Abstract
Objectives
This study aimed to examine the electromyographic activity of superficial masseter and anterior temporal muscles during chewing gum and gummy jelly mastication in healthy subjects to reveal the difference of neuromuscular control of jaw-closing muscles, according to the food texture.
Materials and methods
Electromyographic activity was recorded in 30 adults with Angle Class I occlusion and unimpaired function from the bilateral superficial masseter and anterior temporal muscles during unilateral mastication of two test foods: standardized gummy jelly and color-changeable chewing gum. Differences in normalized electromyographic activity and asymmetry index values between gummy jelly and chewing gum mastication were analyzed during the early, middle, and late phases of mandibular closure. Furthermore, changes among the three closing phases were compared for each test food.
Results
High electromyographic activity of both muscles tended to occur bilaterally during the middle and late closing phases during gummy jelly mastication, but increased muscle activity in the late closing phase was not observed during chewing gum mastication. The asymmetry index of the superficial masseter muscle increased significantly from early to late closure, regardless of the food texture, but it tended to decrease for the anterior temporal muscle during gummy jelly mastication.
Conclusion
The different aspects of the chewing process between the comminution and mixing test measures are necessary to elicit the different human neuromuscular strategies of chewing for different test foods.
Clinical relevance
These characteristic EMG activities of the superficial masseter and anterior temporalis muscles may be used as supporting diagnostic information during patient assessments and a reference during evaluation of masticatory system disharmony or dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surface electromyographic (EMG) recording provides non-invasive information regarding muscle properties through electrodes located over the skin [1]. This information is useful to assess the neuromuscular control of jaw-closing muscles during mastication. Previous studies have demonstrated that the EMG activity of masticatory muscles is associated with the properties of the foods [2, 3], including mechanical properties such as the particle size, hardness, and elasticity [4,5,6]. In addition, the masseter/temporal activity ratio is associated with the mechanical hardness of the foods during unilateral mastication [7]. It has been reported that the pattern of relative activation of the masseter and temporal muscles is mainly determined by isometric contraction with a small gape during the slow power phase [8, 9], indicating that, with a decrease in the gape, the chewing/non-chewing ratio becomes more asymmetric for the masseter and more symmetric for the posterior temporal muscle. These previous findings collectively suggest that the masseter and temporal muscles employ different neuromuscular strategies for masticatory function.
In the masticatory process of natural foods, the food bolus is first cut and grinded, following which the softened small particles are mixed with saliva to facilitate swallowing [10]. To date, chewing gum has been predominantly used as an artificial test food for assessing mandibular movements during mastication [11,12,13,14,15] because stable jaw movements and EMG activity are suitable for analyzing an individual’s chewing pattern. On the other hand, swallowable standardized gummy jelly, which has a reproducible texture and constant shape and size, is often used because its changing food properties are similar to changes occurring during daily natural chewing, and it is superior for examining the role of intraoral inputs in adapting masticatory behavior under changing bolus conditions [5, 6, 13, 16,17,18,19,20]. Color-changeable gum [21,22,23] and standardized gummy jelly [24,25,26,27] have recently been applied for the evaluation of masticatory performance, with the former used to evaluate the ability to mix and knead (i.e., mixing ability) a food bolus and the latter used to evaluate the degree of breakdown of a food bolus (i.e., comminution). A previous study suggested that the comminution test detected masticatory performance differences in a wider range than the mixing ability test; however, the mixing ability test can be used to assess masticatory performance in children [28].
The different aspects of the masticatory process between the comminution test and the mixing test measure seem to induce different human neuromuscular strategies in chewing for each type of artificial test food. However, the changes in masticatory muscle activity that occur during the functional phase of the chewing cycle, including final mandibular closure, of gummy jelly and chewing gum mastication remain unknown.
The aim of the present study was to examine the EMG activity of the bilateral superficial masseter and anterior temporal muscles during unilateral mastication of chewing gum and gummy jelly in healthy subjects to assess the differences in the neuromuscular control of the jaw-closing muscles according to food texture. Our null hypothesis was that no difference exists between gummy jelly and chewing gum mastication in determining the masticatory muscle activity involved in different human neuromuscular strategies.
Materials and methods
Subjects
All subjects were clinically assessed using radiographs and dental casts. Furthermore, clinical signs and symptoms of temporomandibular disorders (TMDs; e.g., temporomandibular joint [TMJ] clicking, tiredness/stiffness, pain, difficulties in wide mouth opening, and TMJ locking) were evaluated using clinical examinations and questionnaires, based on the Research Diagnostic Criteria for TMD Axis I [29]. The inclusion criteria for the study were as follows: Angle Class I canine and molar relationship, with no skeletal asymmetry, normal overjet and overbite; complete eruption of permanent teeth, excluding the third molars; no missing or extracted teeth; no root-filled teeth or dental prostheses; no signs or symptoms of TMDs; minimal crowding and rigid intercuspation, without crossbite or scissors-bite; and no current/ongoing dental or orthodontic treatment. Before participation, all subjects provided written informed consent after receiving an explanation of the study goals and structure, which were independently reviewed and approved by our Ethics Committee (#25–116). The study was conducted in accordance with ethical principles, including the tenets of the World Medical Association Declaration of Helsinki.
