Abstract
Objectives
This study aims to investigate the additional influence of multiple applications of antimicrobial photodynamic therapy (aPDT) in smokers with chronic periodontitis.
Materials and methods
Twenty smokers with chronic periodontitis were treated in a split-mouth design study with aPDT adjunct to Scaling and Root Planing (SRP) or SRP. aPDT was performed by using a laser light source with 660 nm wavelength associated with a photosensitizer. The applications were performed in four episodes (at days 0, 2, 7, and 14). All patients were monitored for 90 days. Plaque index, probing depth, clinical attachment level, and bleeding on probing were performed at baseline, 30, and 90 days after the SRP. Gingival crevicular fluid and subgingival plaque samples were collected for immunological and microbiological analysis, respectively. Data obtained were statistically analyzed.
Results
aPDT as an adjunct to SRP did not demonstrate statistically significant advantages on clinical parameters when compared with SRP alone. No statistic significant differences between groups were observed (p < 0.05). Levels of anti-inflammatory cytokines and bacterial species were comparable in both groups at day 90 after treatment.
Conclusion
Periodontal treatment with SRP + aPDT in multiples episodes was not able to promote additional clinical, immunological, and microbiological benefits in smokers when compared SRP alone in patients with chronic periodontitis.
Clinical relevance
Multiple episodes of aPDT adjunctive to non-surgical treatment did not improve significantly the clinical, immunological, and microbiological parameters when compared with SRP alone. More randomized clinical trials are needed to evaluate adjuvant therapies for scaling and root planning in smokers with chronic periodontitis. ClinicalTrials.gov Identifier: NCT03039244
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high frequency and severity of periodontal disease are related to risk factors such as smoking, which has presented substantial evidence of its detrimental effect on periodontal health [1, 2]. Smokers are more prone to periodontal pocket depth, attachment loss, bone loss, and high numbers of pathogenic bacteria [3, 4]. The clinical and microbiological conditions of smokers are related to deficiency in the host inflammatory response caused by changes in the immune system including vascular function, neutrophil activities, and release of inflammatory mediators [4].
Clinical trials agree that is harder to affect subgingival pathogens in smokers by mechanical periodontal therapy and to keep them under control in these individuals, in comparison with non-smokers [5, 6]. Scaling and root planning (SRP) are the most commonly used periodontal treatment. However, this procedure does not frequently lead to the microbiological changes to maintain the long term stability of the clinical benefits achieved initially [7].
To maximize the effects of SRP, mechanical therapy protocols associated with systemic antibiotics have been used in the periodontal therapy [8, 9], with clinical and biological benefits in smokers [10]. Moreover, antibiotic therapy may be accompanied by side effects such as gastrointestinal disorders and the development of bacterial resistance [11].
In the context, to develop alternative approaches to manage patients with periodontitis, antimicrobial photodynamic therapy (aPDT) has been investigated as a therapeutic alternative [12,13,14]. Specifically in smokers, there are not sufficient studies to affirm the potential benefits of aPDT an adjuvant to SRP.
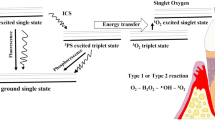
The aPDT is based on the principle that a photosensitizer binds to the target cell and is activated by suitable wavelength light [12]. The light action forms free radicals of singlet oxygen, producing a toxic effect and resulting in cell death [12, 15].Unlike antibiotics, bacterial resistance in individuals who undergo this therapy would be improbable [12].
Single application of aPDT protocols has shown positive results [16, 17]. However, in other studies, additional benefit to SRP was not statistically observed when evaluating clinical parameters and cytokine levels in gingival crevicular fluid (GCF) [12, 18, 19]. It is speculated that the effect of a single aPDT application may be insufficient to prevent recolonization of treated sites [13, 20]. Because of these facts, the use of repeated episodes of aPDT has been suggested [20, 21].
Since the Smoking exerts a negative influence on the outcome of periodontal treatment, the aim of this study is to evaluate the use of multiple episodes of aPDT as adjuvant to non-surgical treatment in smokers with chronic periodontitis. The authors hypothesized that repeated aPDT adjunctive SRP would reduce periodontal pathogens, improving the host immune-inflammatory response and clinical periodontal conditions.
