incoming demographic data were collected through the patient’s anamnesis: gender (female/male), systemic condition (ASA classification), tobacco habit (non-smoker, former smoker, or current smoker), previous history of periodontal disease (yes/no), periodontal disease recurrence along the study period (yes/no), history of periodontal disease severity (mild/moderate/severe) [20], history of periodontal disease extension (localized/generalized) [20], and implant position (maxilla/mandible and incisors/canines and premolars/molars). The implant system was further recorded as well as the type of prosthesis (cemented/screw-retained).
Clinical measurements
The following clinical parameters were assessed at six sites for each implant by a single calibrated examiner (CV) at baseline and at the 12 months of follow-up using a periodontal probe (PCP-UNC 15; Hu-Friedy, Chicago, IL, USA):
Plaque index (PlI): presence or absence of plaque along the mucosal margin [21].
Bleeding on probing (BoP): presence or absence of bleeding 15 s after gentle probing.
Suppuration on probing (SoP): presence or absence of suppuration after probing.
Peri-implant pocket depth (PPD): distance (mm) from the mucosal margin to the base of the probable pocket.
Gingival recession (REC): distance (mm) from the mucosal margin and the implant abutment interface.
Keratinized mucosa (KM): distance (mm) from the mucosal margin and the mucogingival junction.
Radiographic examination
A periapical radiograph was obtained using the long-cone parallel technique and a film holder (Dürr Dental AG, Bietigheim-Bissingen, Germany) at baseline and at 12-month follow-up visit. All radiographs were standardized in their exposure (7 mA-60 kV/20 ms).
The following measurements were recorded by an independent previously calibrated examiner (RP) (intra-class correlation coefficient 0.982) at the mesial and distal aspects of the treated implants:
Bone level (BL): distance (mm) between the implant shoulder and the base of the defect.
Intra-bony defect (ID): distance (mm) between the bottom of the defect and the line connecting the distal and mesial interproximal bone crest.
Intra-bony defect width (WD): distance (mm) between the distal and mesial interproximal bone crest and the implant surface.
Angulation of the intra-bony defect (AD): angle resulted from a vertical line along the outer implant surface and a line extending along the peri-implant bone defect.
The measurements were determined using an image-processing program (ImageJ; NIH, Bethesda, MA, USA). The radiographs were calibrated using the known dimensions of the implant as reference values. Mean values were calculated from the mesial and distal aspects.
Success criteria
The following criteria were considered for therapeutic success [22]:
Implant survival.
Absence of probing pocket depth ≥ 5 mm with concomitant BoP and/or SoP.
Absence of progression of peri-implant bone loss.
Statistical analysis
The primary outcome parameter was the change in PPD over time. Analysis was performed at patient level (i.e., the implant with the deepest PPD at baseline was selected for analysis). Descriptive statistical analysis included mean values and standard deviations (SD) of quantitative variables, while qualitative variables were expressed with frequencies and valid percentages. Changes versus baseline were analyzed using the non-parametric Wilcoxon test. Finally, treatment success was defined as the absence of PPD ≥ 5 mm with BoP/SoP and no additional peri-implant bone loss at the end of the evaluation period. The SPSS version 19.00 software (SPSS Inc., Chicago, IL, USA) was used for all analyses. The level of significance was set at p < 0.05.
Results
Patient characteristics
Twenty-four healthy patients were enrolled in the study. However, three patients were lost in the follow-up period: two patient’s radiographs did not meet the standards, and another patient lost the treated implant. Thus, the sample included 21 patients (2 males and 19 females) with a mean age of 53 ± 11.74 years. Of these patients, 16 (76.19%) of them were ASA type 1, while 5 (23.81%) were ASA type 2 (4 patients with well-controlled arterial hypertension and 1 diabetic patient). In regard to tobacco use, one patient (4.76%) was light smoker (< 10 cigarettes per day) and five were former smokers (23.81%). The majority of the recruited patients (71.43%) had a previous history of periodontitis. The characteristics of the study participants are presented in Table 1.
In this investigation, only one implant placed in pristine bone per patient was included in the analysis. Two patients had two implants with peri-implantitis; however, the implant with the most severe condition was included. Therefore, a total of 21 dental implants were treated throughout the study: 7 (33.3%) maxillary implants and 14 (66.7%) mandibular implants. The mean time of implants in function was 7.11 ± 3.20 years. In all the cases, post-operative healing was considered as uneventful.