Artificial test foods
Standardized gummy jelly (UHA Mikakuto Co., Ltd., Osaka, Japan), which is used to evaluate comminution or the degree of breakdown of food, was selected as the test food (dimensions: width, 20 mm; length, 20 mm; thickness, 10 mm; weight: 5.5 g) [26, 27]. For comparison, a piece of color-changeable chewing gum (Xylitol; Lotte Co., Ltd., Saitama, Japan), which is used for the evaluation of mixing ability, was used (dimensions: width, 20 mm; length, 35 mm; thickness, 4.0 mm; weight: 3.0 g) [23]. The texture of the gummy jelly before chewing and that of the color-changeable chewing gum after chewing 60 times were analyzed using a creep meter (RE2-33005S; Yamaden Co., Ltd., Tokyo, Japan). During this procedure, the two test foods were placed on a plate and elevated toward a polyacetal plunger (12-mm diameter) at a speed of 10 mm/s. The plunger was connected to a load cell that pressed the sample twice (80% compressibility at 37 °C). This procedure was repeated five times to obtain a mean value for each parameter. The hardness on the first and second consecutive compression, cohesiveness, and adhesiveness values were 7.86 N and 6.17 N (first and second compression), 0.38 and 3888 J/m3, respectively, for the chewing gum and 7.89 N and 7.25 N (first and second compression), 0.86 and 88 J/m3, respectively for the gummy jelly.
Recording system for jaw movements and electromyography
For recording mandibular movements and muscle activity, an optoelectric jaw tracking system with six degrees of freedom (Fig. 1) was used, as previously described [13, 17,18,19,20]. The system comprised a head frame, a face bow, a pointer, light-emitting diodes (LEDs), charge-coupled device (CCD) cameras, an amplifier, and a personal computer (Gnathohexagraph system, version 1.31; OnoSocki Ltd., Kanagawa, Japan). The sampling frequency for mandibular incisor movements was 89.3 Hz. Accuracy tests for the equipment showed that the three-dimensional accuracy in the mean difference ± the standard deviation (SD) was 0.12 mm ± 0.06 mm [30]. The dental clutch was bent to ensure minimal inhibition of movements of the mandible and lips. For each subject, EMG activity was recorded from the left and right superficial masseter and anterior temporal muscles using a multichannel EMG device (Polygraph system 360; NEC, Tokyo, Japan) [20, 31]. The biosignal data were sampled using a measurement system for oral function (part of the Gnathohexagraph II system). The sampling frequency was 2.5 kHz. Before attaching the electrodes, the skin was lightly abraded by rubbing with a skin preparation gel (Skin Pure; Nihon Kohden, Tokyo, Japan) and cleaned with alcohol swabs to decrease the electrode-to-skin impedance, which was lower than 5 kΩ in all participants. Silver/silver chloride (Ag/AgCl) disposable electrodes (Blue Sensor; METS Co, Tokyo, Japan) with an interelectrode distance of 20 mm were then fixed parallel to the orientation of the fibers of each muscle. A disposable body earth electrode was secured behind the neck. The EMG signals were filtered by a 30–1000-Hz band-pass filter with 3 dB using a 60-Hz notch filter. Each participant was seated on a chair in an upright, but comfortable, position with the head in a natural orientation. During recording, the participant gazed at a red point on the CCD camera. Prior to performing the experimental task, the EMG activity was recorded with the mandible in the rest position for at least 30 s. A gummy jelly or a piece of chewing gum that was well softened by self-chewing (chewing test food by oneself) was placed on the tongue, after which the patients were asked to achieve maximum intercuspation. This maximum occlusion of opposing teeth was recorded as the centric occlusion (CO) for each measurement. Two chewing sessions were performed by each subject: (1) a training session in which the subject chewed freely two test foods under experimental conditions and perceived the properties (taste, smell, and texture property) of the two test foods and (2) deliberate unilateral chewing of chewing gum and gummy jelly, in the same order, on the preferred chewing side, with a 5-min interval between foods. The complete masticatory sequence was recorded until the final swallowing of the gummy jelly and during chewing gum mastication for 30 s. The second session was used for analysis of masticatory jaw movement and EMG activity. All preparation and experiment procedures were performed by one chief examiner and his assistants for all subjects.