Materials and methods
Patient selection
Twenty-two patients (6 males and 16 females, aged 39 to 61 years; mean age 48 ± 6.4) were enrolled from the periodontal clinic of the School of Dentistry of Ribeirao Preto, University of Sao Paulo, but only 20 were analyzed. Two patients were excluded because they used antibiotics for other health reasons. The study protocol was reviewed and approved by the Research Ethics Committee of the same institution (Protocol 36933014.4.0000.5419) and registered at ClinicalTrials.gov (NCT03039244).
Sample size calculation
The sample size was determined to provide 80% power to recognize a significant difference of 1 mm (δ) between groups with a 95% confidence interval (α = 0.05) and standard deviation (σ) of 1.0 mm [22] considering the changes in mean clinical attachment level (CAL) as the primary outcome variable and [Zα (1.96) + Zβ (0.84)]2 = 7.84. Size calculation was based on the following formula: n = {2[(σ)2/(δ)2]} × (Zα + Zβ)2. Hence, a total of 16 patients were required. However, the number of patients enrolled in this study was 22, considering the possibility of dropouts.
Inclusion criteria
The inclusion criteria were as follows: (1) diagnosis of generalized chronic periodontitis [23], (2) interproximal periodontal pockets ≥ 5 mm in contralateral teeth, (3) smoking ≥ 10 cigarettes per day during at least 5 years, and (4) good general health.
Exclusion criteria
The exclusion criteria were as follows: (1) positive history of antibiotic therapy in the last 6 months, (2) positive history of non-surgical periodontal treatment in the last 6 months, (3) systemic involvement that might interfere in disease progression or treatment response (e.g., diabetes, immune disorders), (4) need for antibiotic prophylaxis for performing routine dental procedures, (5) long-term administration of anti-inflammatory drugs, and (6) pregnant. All patients received detailed information about experiment and signed with a consent form.
Experimental design, randomization, and allocation
This study was a split-mouth, double-masked, randomized, controlled clinical trial. In each patient, the upper quadrants were allocated randomly to one of two treatment protocols: SRP + aPDT or SRP alone. An investigator (MRM) with no clinical involvement in the trial generated the allocation sequence using a permuted block design with a computer random-number generator. The allocation sequence was concealed in opaque, sealed envelopes, and the details of the series were unknown to the investigators and patient of the study (examiners, coordinator, outcome assessors, and biostatistician). An investigator (MMI) not involved in data collection and treatment performed the enrollment of the patients and the assignment of sealed envelopes containing the treatment modalities for each quadrant. For each patient, the envelope was opened immediately before the use of the application of photodynamic therapy.
Treatment protocol
Contralateral pairs of upper teeth in the maxillary quadrants with proximal sites presenting probing depth (PD) and CAL ≥ 5 mm were selected for the clinical, microbiologic, and immunologic evaluations. Seven days before the interventions, the selected individuals received specific oral hygiene instructions and supragingival scaling on all teeth (Baseline). One week after the beginning of the study, select individuals were recalled to undergo the next phase of the treatment (day 0). Each patient was subjected to two parallel treatments: The teeth of the test side received SRP + aPDT, whereas the control side received SRP alone. SRP was performed using hand instruments (Hu-Friedy, Chicago, IL, USA) and an ultrasonic device. No systemic antibiotic was prescribed. The subgingival scaling was performed by one trained periodontist (MSMS) who was not informed about the treatment allocation. In the test group, immediately after the SRP procedure, the photosensitizerFootnote 1 was applied. After 1 min (min), the pockets were rinsed. The diode soft-laserFootnote 2 light with a wavelength of 660 nm and a power of 70 mW was used subgingivally. The irradiation was performed using an optical fiber probe of 0.6 mm in diameter.Footnote 3 Light was applied in six sites of each tooth. Each site was irradiated for 10 s (power density of 28 mW/cm2), delivering a total energy of 2.79 J/cm2 per site (16.72 J/cm2 per tooth). aPDT was repeated after 2, 7, and 14 days. A simulation of aPDT (sham procedure) was performed simultaneously in selected contralateral teeth (control group). Individuals were not informed about the type of treatment performed on each tooth. Patients received professional prophylaxis every 15 days for 3 months after non-surgical periodontal treatment. All patients were monitored for 90 days.
Examiner calibration
All clinical parameters were measured by a calibrated examiner (CAB). At two different sessions within 48 h, duplicate measurements of PD and CAL were obtained from 10 patients who were not related to this study. Calibration was accepted if the percentage agreement between measurements was ≥ 90%.