According to the type of implant-abutment connection, 3 patients (14.29%) carried cemented prosthesis, while 18 patients (85.71%) were rehabilitated with screw-supported prosthesis. The following implant systems were treated: six implants Nobel Biocare® (Nobel Biocare AB, Göteborg, Sweden), five implants BioHorizons® (Maestro Dental Implants, Birmingham, AL, USA), three Straumann® (Straumann Institute, Waldenburg, Switzerland), one implant Lifecore Restore® (Lifecore Biomedical Inc., Chaska, MN), one implant Neodent® (Neodent Ltda, Curitiba, Paraná, Brazil), one implant Bioner® (Bioner, Sistemas Implantológicos, Barcelona, Spain), one implant MIS® (MIS Implants Technologies Ltd., Bar-Lev Industrial Park, Israel), one implant Microdent® (MicrodentSystem, SL, Barcelona, Spain), and one implant Klockner® (Klockner Implant System SA, Barcelona, Spain).
Clinical measurements
Table 2 depicts the clinical parameters measured throughout the study. One implant failure occurred during the follow-up period (overall implant survival rate 95.24%). Mean PlI values were 68.17 ± 26.68% at baseline and 40.91 ± 29.87% at 12 months. A statistically significant reduction in plaque levels was found (p = 0.001). The percentage of BoP-positive sites decreased from 78.78 ± 28.26% to 21.22 ± 24.76% at the end of the follow-up examination. Again, the difference was statistically significant (p < 0.001). Similarly, clinical examination at 12 months revealed a statistically significant reduction of SoP (p < 0.001).
At baseline, the mean PPD was 5.34 ± 1.29 mm. A statistically significant (p < 0.001) reduction between baseline and 12-month follow-up (3.69 ± 0.70 mm) was observed. A significant increase in mean marginal recession was also reported following the non-surgical therapy (baseline 0.17 ± 0.47 mm; 12 months 0.79 ± 0.72 mm; p < 0.001).
Radiographic outcomes
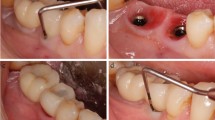
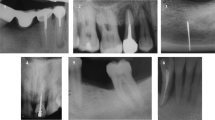
The mean distance between the implant shoulder and the base of the defect (BL) at baseline was 3.76 ± 1.26 mm, and 12 months after non-surgical therapy, this value was reduced to 2.45 ± 1.26 mm. This difference was statistically significant (p < 0.001) (Table 3). Radiographic bone changes are shown in Figs. 1, 2, and 3.
Non-surgical therapy also affected the distance between the bottom of the defect and the interproximal bone crest (Table 3). The mean ID showed a decrease from 1.87 ± 1.10 mm at baseline to 1.60 ± 1.19 mm at the 12-month follow-up. However, these differences did not reach statistical significance (p = 0.057).
Moreover, between baseline and 12-month follow-up, a statistical reduction in the horizontal component of the defect occurred (p < 0.001). In addition, there was a concomitant significant increase in the angle of the defect (p < 0.001) (Table 3).
Therapeutic success at 12-month examination
According to the therapeutic success criteria applied, 40.90% of the peri-implantitis were arrested and resolved, while 59.10% presented with at least one probed site with BoP. Furthermore, all implants except one (95.45%) exhibited PPD < 5 mm, and none of the implants presented a progressive bone loss.
Discussion
Principal findings
The effectiveness of different therapies for peri-implantitis has been a topic for much debate [23]. Due to the vague long-term efficacy of the surgical therapy [24] and increased cost [25] and morbidity [26], the use of non-surgical therapy as a single treatment could be considered to reduce inflammation and enhance implants prognosis. The present case series study has shown that non-surgical debridement and implant-supported prosthesis modification combined with antibiotic therapy in vertical defects followed by supportive PIMT is completely effective to resolve peri-implantitis in ~ 40% of the cases treated. Interestingly, it showed to be efficient to reduce PPD and arrest peri-implant bone loss in the vast majority of the implants. Along these lines, it is worth mentioning that BoP positive might lead to false-positive results, masking non-pathologic conditions [27].