Data analysis
For analysis of the mandibular incisor path and EMG data during deliberate unilateral mastication, 10 chewing cycles were selected using original software (University of Kagoshima, Kagoshima, Japan). After confirming that the intraindividual variation was significantly lower than the interindividual variation for the measurements of the masticatory jaw movements in each chewing cycle (maximum opening, minimum closing, and chewing cycle duration) via analysis of variance, we used the mean 10 chewing cycles as the representative value for each subject. If the intraindividual variation was larger, the subject was excluded from the study. The masticatory path and time-consistent EMG data were analyzed in the initial stage of gummy jelly mastication and in the later stage of chewing gum mastication (i.e., after self-chewing). A chewing cycle was excluded if at least one of the following features was observed: maximum opening < 5.0 mm, minimum closing > 4.0 mm, and chewing cycle duration < 300 ms [20]. For EMG data analysis synchronized with masticatory jaw movements, the mean chewing cycle of 10 chewing cycles was divided into the opening phase and the closing phase, based on the vertical displacement of the mandibular central incisor in the frontal view. The opening phase was the phase from the most superior position to the most inferior position in the chewing cycle. The closing phase was the phase from the most inferior position to the most superior position of the chewing cycle. The closing phase was further divided into three phases of equal durations: early closing phase, middle closing phase, and late closing phase [32]. A schematic of the chewing cycle for the chewing gum and gummy jelly in the frontal, sagittal, and horizontal views is shown in Fig. 2. The EMG data were full-wave rectified and subsequently averaged with a moving interval of 1 ms and a window time of 20 ms. The muscle activity measured (in microvolts) in the mandibular rest position and during mastication of the two test foods was normalized by using the muscle activity during 3 s of maximum voluntary clenching in CO. In all subjects, the normalized EMG activities (%EMG) of the superficial masseter and anterior temporal muscles on both sides were time integrated in the early, middle, and late closing phases of each of the 10 chewing cycles. The %EMG of all four muscles in the mandibular rest position was confirmed to be less than 10% (right side: superficial masseter, 2.85 ± 1.74, anterior temporal muscle, 4.54 ± 1.90; left side: superficial masseter, 3.77 ± 1.41, anterior temporal muscle, 4.11 ± 1.70). In addition, the asymmetry index (AI) was calculated for both muscles using the following formulae [33, 34]:
AI of the superficial masseter muscle = (superficial masseter muscle %EMG on the chewing side − superficial masseter muscle %EMG on the non-chewing side) / (superficial master muscle %EMG on the chewing side + superficial masseter muscle %EMG on the non-chewing side) × 100%
AI of the anterior temporal muscle = (anterior temporal muscle %EMG on the chewing side − anterior temporal muscle %EMG on the non-chewing side) / (anterior temporal muscle %EMG on the chewing side + anterior temporal muscle %EMG on the non-chewing side) × 100%
Statistical analysis
In a priori power analyses, we performed a sample size calculation [35] using data derived from a pilot study that involved eight subjects. Based on the parameters of interest (EMG activity in two muscles on both sides), the effect size f was estimated. Assuming a significance level of 0.05 and a power of 90%, with an effect size f of 0.68 for comparison of two test foods, the sample size calculation indicated that the required number of subjects is 25. For comparison of %EMG values for the superficial masseter and anterior temporal muscles during mandibular closure between gummy jelly and chewing gum mastication, an unpaired t test or the Mann–Whitney U test was used, according to data distribution. The differences in %EMG and AI values between gummy jelly and chewing gum mastication and those among the early, middle, and late closing phases were analyzed using two-way ANOVA and a post hoc Bonferroni multiple comparison of mean test. The observed significance level of the test, i.e., probability (P), was calculated for each comparison. A P value of < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 20 for Windows.
Results
Thirty healthy young adults (mean age, 21.5 years; range, 20–25 years; 15 men and 15 women) with Angle Class I occlusion were selected for this study.
Table 1 shows the mean %EMG values for the bilateral superficial masseter and anterior temporal muscles during gummy jelly and chewing gum mastication (Fig. 3). The %EMG of the superficial masseter muscle on the chewing and non-chewing sides was significantly higher during gummy jelly mastication than during chewing gum mastication. The %EMG values for the anterior temporal muscle showed a similar trend (Fig. 4).