Clinical measurements
At baseline, 0, 30, and 90 days after SRP, the clinical parameters were recorded. A computerized periodontal probeFootnote 4 was used to perform the periodontal measurements in six sites per tooth. Plaque index (PI) [24] was used to assess the oral hygiene status of the individuals. Bleeding on probing (BOP) and Marginal Bleeding (MB) [25] were recorded based on the presence or absence of bleeding up to 30 s after probing at the experimental sites. PI, BOP, and MB were scored as plaque and bleeding being absent or present. PD was measured from the free gingival margin to the bottom of the periodontal pocket. CAL was measured as distance from the cementoenamel junction to the bottom of the pocket. BOP, PD, MG, and CAL were measured at six sites per tooth.
Micobiological monitoring
Subgingival plaque samples from interproximal sites were collected at baseline, 0, 30, and 90 days after SRP. Plaque samples were collected from contralateral sites of proximal tooth surface with PD ≥ 5 mm. The samples were collected from the same sites initially selected and grouped in test and control. Briefly, supragingival plaque was removed, and subgingival samples were collected with individual sterile Gracey curettes and immediately placed in separate Eppendorf tubes containing 0.15 ml of buffer solution (10 mM Tris-HCl, 1 mM EDTA, pH 7.6). One hundred microliters of 0.5 M NaOH were added to each tube, and the samples were dispersed using a vortex mixer. Counts of 40 bacterial species were performed in each sample, using the checkerboard DNA–DNA hybridization technique [10]. A total of 190 samples were analyzed.
Immunologic monitoring
GCF was collected from contralateral sites of proximal tooth surface with PD ≥ 5 mm using filter paper stripsFootnote 5 at days 0, 14, 30, and 90 after SRP as described previously by Moreira et al. [21]. The samples were collected from the same sites initially selected and grouped in test and control considering subsets of sites according to initial PD of 5 to 6 mm (moderate periodontal pockets) or ≥ 7 mm (deep periodontal pockets). The amount of total protein of each sample was determined by conventional enzyme-linked immunosorbent assays, using commercially available kits. The cytokines IL-1b, IL-10, and TNF-α were determined using high-sensitivity kitsFootnote 6 and the multiplexing instrumentFootnote 7 according to the recommendations of the manufacturer. The samples were evaluated individually, and the concentrations of each cytokine were estimated from the standard curve using a five-parameter polynomial equation with specific software. The mean concentration of each marker was calculated and expressed as picograms per milliliter, and subsequently, the ratio of cytokine concentration to the amount of total protein was performed as picograms per milliliter. The cytokine levels in each sample were calculated from a standard curve and normalized by total protein [26]. Cotinine levels were performed by immunoenzymatic analysis using a kit for the quantitative analysis of cotinine in salivaFootnote 8 according to the manufacturer’s instructions.
Outcome variables
The mean CAL at 90 days after SRP was defined as the primary outcome variable of the study. Secondary outcome variables were differences between group in the following parameters: (1) PD, (2) PI, (3) BOP, (4) MB, (5) counts and proportions of the 40 bacterial species analyzed, (6) mean levels of each cytokine analyzed, (7) number of residual periodontal pockets at 90 days after SRP, and (8) future necessity of surgery.
Statistical analyses
Changes in PD, CAL, and levels of cytokines were examined in subsets of sites according to initial PD of 5 to 6 mm (moderate pockets) or ≥ 7 mm (deep pockets). Normality of distribution of the variables was tested with Shapiro-Wilk tests. The significance level was set at 5%. The significance of differences between groups for PD and CAL was determined using a two-way ANOVA test with Bonferroni post-test. Microbiologic analysis was presented as mean counts of individual bacterial species in both groups. Bacterial species were also grouped into complexes, according to Socransky et al. [27]. A Wilcoxon test was used to detect significant differences between groups for mean counts of bacterial species and cytokines levels. Analyses were performed after adjustments for multiple comparisons [28]. Kruskal-Wallis test was used to detect significant differences within each group. A Fisher’s exact test and Friedman test were used to evaluate differences in BOP, MG, PI, and residual periodontal pockets between groups and intragroup, respectively.
Results
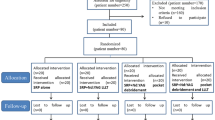
The study was conducted between August 2014 and July 2017. Three hundred thirty-two individuals were assessed for eligibility. Two individuals were lost to follow-up in the study because they used antibiotics. Figure 1 presents the flow chart of the study design. Twenty individuals presented the following demographic characteristics: (1) mean ± SD age, 47.8 ± 5.6 years; (2) 6 males and 14 females (70%); (3) mean and standard deviation of number of cigarettes per day, 19 ± 5.7; and (4) mean and standard deviation of salivary cotinine level was 431.9 ± 180.8 ng/ml. No adverse effects were observed with the use of aPDT.