Agreements and disagreements with previous findings
To date, there is scarce evidence concerning the non-surgical therapy of peri-implantitis when compared with the treatment of periodontitis. Recent data suggest the improvement in the clinical and radiographic conditions. For instance, Mettraux et al. showed a significant reduction in BoP and SoP from 100 to 43% and from 87 to 0% when combining non-surgical debridement with diode laser [11]. Bassetti et al. showed the statistically significant reduction for PPD, BoP, bacterial counts, and IL-1B regardless of the use of photodynamic therapy for the non-surgical treatment of peri-implantitis [15]. Likewise, Roos-Jansåker et al. showed a significant reduction of BoP sites from 97 to 38% when applied chloramine as adjunct [28]. Promising outcomes have been also achieved when using air-polishing devices [29]. Nevertheless, a complete disease resolution was not frequently obtained in these studies. Our findings are congruent with previous results. These are suggestive that non-surgical debridement therapy combined with post-operative metronidazole and an adequate adherence of professionally administered PIMT is effective to arrest bone loss and reduce PPD in the vast majority of the cases. Yet, non-surgical therapy was ineffective to completely resolve BoP around dental implants.
Interestingly, a systematic review revealed that non-surgical debridement combined with antibiotics exhibited superior outcomes by means of PPD [5]. In fact, the use of antibiotics is conceivable in the treatment of periodontal disease based on the theoretical understanding that specific bacterial loading is responsible to activate bone metabolism [30]. On the other side, it seems that the peri-implantitis microbiome is more heterogeneous. A recent systematic review has highlighted that peri-implantitis represents a heterogeneous mixed infection that includes periodontopathic microorganisms, uncultivable asaccharolytic anaerobic gram+ rods, and other cultivable gram− rods as well as opportunistic microorganisms [31]. Nonetheless, assuming that peri-implantitis lesions are populated by putative anaerobic bacteria, the use of antibiotics can potentially benefit the treatment outcome. Current evidence, however, has failed to demonstrate the adjuvant positive effect of antibiotics to non-surgical and surgical peri-implantitis therapy [7, 9, 10]. Due to the single-arm nature of the present study, the findings achieved cannot be attributed to the use of antibiotics. Hence, in the future, it is encouraged to test in long-term randomized clinical trials the effect of antibiotics versus placebo as adjunct to non-surgical therapy in the treatment of peri-implantitis.
Clinical implications
Findings from the present case series study are promising in the therapy of peri-implantitis. In the context, it should be taken into consideration that non-surgical therapy implies many advantages compared with surgical treatments. As such, reduced cost, limited morbidity, and less operative time might tilt the balance towards the patient’s preferable treatment option to manage this disease. Interestingly, a cost-effectiveness analysis revealed that debridement alone proved preferable only if a decision-maker is willing to pay less than 5.7 € per millimeter of PPD reduction [32]. This fact could increase patient adherence and willingness for the treatment. On the other side, it is speculated that the limited cost could infer in lower awareness of the patients, and this might influence on the post-operative care (i.e., personal and professional-administered PIMT). Furthermore, it is worth noting that in the present case series study, the prostheses were modified with the goal of facilitating the cleansability. This fact highlights the positive impact of providing access to improve the capability of the patients to achieve more efficient personal-administered oral hygiene measures.
Further, in the present case series, metal-made instruments, including ultrasonic tips and curettes, were used to manage the peri-implantitis defects. The features as well as the material are made the instruments for the curettage on dental implants has been a subject of controversy due to the damage by means of roughness associated to these instruments upon the implant surface [28, 33]. Nevertheless, it is the authors’ opinion that the relevance of surface modification is surpassed by the potential to disrupt the biofilm and the effectiveness to remove the inflammatory tissue.
It is important to remark that the cases selected for the present study displayed vertical intra-bony defects. Hence, these outcomes cannot be extrapolated to horizontal peri-implant bone loss as these are less prone to repair. This has been extensively demonstrated in the treatment of periodontal diseases [34,35,36]; however, literature within the therapy of peri-implantitis is scarce [37].
In addition, the most effective therapy for peri-implantitis is yet to be conclusively identified. As such, over the last decade, dentist from all over the globe have been performing empirical treatment modalities, including the prescription of antibiotics. In consequence, antibiotic resistance by certain peri-implantitis-associated microorganisms has been reported [38]. Not surprisingly, Rams et al. demonstrated that 6.7% of the studied population revealed submucosal species resistant in vitro to amoxicillin and metronidazole. Hence, to date, caution should be exercised when prescribing antibiotics for the treatment of peri-implantitis [38].