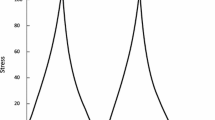
The mean normalized electromyographic activity (%EMG) of the superficial masseter muscle (a) and anterior temporal muscle (b) on the chewing and non-chewing sides during gummy jelly and chewing gum mastication. Measurements were obtained during the early, middle, and late phases of mandibular closure. ― : statistical significance among stage, [ : statistical significance between two test foods
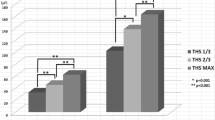
Asymmetry index (AI) of the superficial masseter muscle (a) and anterior temporal muscle (b) during gummy jelly and chewing gum mastication. Measurements were obtained during the early, middle, and late phases of mandibular closure. Π: statistical significance among stage, [ :statistical significance between two test foods
Figure 5 shows the mean %EMG values of the superficial masseter muscle in each closing phase on the chewing and non-chewing sides (Fig. 3a). There were significant differences between gummy jelly and chewing gum mastication and among the three closing phases. The %EMG values in the middle and late closing phases during gummy jelly mastication on both sides were significantly higher than those in the same phases during gum mastication on both sides. On the chewing side, the %EMG values of the superficial masseter muscle in the middle and late closing phases during gummy jelly mastication were higher than those in the early closing phase, whereas they were significantly higher in the middle phase than in the early phase during chewing gum mastication. On the non-chewing side during gummy jelly mastication, the %EMG values of the superficial masseter muscle showed a significant increase from the early phase to the middle phase, followed by a significant decrease from the middle phase to the late phase. With regard to chewing gum mastication, there were no significant differences among the three closing phases on the non-chewing side.
Figure 6 shows the mean %EMG values for the anterior temporal muscles in each closing phase on the chewing and non-chewing sides (Fig. 3b). There were significant differences between gummy jelly and chewing gum mastication and among the three closing phases. The %EMG values in the middle and late closing phases on both sides were significantly higher during gummy jelly mastication than during chewing gum mastication. On the chewing side, the %EMG values during gummy jelly or chewing gum mastication were significantly increased from the early closing phase to the middle closing phase, followed by a significant decrease from the middle closing phase to the late closing phase. On the non-chewing side during gummy jelly mastication, the %EMG values in the middle and late closing phases were higher than those in the early closing phase. During chewing gum mastication, the %EMG values increased from the early closing phase to the middle closing phase.
Figure 7 shows the mean AI values of the superficial masseter and anterior temporal muscles in each closing stage during gummy jelly and chewing gum mastication (Fig. 4). There were significant differences between gummy jelly and chewing gum mastication and among the three closing phases. The AI of the superficial masseter muscle was significantly lower during gummy jelly mastication than during chewing gum mastication in the middle phase (Fig. 4a). However, the AI of the anterior temporal muscle was significantly higher during gummy jelly mastication than during chewing gum mastication in the middle phase (Fig. 4b). The AI of the superficial masseter muscle significantly increased from the early closing phase to the middle and late closing phases during gummy jelly mastication, and it significantly increased from the early closing phase to the late closing phase during chewing gum mastication. However, the AI of the anterior temporal muscle was significantly lower in the late closing phase than in the early and middle closing phases during gummy jelly mastication, with no significant differences among the three phases during chewing gum mastication.
Discussion
For the estimation of EMG activity of the jaw-closing muscles during unilateral mastication, it is important that any test food selected has a homogeneous structure, a reproducible texture, a constant shape and size, and the ability to exhibit a complete texture value [36]. In the present study, we used two artificial test foods that are routinely used for the mixing ability test (i.e., chewing gum) and the comminution test (i.e., gummy jelly); both foods satisfied the aforementioned criteria. It has been shown that muscle activity adapts to changes in the rheological properties of food throughout the masticatory sequence [16, 37]. For gummy jelly mastication, we selected 10 chewing cycles at the initial stage without a first cycle to investigate the processing stage of the breakdown of a food bolus. With regard to chewing gum mastication, significant time-dependent changes in the food texture occur during the initial stage because chewing gum texture is a thin, flat form, has low cohesiveness, and is softened by the intraoral temperature, which affect the masticatory path and muscle activity. Therefore, we selected 10 chewing cycles after the chewing gum was softened by self-chewing, which resulted in negligible changes in rheological properties. To estimate the aspect of jaw-closing muscle activity during mixing and commute mastication, our selection of 10 chewing cycles during the mastication of each test food minimized the influence of time-dependent changes in muscle activity during masticatory presses; moreover, it was relatively easy to select 10 stable chewing cycles for each subject.