Clinical monitoring
Means and standard deviations of PD and CAL are presented in Table 1. Absolute and relative frequencies of BOP, MB, and PI are presented in Table 2. In general, no significant differences were observed between groups in relation to the clinical parameters evaluated (p > 0.05). All therapies led to a decrease in mean values of PD, CAL, PI, and BOP (Tables 1 and 2). When analyzing moderate pockets, both groups presented significant reduction in PD (p < 0.05). The CAL was significantly different in the test group (p < 0.05) when compared with baseline (Table 1). For deep pockets, the PD reduced significantly in the test group (p < 0.05) and CAL reduced significantly in both groups (p < 0.05).
Residual periodontal pockets and number of patient identified with periodontal access surgery needs at day 90 (%) are presented in Table 3. No significant differences were observed in the frequency of residual periodontal pockets (p > 0.05).
Microbiologic monitoring
All groups presented high counts of periodontal pathogens of the orange and red complexes at baseline. Fusobacterium periodonticum, Porphyromonas gingivalis, and Tannerella forsythia reduced significantly in both groups when compared with baseline at day 30 (p < 0.05). Figure 2 shows changes in the proportions of microbial complexes at baseline, 0, 30, and 90 days after SRP. Microbial profiles were changed, but no statistical differences between groups at day 30 and 90 (p > 0.05) were observed. Significant differences were observed between groups in the mean counts of species at baseline for orange and green complexes (p > 0.05). The control group in red complex showed a significant reduction when compared with baseline at day 30 (p < 0.05).
Pie charts of the mean proportions of each microbial complex at baseline, + 0, + 30, and + 90 days in test and control groups. The colors represent different microbial complexes (blue: Actinomyces; yellow: Streptococcus; green: E. corrodens, C. gingivalis, C. sputigena, C. ochracea; light green: A. actinomycetemcomitans; purple: V. parvula, A. odontolyticus; orange: S. constellatus, C. gracillis, C. rectus, C. showae, E. nodatum, P. intermedia, P. nigrescens, P. micros, F. nuc. vincentii, F. nuc. nucleatum, F. nuc. polymorphum, F. periodonticum; red: P. gingivalis, T. forsythia, T. denticola; gray: E. saburreum, G. morbillorum, L. buccalis, P. acnes, P. melaninogenica, N. mucosa, S. anginosus, S. noxia, and T. socranskii) [27]. *Significant difference when compared to baseline, Kruskal-Wallis test. †Significant difference between groups in the same period of analysis, Wilcoxon test (p < 0.05)
Immunologic monitoring
GCF levels of IL-1b, IL-10, and TNF-α cytokines are shown in Fig. 3 considering moderate and deep periodontal pockets. Both groups showed comparable levels of available cytokines at day 0. No significant differences between groups were observed in mean levels of cytokines in deep pockets at day 90 after periodontal treatment (p > 0.05). In moderate pockets, IL-10 levels were higher with a statistically significant difference when compared to the control group at day 30. Statistical differences intra-group was observed in IL-1b levels in moderate pockets at day 30 (p < 0.05).
Mean levels (picograms per milligram) and SDs of IL-1b, IL-10, and TNF-a in the control and test groups at 0, 14, 30, and 90 days after SRP when considering moderate (A, C, and E) and deep (B, D, and F) periodontal pockets. *P < 0.05 when compared to baseline, Kruskal-Wallis test. †Statistical difference between groups in the same period of analysis was observed, Wilcoxon test
Discussion
The present randomized controlled trial was designed to evaluate the effectiveness of adjunctive aPDT with multiple episodes for treating chronic periodontitis in smokers. Similar randomized controlled trials on aPDT in smokers have been published [16, 29], but with a single episode. This study is the first clinical trial with aPDT using multiple episodes to treat chronic periodontitis in smokers.
The rationale to use a split-mouth study design was the decreases in a lot of inter-individual variability from the estimates of the treatment effect providing a powerful tool for the comparison of periodontal treatments [30]. Although the split-mouth design has disadvantages such as the possibility of intra-oral translocation of periodontopathogens from the untreated sites to the treated pockets [31], it is important to emphasize that in the present study, the patient received full mouth disinfection prior to photodynamic therapy, in order to reduce the incidence of such a recontamination.