Limitations of the study
It must be pointed out the methodological limitations present in the current case series study with respect to the study design and due to the absence of standardized clinical and radiographic examinations. On the other hand, the prosthesis modification was performed simultaneously with the subgingival instrumentation; thus, the results achieved in the present investigation could be the response of the combination of both procedures. The therapy used in the current protocol needs to be evaluated clinically in a large sample, over a longer follow-up, and in randomized controlled setting.
Conclusion
The present case series study suggested that non-surgical therapy of peri-implantitis is effective to arrest progressive bone loss, to reduce probing pocket depth and suppuration, and to achieve radiographic bone gain in the majority of cases. Nevertheless, it failed to be completely efficacious in the achievement of successful therapeutic outcomes as bleeding on probing frequently remained present.
References
Derks J, Schaller D, Hakansson J, Wennstrom JL, Tomasi C, Berglundh T (2016) Peri-implantitis—onset and pattern of progression. J Clin Periodontol 43:383–388
Khoshkam V, Chan HL, Lin GH, MacEachern MP, Monje A, Suarez F, Giannobile WV, Wang HL (2013) Reconstructive procedures for treating peri-implantitis: a systematic review. J Dent Res 92:131S–138S
Chan HL, Lin GH, Suarez F, MacEachern M, Wang HL (2014) Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol 85:1027–1041
Mailoa J, Lin GH, Chan HL, MacEachern M, Wang HL (2014) Clinical outcomes of using lasers for peri-implantitis surface detoxification: a systematic review and meta-analysis. J Periodontol 85:1194–1202
Faggion CM Jr, Listl S, Fruhauf N, Chang HJ, Tu YK (2014) A systematic review and Bayesian network meta-analysis of randomized clinical trials on non-surgical treatments for peri-implantitis. J Clin Periodontol 41:1015–1025
Schou S, Berglundh T, Lang NP (2004) Surgical treatment of peri-implantitis. Int J Oral Maxillofac Implants 19(Suppl):140–149
Carcuac O, Derks J, Charalampakis G, Abrahamsson I, Wennstrom J, Berglundh T (2016) Adjunctive systemic and local antimicrobial therapy in the surgical treatment of peri-implantitis: a randomized controlled clinical trial. J Dent Res 95:50–57
Carcuac O, Derks J, Abrahamsson I, Wennstrom JL, Petzold M, Berglundh T (2017) Surgical treatment of peri-implantitis: 3-year results from a randomized controlled clinical trial. J Clin Periodontol 44:1294–1303
Berglundh T, Wennstrom JL, Lindhe J (2018) Long-term outcome of surgical treatment of peri-implantitis. A 2-11-year retrospective study. Clin Oral Implants Res 29:404–410
Heitz-Mayfield LJA, Salvi GE, Mombelli A, Faddy M, Lang NP (2012) Anti-infective surgical therapy of peri-implantitis. A 12-month prospective clinical study. Clin Oral Implants Res 23:205–210
Mettraux GR, Sculean A, Burgin WB, Salvi GE (2016) Two-year clinical outcomes following non-surgical mechanical therapy of peri-implantitis with adjunctive diode laser application. Clin Oral Implants Res 27:845–849
Teles RP, Haffajee AD, Socransky SS (2006) Microbiological goals of periodontal therapy. Periodontol 42:180–218
Roccuzzo M, Layton DM, Roccuzzo A, Heitz-Mayfield LJ (2018) Clinical outcomes of peri-implantitis treatment and supportive care: a systematic review. Clin Oral Implants Res 29(Suppl 16):331–350
Claffey N, Clarke E, Polyzois I, Renvert S (2008) Surgical treatment of peri-implantitis. J Clin Periodontol 35:316–332
Bassetti M, Schar D, Wicki B, Eick S, Ramseier CA, Arweiler NB, Sculean A, Salvi GE (2014) Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res 25:279–287
Haffajee AD, Socransky SS, Gunsolley JC (2003) Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol 8:115–181
Stein JM, Hammacher C, Michael SS (2018) Combination of ultrasonic decontamination, soft tissue curettage, and submucosal air polishing with povidone-iodine application for non-surgical therapy of peri-implantitis: 12 month clinical outcomes. J Periodontol 89:139–147
Rodrigo D, Sanz-Sánchez I, Figuero E, Llodrá JC, Bravo M, Caffesse RG, Vallcorba N, Guerrero A, Herrera D (2018) Prevalence and risk indicators of peri-implant diseases in Spain. J Clin Periodontol 45(12):1510–1520