In the present study, the EMG activity of the superficial masseter and anterior temporal muscles on the chewing and non-chewing sides showed that the muscle activity in the middle and late closing phases was significantly greater during gummy jelly mastication than during chewing gum mastication, which is interpreted to require more forceful mastication for gummy jelly than for chewing gum. It is well known that the muscle activation during the mastication of different textures increases with increasing hardness [4, 6, 14]. It has also been demonstrated that EMG activity of the masseter and temporal muscles during unilateral mastication on the functional side and on the non-functional side is modified by the food texture, which indicates that mastication of tougher foods increases the activity of the superficial masseter and anterior temporal muscles on the non-functional side [7, 14]. Our texture analysis of the two test foods showed that on the second compression, the hardness of gummy jelly was higher than that of chewing gum, although on the first compression, their hardness values were comparable. These results indicated that the hardness of gummy jelly remains constant because of the higher cohesiveness during the masticatory sequence, whereas the hardness of chewing gum does not remain constant. Furthermore, the gummy jelly volume was higher than that of chewing gum. In another study, the muscle activity during mastication of a large gummy jelly was higher than that of a small gummy jelly [13]. It is considered that compared to chewing gum, gummy jelly has a constant hardness value because of its higher cohesiveness and larger size, and thus, greater muscle activity is required to cut and grind the food bolus.
Our results showed that EMG activity of the superficial masseter and anterior temporal muscles on both sides increased from the early closing phase to the middle and late closing phases during gummy jelly mastication. However, increased muscle activity in the late closing phase was not observed during chewing gum mastication, although an increase in muscle activity on the chewing side in the middle closing phase was confirmed. These results suggested that gummy jelly mastication required induction of greater bite force at the final closure of the mandible. During the masticatory process of gummy jelly, large food particles need to be broken into smaller particles with the premolar and/or molar teeth. In this process, neuromuscular control of the masticatory closure muscles requires precise holding of the food between the upper and lower molars and subsequent forceful biting of the food bolus because the high cohesiveness of gummy jelly resists breakage of the gummy jelly into small particles. By contrast, color-changeable gum after self-chewing is easily formed by each cycle and is easy to hold and manipulate between the upper and lower teeth because of its low cohesiveness and high adhesiveness. Therefore, these texture properties of chewing gum do not require a forceful bite at the final closure of the mandible, which makes chewing gum, as a test food, suitable for measuring masticatory performance among children with mixed dentition [28] or adults with compromised dentition [38, 39]. A broader occlusal contact area is suitable for achieving a stable hold and forceful biting of a food bolus at final closure of the mandible. The occlusal condition may be more strongly associated with the efficiency of gummy jelly mastication, compared to that of chewing gum mastication, as suggested by a previous report [38, 39].
Other test foods (such as silicone tablets (Optosil) [28] or natural foods (peanuts) [40, 41]) were also used to estimate the commuting ability, which is called as crushing capacity [41]. These breakable test foods have different rheological properties compared to the two test foods used in this study. It has been reported that the objective hardness values for peanut are almost constant at various percentages of compression [40], whereas the objective hardness for gummy jelly increases with an increase in the percentage of compression. Additionally, it has been suggested that the crushing capacity estimated by using brittle test foods correlates with tongue and lip function, whereas the shearing capacity estimated by using elastic test foods does not [41]. Furthermore, the difference in rheological properties, i.e., elasticity and plasticity, affect the masticatory movement of the mandible [4]. The different neuromuscular pattern is assumed to exist to chew these breakable test foods, estimating the crushing capacity, compared to gummy jelly and chewing gum.
Related studies recently reported that relative jaw muscle activation is associated with the interocclusal distance during unilateral biting and chewing [8, 9, 42]. With decreasing jaw gape, the working and balancing ratio of the masseter muscles become asymmetrical [9]. In line with these previous reports, the superficial masseter AI increased significantly from the early closing phase to the late closing phase, regardless of the food texture. This increasing superficial masseter AI occurs because of constant high muscle activity on the chewing side and decreasing muscle activity on the non-chewing side from the middle to the late closing phases. In contrast to the pattern of the superficial masseter AI, the anterior temporal AI of gummy jelly mastication was significantly symmetrical between the chewing and non-chewing sides from the early closing to the late closing phases. The decreasing anterior temporal AI is derived from the constant high muscle activity on the non-chewing side and the decreasing muscle activity on the chewing side from the middle to the late closing phase. These contrasting patterns of the superficial masseter and anterior temporal AIs were more clearly observed during gummy jelly mastication than during chewing gum mastication. The direction of the bite force is a factor that affects the pattern of relative temporal muscle activation [43]. It has been suggested that jaw closure from the anterior direction in the sagittal view resulted in increased activity of the elevator muscles, whereas that from the posterior direction resulted in decreased elevator muscle activity during unilateral chewing [44]. These previous studies demonstrated that the direction of mandibular incisor closure affected the activity of the elevator muscles during unilateral chewing. In our study, during gummy jelly mastication, the mandibular incisor was positioned more laterally on the chewing side and the direction of the bite force was more medial throughout the three closing phases, compared to the masticatory path during chewing gum mastication. A more lateral and wide masticatory pathway may contribute to the relative activation of the anterior temporal muscles on both sides. Therefore, our finding of relative activation of the jaw-closing muscles on both sides suggested that, as the mandible approached CO during each unilateral chewing cycle, the functional/non-functional side ratio of the anterior temporal muscle became symmetrical and that of the superficial masseter muscle became asymmetrical. This neuromuscular pattern may reflect the functional aspects of jaw-closing muscles. That is, the temporal muscle controls mandibular movements during mastication and is considered a positioning muscle, whereas the masseter muscle contributes to forceful biting during the power phase on the functional side and is considered a force-generating muscle.