The effect of aPDT on the primary outcome showed no statistically significant gain in the CAL between groups as well as for PD, MB, BOP, and PI at day 90 after treatment. Queiroz et al. [16] evaluated the efficacy of the adjunctive effect of aPDT associated with SRP with single episode in smokers, and no significant differences were observed for the between group comparisons. In counterpart, Al-Zahrani and Austah [29] conducted a randomized controlled clinical trial with similar methods and showed a statistically significant greater reduction in PD and CAL in the test when compared to the control group, but in their study, the parameters related to the laser therapy such as energy fluence, power output, and duration of light application were lacking. The characteristics related to the laser can modify the actual amount of energy released during the process, potentially affecting the antimicrobial efficacy and hence anti-inflammatory response to the periodontal treatment [32, 33].
The absence of sites with PD ≥ 5 mm after treatment is an important clinical endpoint of therapy [34]. The present study demonstrated that the test group presented a percentage of residual periodontal pockets lower than the control group but not statistically significant at 90 days after treatment. Residual pockets represent bacterial niches that can act as the focus of reinfection [35]. Future studies should be carried out to demonstrate if aPDT is an effective adjunct to open flap debridement in the surgical treatment of smokers.
A systematic review related to the clinical efficacy of aPDT adjunctive to SRP in the treatment of chronic periodontitis showed no significant clinical improvements in PD and CAL for studies that included smokers [36]. Furthermore, the results of subgroup analysis revealed a negative impact of smoking on the clinical efficacy of the combined therapy using both SRP and aPDT [36]. On the other hand, a protocol of multiple aPDT applications adjunctive to SRP showed better results than SRP alone in periodontal patients with type II diabetes and aggressive periodontitis [21, 37]. Some aspects should be considered, the negative effects of smoking on the bacterial challenge [38, 39], the reduced immune-inflammatory response [40], the disturbance on the balance between reactive oxygen species (ROS), and antioxidants that depletes systemic endogenous antioxidant capacity [41].
Concerning the microbiologic parameters in this present study, the bacterial species of the blue complex increased and of the red complex decreased in both groups at day 90, but no statistical differences were observed between groups. Queiroz et al. [42] investigated the microbiologic effects of adjunctive aPDT in a single episode, and the results showed that SRP or SRP + aPDT was not able to reduce levels of 40 subgingival species in smokers with chronic periodontitis. Several mechanisms have been proposed to explain the influence of cigarettes on the response of bacteria. These include the effects of harmful components of smoke that modify the oral bacterial composition, influencing the level of oxygenation and, consequently, the growth of beneficial aerobic bacteria [43].
Considering immunological findings, similar results in both groups were found at day 90, indicating that aPDT does not have an additional effect on the proinflammatory and anti-inflammatory cytokines assessed in smokers. IL-10 and TNF-α cytokines were significantly increased in the test group when compared to the control group at day 30 but showed comparable levels at day 90. The impairment of the host immune system may be due to changes in polymorphonuclear leukocyte function such as chemotaxis, phagocytosis, and oxidative burst which are decreased by substances in cigarette smoke [44]. Site-specific mapping of inflammatory markers, in smokers with chronic periodontitis, reports conflicting results [45]. Most of the studies have reported decreased local expression of some proinflammatory cytokines and chemokines in smokers [46, 47]. Conversely, elevated expressions of chemokines and proinflammatory cytokines have also been reported in smokers [48].
The results of this study provide additional evidence that smokers exhibit impaired antimicrobial function, which is consistent with the greater level of periodontal pathogens and severity of periodontal disease even after treatment with aPDT in multiples episodes. A systematic review with meta-analysis that assessed the effect of systemic antibiotics in the periodontal treatment of smokers showed that systemic antibiotic therapy favors CAL and PD reduction when compared with SRP alone, but with modest results although statistically significant, and appeared to be of little clinical relevance [49]. It should be taken into consideration that even when systemic antibiotics were used in smokers with chronic periodontitis, the effect of the periodontal therapy in reducing residual sites when compared with no smokers is lower [50, 51].
Conclusion
The multiple applications of aPDT did not promote additional clinical, microbiologic, and immunologic benefits in the treatment of smokers with chronic periodontitis. More randomized clinical trials are needed to evaluate adjuvant therapies in periodontal treatment in smokers.