Monje A, Wang HL, Nart J (2017) Association of preventive maintenance therapy compliance and peri-implant diseases: a cross-sectional study. J Periodontol 88:1030–1041
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4(1):1–6
O’Leary TJ, Drake RB, Naylor JE (1972) The plaque control record. J Periodontol 43(1):38
Heitz-Mayfield LJA, Salvi GE, Mombelli A, Loup PJ, Heitz F, Kruger E, Lang NP (2018) Supportive peri-implant therapy following anti-infective surgical peri-implantitis treatment: 5-year survival and success. Clin Oral Implants Res 29(1):1–6
Koldsland OC, Wohlfahrt JC, Aass AM (2018) Surgical treatment of peri-implantitis: prognostic indicators of short-term results. J Clin Periodontol 45(1):100–113
La Monaca G, Pranno N, Annibali S, Cristalli MP, Polimeni A (2018) Clinical and radiographic outcomes of a surgical reconstructive approach in the treatment of peri-implantitis lesions: a 5-year prospective case series. Clin Oral Implants Res 29(10):1025–1037
Schwendicke F, Stolpe M, Graetz C (2017) Cost comparison of prediction-based decision-making for periodontally affected molars. J Clin Periodontol 44(11):1145–1152
Powell CA, Mealey BL, Deas DE, McDonnell HT, Moritz AJ (2005) Post-surgical infections: prevalence associated with various periodontal surgical procedures. J Periodontol 76(3):329–333
Hashim D, Cionca N, Combescure C, Mombelli A (2018) The diagnosis of peri-implantitis: a systematic review on the predictive value of bleeding on probing. Clin Oral Implants Res 29(Suppl 16):276–293
Roos-Jansåker AM, Almhojd US, Jansson H (2017) Treatment of peri-implantitis: clinical outcome of chloramine as an adjunctive to non-surgical therapy, a randomized clinical trial. Clin Oral Implants Res 28:43–48
Schwarz F, Becker K, Renvert S (2015) Efficacy of air polishing for the non-surgical treatment of peri-implant diseases: a systematic review. J Clin Periodontol 42:951–959
Loesche WJ (1992) The specific plaque hypothesis and the antimicrobial treatment of periodontal disease. Dent Update 19(68):70–2, 74
Lafaurie GI, Sabogal MA, Castillo DM, Rincón MV, Gómez LA, Lesmes YA, Chambrone L (2017) Microbiome and microbial biofilm profiles of Peri-Implantitis: a systematic review. J Periodontol 88:1066–1089
Listl S, Fruhauf N, Dannewitz B, Weis C, Tu YK, Chang HJ, Faggion CM Jr (2015) Cost-effectiveness of non-surgical peri-implantitis treatments. J Clin Periodontol 42:470–477
Fox SC, Moriarty JD, Kusy RP (1990) The effects of scaling titanium implant surface with a metal and plastic instruments: an in vitro study. J Periodontol 61:485–490
Steffensen B, Suzuki H, Caffesse RG, Ash MM (1987) Repair of periodontal angular bony defects evaluated by one- and two-dimensional radiographic analysis. Oral Surg Oral Med Oral Pathol 63:109–114
Steffensen B, Webert HP (1989) Relationship between the radiographic periodontal defect angle and healing after treatment. J Periodontol 60:248–254
Nibali L, Yeh YC, Pometti D, Tu YK (2018) Long-term stability of intrabony defects treated with minimally-invasive non-surgical therapy. J Clin Periodontol 45(12):1458–1464
Renvert S, Roos-Jansäker AM, Claffey N (2008) Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol 35(8 Suppl):305–315
Rams TE, Degener JE, van Winkelhoff AJ (2014) Antibiotic resistance in human peri-implantitis microbiota. Clin Oral Implants Res 225(1):82–90
Funding
The present investigation was a self-funded investigation by the Department of Periodontology of the Universitat Internacional de Catalunya.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This case series study was performed in accordance with the Universitat Internacional de Catalunya Ethical Committee (PERECL201802) and Helsinki Declaration.
Informed consent
All patients read and signed an appropriate informed consent document prior to participation in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nart, J., Pons, R., Valles, C. et al. Non-surgical therapeutic outcomes of peri-implantitis: 12-month results. Clin Oral Invest 24, 675–682 (2020). https://doi.org/10.1007/s00784-019-02943-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02943-8