Considering that electromyographic activity of the superficial masseter and anterior temporal muscles on the chewing and non-chewing sides was significantly different in the comparison between gummy jelly mastication and chewing gum mastication, our null hypothesis was rejected.
There are limitations with respect to EMG analysis of the masticatory muscles. The amplitude of an electromyogram is affected by several factors other than the intensity of muscle activity [1]. The important biological factors, including the skin thickness [45], skeletal morphology [46], malocclusion [19, 2047], occlusal contact on the balancing side [48], and TMDs [49], may have increased the variability of data across subjects. In addition, the use of isometric biting data for dynamic biting may cause overestimation when the chewing force is predicted from masticatory data [50]. These factors should be considered for all attempts in estimating muscle activity using EMG data obtained from jaw-closing muscles.
Conclusions
Gummy jelly mastication requiring high muscle activity of the bilateral superficial masseter and anterior temporal muscles may be advantageous; in that, they allow for estimation of the dynamic neuromuscular control of the jaw-closing muscles, as a functional aspect of the masticatory system. However, the different human neuromuscular strategies of chewing for each artificial test food indicated that analysis using multiple test foods may be useful for obtaining diagnostic information for patient assessments and can provide a reference for the evaluation of disharmony or dysfunction in the masticatory system during clinical examinations.
References
Castroflorio T, Bracco P, Farina D (2008) Surface electromyography in the assessment of jaw elevator muscles. J Oral Rehabil 35:638–645. https://doi.org/10.1111/j.1365-2842.2008.01864.x
Agrawal KR, Lucas PW, Bruce IC, Prinz JF (1998) Food properties that influence neuromuscular activity during human mastication. J Dent Res 77:1931–1938
Peyron MA, Maskawi K, Woda A, Tanguay R, Lund JP (1997) Effect of food texture and sample thickness on mandibular movement and hardness assessment during biting in man. J Dent Res 76:789–795
Foster KD, Woda A, Peyron MA (2006) Effect of texture of plastic and elastic model foods on the parameters of mastication. J Neurophysiol 95:3469–3479
Miyawaki S, Ohkochi N, Kawakami T, Sugimura M (2000) Effect of food size on the movement of the mandibular first molars and condyles during deliberate unilateral mastication in humans. J Dent Res 79:1525–1531
Peyron MA, Lassauzay C, Woda A (2002) Effects of increased hardness on jaw movement and muscle activity during chewing of visco-elastic model foods. Exp Brain Res 142:41–51
Mioche L, Bourdiol P, Martin JF, Noël Y (1999) Variations in human masseter and temporal muscle activity related to food texture during free and side-imposed mastication. Arch Oral Biol 44:1005–1012
Pröschel PA, Jamal T, Morneburg JR (2008) Motor control of jaw muscles in chewing and in isometric biting with graded narrowing of jaw gape. J Oral Rehabil 35:722–728. https://doi.org/10.1111/j.1365-2842.2008.01871.x
Pröschel PA, Morneburg TR (2010) Indications for jaw gape-related control of relative muscle activation in sequent chewing strokes. J Oral Rehabil 37:178–184. https://doi.org/10.1111/j.1365-2842.2009.02036.x
van der Bilt A (2011) Assessment of mastication with implications for oral rehabilitation: a review. J Oral Rehabil 38:754–780. https://doi.org/10.1111/j.1365-2842.2010.02197.x
Anderson K, Throckmorton GS, Buschang PH, Hayasaki H (2002) The effects of bolus hardness on masticatory kinematics. J Oral Rehabil 29:689–696. https://doi.org/10.1046/j.1365-2842.2002.00862.x
Bishop B, Plesh O, McCall WDJ (1990) Effects of chewing frequency and bolus hardness on human incisor trajectory and masseter muscle activity. Arch Oral Biol 35:311–318. https://doi.org/10.1016/0003-9969(90)90048-F
Miyawaki S, Ohkochi N, Kawakami T, Sugimura M (2001) Changes in masticatory muscle activity according to food size in experimental human mastication. J Oral Rehabil 28:778–784
Piancino MG, Bracco P, Vallelonga T, Merlo A, Farina D (2008) Effect of bolus hardness on the chewing pattern and activation of masticatory muscles in subjects with normal dental occlusion. J Electromyogr Kinesiol 18:931–937. https://doi.org/10.1016/j.jelekin.2007.05.006