Notes
Phenothiazine chloride, 10 mg/Ml, HELBO Blue, HELBO Photodynamic Systems, Grieskirchen, Austria
Minilaser 2075F, Helbo Photodynamic Systems
HELBO 3D Pocket Probe, HELBO Photodynamic Systems
Florida Probe, Gainesville, FL
PerioPapers, Interstate Drug Exchange, Amityville, NY
MilliplexMap, Merck Millipore, Billerica, MA
MAGPIX analyzer, Luminex, Austin, TX
Salimetrics Inc., State College, PA, USA
References
Genco RJ, Borgnakke WS (2013) Risk factors for periodontal disease. Periodontol 62:59–94. https://doi.org/10.1111/j.1600-0757.2012.00457.
Zeng J, Williams SM, Fletcher DJ, Cameron CM, Broadbent JM, Shearer DM, Thomson WM (2014) Re-examining the association between smoking and periodontitis in the Dunedin study with an enhanced analytical approach. J Periodontol 85:1390–1397. https://doi.org/10.1902/jop.2014.130577.
Johnson GK, Hill M (2004) Cigarette smoking and the periodontal patient. J Periodontol 75:196–209. https://doi.org/10.1902/jop.2004.75.2.196
Ryder MI (2007) The influence of smoking on host responses in periodontal infections. Periodontol 43:267–277. https://doi.org/10.1111/j.1600-0757.2006.00163.x
van der Velden U, Varoufaki A, Hutter JW, Xu L, Timmerman MF, van Winkelhoff AJ, Loos BG (2003) Effect of smoking and periodontal treatment on the subgingival microflora. J Clin Periodontol 30:603–610
Feres M, Bernal M, Matarazzo F, Faveri M, Duarte PM, Figueiredo LC (2015) Subgingival bacterial recolonization after scaling and root planning in smokers with chronic periodontitis. Aust Dent J 60:225–232. https://doi.org/10.1111/adj.12225
Carvalho LH, D’Avila GB, Leao A, Gonçalves C, Haffajee AD, Socransky SS, Feres M (2005) Scaling and root planning, systemic metronidazole and professional plaque removal in the treatment of chronic periodontitis in a Brazilian population II. Microbiological results. J Clin Periodontol 32:406–411. https://doi.org/10.1111/j.1600-051X.2005.00720.
Herrera D, Sanz M, Jepsen S, Needleman I, Roldan SA (2002) Systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planning in periodontitis patients. J Clin Periodontol 29:136–159
Haffajee AD, Socransky SS, Gunsolley JC (2003) Systemic anti-infective periodontal therapy: a systematic review. Ann Periodontol 8:115–181. https://doi.org/10.1902/annals.2003.8.1.115
Matarazzo F, Figueiredo LC, Cruz SE, Faveri M, Feres M (2008) Clinical and microbiological benefits of systemic metronidazole and amoxicillin in the treatment of smokers with chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol 35:885–896. https://doi.org/10.1111/j.1600-051X.2008.01304.
Pallasch TJ (2003) Antibiotic resistance. Dent Clin N Am 47:623–639
de Oliveira RR, Schwartz-Filho HO, Novaes AB Jr, Taba M Jr (2007) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: a preliminary randomized controlled clinical study. J Periodontol 78:965–973. https://doi.org/10.1902/jop.2007.060494
Novaes AB Jr, Schwartz-Filho HO, de Oliveira RR, Feres M, Sato S, Figueiredo LC (2012) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: microbiological profile. Lasers Med Sci 27:389–395. https://doi.org/10.1007/s10103-011-0901-6
Queiroz AC, Suaid FA, de Andrade PF, Oliveira FS, Novaes AB Jr, Taba M Jr, Palioto DB, Grisi MF, Souza SL (2015) Adjunctive effect of antimicrobial photodynamic therapy to nonsurgical periodontal treatment in smokers: a randomized clinical trial. Lasers Med Sci 30:617–625. https://doi.org/10.1007/s10103-013-1379-1