Takada K, Miyawaki S, Tatsuta M (1994) The effects of food consistency on jaw movement and posterior temporalis and inferior orbicularis oris muscle activities during chewing in children. Arch Oral Biol 39:793–805. https://doi.org/10.1016/0003-9969(94)90009-4
Kitashima F, Tomonari H, Kuninori T, Uehara S, Miyawaki S (2015) Modulation of the masticatory path at the mandibular first molar throughout the masticatory sequence of a hard gummy jelly in normal occlusion. Cranio 33:263–270. https://doi.org/10.1080/08869634.2015.1097275
Miyawaki S, Tanimoto Y, Inoue M, Sugawara Y, Fujiki T, Takano-Yamamoto T (2001) Condylar motion in patients with reduced anterior disc displacement. J Dent Res 80:1430–1435
Miyawaki S, Tanimoto Y, Kawakami T, Sugimura M, Takano-Yamamoto T (2001) Motion of the human mandibular condyle during mastication. J Dent Res 80:437–442
Tomonari H, Ikemori T, Kubota T, Uehara S, Miyawaki S (2014) First molar cross-bite is more closely associated with a reverse chewing cycle than anterior or pre-molar cross-bite during mastication. J Oral Rehabil 41:890–896. https://doi.org/10.1111/joor.12222
Tomonari H, Kubota T, Yagi T, Kuninori T, Kitashima F, Uehara S, Miyawaki S (2014) Posterior scissors-bite: masticatory jaw movement and muscle activity. J Oral Rehabil 41:257–265. https://doi.org/10.1111/joor.12148
Hama Y, Kanazawa M, Minakuchi S, Uchida T, Sasaki Y (2014) Properties of a color-changeable chewing gum used to evaluate masticatory performance. J Prosthodont Res 58:102–106. https://doi.org/10.1016/j.jpor.2013.12.005
Komagamine Y, Kanazawa M, Minakuchi S, Uchida T, Sasaki Y (2011) Association between masticatory performance using a colour-changeable chewing gum and jaw movement. J Oral Rehabil 38:555–563. https://doi.org/10.1111/j.1365-2842.2011.02204.x
Tarkowska A, Katzer L, Ahlers MO (2017) Assessment of masticatory performance by means of a color-changeable chewing gum. J Prosthodont Res 61:9–19. https://doi.org/10.1016/j.jpor.2016.04.004
Ikebe K, Matsuda K, Kagawa R, Enoki K, Okada T, Yoshida M, Maeda Y (2012) Masticatory performance in older subjects with varying degrees of tooth loss. J Dent 40:71–76. https://doi.org/10.1016/j.jdent.2011.10.007
Kurushima Y, Ikebe K, Matsuda K, Enoki K, Ogata S, Yamashita M, Murakami S, Maeda Y, Osaka Twin Research Group (2015) Examination of the relationship between oral health and arterial sclerosis without genetic confounding through the study of older Japanese twins. PLoS One 10:e0127642. https://doi.org/10.1371/journal.pone.0127642
Kuninori T, Tomonari H, Uehara S, Kitashima F, Yagi T, Miyawaki S (2014) Influence of maximum bite force on jaw movement during gummy jelly mastication. J Oral Rehabil 41:338–345. https://doi.org/10.1111/joor.12149
Okiyama S, Ikebe K, Nokubi T (2003) Association between masticatory performance and maximal occlusal force in young men. J Oral Rehabil 30:278–282
Kaya MS, Guclu B, Schimmel M, Akyuz S (2017) Two-color chewing gum mixing ability test for evaluating masticatory performance in children with mixed dentition: validity and reliability study. J Oral Rehabil 44:827–834. https://doi.org/10.1111/joor.12548
Dworkin SF, LeResche L (1992) Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 6:301–355
Tokiwa H (2001) Evaluation of the clinical accuracy of an optical recording system for mandibular movement. J Jpn Soc Stomatognath Funct 7:13–25. https://doi.org/10.7144/sgf.7.13
Ohmure H, Miyawaki S, Nagata J, Ikeda K, Yamasaki K, Al-Kalaly A (2008) Influence of forward head posture on condylar position. J Oral Rehabil 35:795–800. https://doi.org/10.1111/j.1365-2842.2007.01834.x
Tomonari H, Kwon S, Kuninori T, Miyawaki S (2017) Differences between the chewing and non-chewing sides of the mandibular first molars and condyles in the closing phase during chewing in normal subjects. Arch Oral Biol 81:198–205. https://doi.org/10.1016/j.archoralbio.2017.05.006
Naeije M, McCarroll RS, Weijs WA (1989) Electromyographic activity of the human masticatory muscles during submaximal clenching in the inter-cuspal position. J Oral Rehabil 16:63–70
Kimoto K, Fushima K, Tamaki K, Toyoda M, Sato S, Uchimura N (2000) Asymmetry of masticatory muscle activity during the closing phase of mastication. Cranio 18:257–263
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Woda A, Foster K, Mishellany A, Peyron MA (2006) Adaptation of healthy mastication to factors pertaining to the individual or to the food. Physiol Behav 89:28–35. https://doi.org/10.1016/j.physbeh.2006.02.013
Iguchi H, Magara J, Nakamura Y, Tsujimura T, Ito K, Inoue M (2015) Changes in jaw muscle activity and the physical properties of foods with different textures during chewing behaviors. Physiol Behav 152(Pt A):217–224. https://doi.org/10.1016/j.physbeh.2015.10.004
Speksnijder CM1, Abbink JH, van der Glas HW, Janssen NG, van der Bilt A (2009) Mixing ability test compared with a comminution test in persons with normal and compromised masticatory performance. Eur J Oral Sci 117(5):580–586. https://doi.org/10.1111/j.1600-0722.2009.00675.x
van der Bilt A, Mojet J, Tekamp FA, Abbink JH (2010) Comparing masticatory performance and mixing ability. J Oral Rehabil 37:79–84. https://doi.org/10.1111/j.1365-2842.2009.02040.x
Sugiura T, Fueki K, Igarashi Y (2009) Comparisons between a mixing ability test and masticatory performance tests using a brittle or an elastic test food. J Oral Rehabil 36(3):159–167. https://doi.org/10.1111/j.1365-2842.2008.01917.x
Yamada A, Kanazawa M, Komagamine Y, Minakuchi S (2015) Association between tongue and lip functions and masticatory performance in young dentate adults. J Oral Rehabil 42(11):833–839. https://doi.org/10.1111/joor.12319
Morneburg TR, Döhla S, Wichmann M, Pröschel PA (2014) Afferent sensory mechanisms involved in jaw gape-related muscle activation in unilateral biting. Clin Oral Investig 18:883–890. https://doi.org/10.1007/s00784-013-1024-1
Mao J, Osborn JW (1994) Direction of a bite force determines the pattern of activity in jaw-closing muscles. J Dent Res 73:1112–1120
Kimoto K, Tamaki K, Yoshino T, Toyoda M, Celar AG (2002) Correlation between elevator muscle activity and direction of sagittal closing pathway during unilateral chewing. J Oral Rehabil 29:430–434
van der Glas HW, Lobbezoo F, van der Bilt A, Bosman F (1996) Influence of the thickness of soft tissues overlying human masseter and temporal muscles on the electromyographic maximal voluntary contraction level. Eur J Oral Sci 104:87–95
Saifuddin M, Miyamoto K, Ueda HM, Shikata N, Tanne K (2003) An electromyographic evaluation of the bilateral symmetry and nature of masticatory muscle activity in jaw deformity patients during normal daily activities. J Oral Rehabil 30:578–586
Ferrario VF, Sforza C, Serrao G (1999) The influence of crossbite on the coordinated electromyographic activity of human masticatory muscles during mastication. J Oral Rehabil 26:575–581
Schubert D, Pröschel P, Schwarz C, Wichmann M, Morneburg T (2012) Neuromuscular control of balancing side contacts in unilateral biting and chewing. Clin Oral Investig 16:421–428. https://doi.org/10.1007/s00784-011-0542-y
Suvinen TI, Kemppainen P (2007) Review of clinical EMG studies related to muscle and occlusal factors in healthy and TMD subjects. J Oral Rehabil 34:631–644
Proeschel PA, Morneburg T (2002) Task-dependence of activity/bite-force relations and its impact on estimation of chewing force from EMG. J Dent Res 81:464–468
Acknowledgments
The authors would like to thank Specially Appointed Professor T. Nokubi from the Development Center for Evaluating Masticatory Function at Osaka University (Suita, Japan) for his technical suggestions on the use of gummy jelly. We also thank UHA Mikakuto Co., Ltd. (Osaka, Japan) for providing the gummy jelly.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI grant program (26463099, 17K11945, and 15H05051).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tomonari, H., Seong, C., Kwon, S. et al. Electromyographic activity of superficial masseter and anterior temporal muscles during unilateral mastication of artificial test foods with different textures in healthy subjects. Clin Oral Invest 23, 3445–3455 (2019). https://doi.org/10.1007/s00784-018-2754-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2754-x








 : statistical significance between two test foods
: statistical significance between two test foods
 : statistical significance between two test foods
: statistical significance between two test foods
 : statistical significance between two test foods
: statistical significance between two test foods