Meisel P, Kocher T (2005) Photodynamic therapy for periodontal diseases: state of the art. J Photochem Photobiol B 79:159–170. https://doi.org/10.1016/j.jphotobiol.2004.11.023
Braun A, Dehn C, Krause F, Jepsen S (2008) Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. J Clin Periodontol 35:877–884
Betsy CS, Prasanth KV, Baiju J, Prasanthila N, Subhash (2014) Efficacy ofantimicrobial photodynamic therapy in the management of chronicperiodontitis: a randomized controlled clinical trial. J Clin Periodontol 41:573–581
de Oliveira RR, Schwartz-Filho HO, Novaes AB Jr, Garlet GP, de Souza RF, Taba M Jr, Scombatti SLS, Ribeiro FJ (2009) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: cytokine profile in gingival crevicular fluid, preliminary results. J Periodontol 80:98–105. https://doi.org/10.1902/jop.2009.070465
Balata ML, de Andrade LP, Santos DB, Cavalcanti AN, Tunes Uda R, Ribeiro EP, Bittencourt S (2013) Photodynamic therapy associated with full-mouth ultrasonic debridement in the treatment of severe chronic periodontitis: a randomized controlled clinical trial. J Appl Oral Sci 21:208–214. https://doi.org/10.1590/1678-7757201302366
Petelin M, Perkic K, Seme K, Gašpirc B (2014) Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med Sci 30:1647–1656. https://doi.org/10.1007/s10103-014-1632-2
Moreira AL, Novaes AB Jr, Grisi MF, Taba M Jr, Souza SL, Palioto DB, de Oliveira PG, Casati MZ, Casarin RC, Messora MR (2015) Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: a split-mouth randomized controlled trial. J Periodontol 86:376–386. https://doi.org/10.1902/jop.2014.140392
Dilsiz A, Canakci V, Aydin T (2013) Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: a randomized controlled clinical trial. J Periodontol 84:278–286. https://doi.org/10.1902/jop.2012.120096
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4:1–6. https://doi.org/10.1902/annals.1999.4.1.1.
O'Leary TJ, Drake RB, Naylor JE (1972) The plaque control record. J Periodontol 43:38. https://doi.org/10.1902/jop.1972.43.1.38
Ainamo J, Bay I (1976) Periodontal indexes for and in practice. Tandlaegebladet 80:149–152
Tirachaimongkol C, Pothacharoen P, Reichart PA, Khongkhunthian P (2016) Relation between the stability of dental implants and two biological markers during the healing period: a prospective clinical study. Int J Implant Dent 2(1):27
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr (1998) Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144
Socransky SS, Haffajee AD, Smith C, Dibart S (1991) Relation of counts of microbial species to clinical status at the sampled site. J Clin Periodontol 8:766–775
Al-Zahrani MS, Austah ON (2011) Photodynamic therapy as an adjunctive to scaling and root planing in treatment of chronic periodontitis in smokers. Saudi Med J 32:1183–1188
Lesaffre E, Philstrom B, Needleman I, Worthington H (2009) The design and analysis of split-mouth studies: what statisticians and clinicians should know. Stat Med 28(28):3470–3482
Quirynen M, De Soete M, Dierickx K, van Steenberghe D (2001) The intra-oral translocation of periodontopathogens jeopardises the outcome of periodontal therapy. A review of the literature. J Clin Periodontol 28(6):499–507
Street CN, Pedigo LA, Loebel NG (2010) Energy dose parameters affect antimicrobial photodynamic therapy-mediated eradication of periopathogenic biofilm and planktonic cultures. Photomed Laser Surg 28:S61–S66. https://doi.org/10.1089/pho.2009.2622.
Akram Z, Abduljabbar T, Sauro S, Daood U (2016) Effect of photodynamic therapy and laser alone as adjunct to scaling and root planing on gingival crevicular fluid inflammatory proteins in periodontal disease: a systematic review. Photodiagn Photodyn Ther 16:142–153. https://doi.org/10.1016/j.pdpdt.2016.09.004
Matuliene G, Studer R, Lang NP, Schmidlin K, Pjetursson BE, Salvi GE, Brägger U, Zwahlen M (2010) Significance of periodontal risk assessment in the recurrence of periodontitis and tooth loss. J Clin Periodontol 37:191–199. https://doi.org/10.1111/j.1600-051X.2009.01508.x
Matuliene G, Pjetursson BE, Salvi GE, Schmidlin K, Brägger U, Zwahlen M, Lang NP (2008) Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodontol 35:685–695. https://doi.org/10.1111/j.1600-051X.2008.01245.x
Xue D, Tang L, Bai Y, Ding Q, Wang P, Zhao Y (2017) Clinical efficacy of photodynamic therapy adjunctive to scaling and root planning in the treatment of chronic periodontitis: a systematic review and meta-analysis. Photodiagn Photodyn Ther 18:119–127. https://doi.org/10.1016/j.pdpdt.2017.01.183
Ramos UD, Ayub LG, Reino DM, Grisi MF, Taba M Jr, Souza SL, Palioto DB, Novaes AB Jr (2016) Antimicrobial photodynamic therapy as an alternative to systemic antibiotics: results from a double-blind, randomized, placebo-controlled, clinical study on type 2 diabetics. J Clin Periodontol 43:147–155. https://doi.org/10.1111/jcpe.12498
Haffajee AD, Socransky SS (2001) Relationship of cigarette smoking to the subgingival microbiota. J Clin Periodontol 28:377–388
Kumar PS (2012) Smoking and the subgingival ecosystem: a pathogen-enriched community. Future Microbiol 7:917–919. https://doi.org/10.2217/fmb.12.71
Boström L, Linder LE, Bergström J (1998) Clinical expression of TNF-alpha in smoking-associated periodontal disease. J Clin Periodontol 25:767–777
Akpinar A, Toker H, Ozdemir H, Bostanci V, Aydin H (2013) The effects of non-surgical periodontal therapy on oxidant and anti-oxidant status in smokers with chronic periodontitis. Arch Oral Biol 58:717–723. https://doi.org/10.1016/j.archoralbio.2012.11.009
Queiroz AC, Suaid FA, de Andrade PF, Novaes AB Jr, Taba M Jr, Palioto DB, Grisi MF, Souza SL (2014) Antimicrobial photodynamic therapy associated to nonsurgical periodontal treatment in smokers: microbiological results. J Photochem Photobiol B 141:170–175. https://doi.org/10.1016/j.jphotobiol.2014.10.017
Lee J, Taneja V, Vassallo R (2012) Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res 91:142–149. https://doi.org/10.1177/0022034511421200
Nociti FH Jr, Casati MZ, Duarte PM (2015) Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontol 67:187–210. https://doi.org/10.1111/prd.12063
Johannsen A, Susin C, Gustafsson A (2014) Smoking and inflammation: evidence for a synergistic role in chronic disease. Periodontol 64:111–126. https://doi.org/10.1111/j.1600-0757.2012.00456.x
Rawlinson A, Grummitt JM, Walsh TF, Ian Douglas CW (2003) Interleukin 1 and receptor antagonist levels in gingival crevicular fluid in heavy smokers versus non-smokers. J Clin Periodontol 30:42–48
Tymkiw KD, Thunell DH, Johnson GK, Joly S, Burnell KK, Cavanaugh JE, Brogden KA, Guthmiller JM (2011) Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J Clin Periodontol 38:219–228. https://doi.org/10.1111/j.1600-051X.2010.01684.x
Giannopoulou C, Kamma JJ, Mombelli A (2003) Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J Clin Periodontol 30:145–153
Assem NZ, Alves MLF, Lopes AB, Gualberto EC Jr, Garcia VG, Theodoro LH (2017) Antibiotic therapy as an adjunct to scaling and root planning in smokers: a systematic review and meta-analysis. Braz Oral Res 3(31):e67. https://doi.org/10.1590/1807-3107BOR-2017.vol31.0067.
Faveri M, Rebello A, de Oliveira DR Borges I Jr, Duarte PM, Figueiredo LC, Feres M (2014) Clinical and microbiologic effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized chronic periodontitis: smokers versus non-smokers. J Periodontol 85:581–591. https://doi.org/10.1902/jop.2013.130278
Chambrone L, Vargas M, Arboleda S, Serna M, Guerrero M, de Sousa J, Lafaurie GI (2016) Efficacy of local and systemic antimicrobials in the non-surgical treatment of smokers with chronic periodontitis: a systematic review. J Periodontol 87:1320–1332. https://doi.org/10.1902/jop.2016.160268
Funding
The fiber tips and photosensitizers were donated by helbo (bredent medical GmbH & Co KG, Walldorf, Germany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Principal findings
Multiple episodes of aPDT adjunctive to non-surgical treatment did not improve significantly the clinical, immunological, and microbiological parameters when compared with SRP alone in smokers.
Rights and permissions
About this article
Cite this article
de Melo Soares, M.S., D’Almeida Borges, C., de Mendonça Invernici, M. et al. Antimicrobial photodynamic therapy as adjunct to non-surgical periodontal treatment in smokers: a randomized clinical trial. Clin Oral Invest 23, 3173–3182 (2019). https://doi.org/10.1007/s00784-018-2740-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2740-3