Abstract
Objectives
Cold atmospheric plasma (CAP), a room temperate ionized gas, seems to be a possible way to enhance tissue recovery. An in vitro study was conducted to investigate the influence of medical CAP on the regenerative capacity of human periodontal ligament (PDL) cells.
Material and methods
Human PDL cells were subjected to CAP at various intensities, distances, and durations. The effects of CAP on a number of specific markers were studied at transcriptional level using real-time PCR. Additionally, an in vitro wound healing assay was applied to PDL cell monolayers either in the presence or absence of CAP by using JuLI™ Br Live Cell Analyzer and software. Finally, cell viability of CAP-treated cells was analyzed by an XTT assay.
Results
CAP treatment enhanced significantly the expression of the cytokines tumor necrosis factor (TNF)α, cyclooxygenase (COX)2, interleukin (IL)-1β, IL-6, IL-8, collagen (COL)1α, and matrix metalloproteinase (MMP)1, as well as the proliferation markers Ki67 and proliferating cell nuclear antigen (PCNA), but downregulated apoptotic markers Apaf1 and p53. Additionally, the in vitro wound healing rate was significantly enhanced after CAP application. Moreover, CAP treatment resulted in a significantly increased cell viability in the XTT assay.
Conclusion
This in vitro study shows that CAP has regulatable effects on markers of periodontal wound healing thereby underlining the potential use of CAP as a benefit treatment strategy.
Clinical relevance
Our study demonstrates the application of CAP in the treatment of oral pathologies suggesting a promising future treatment approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical wound healing is a multifactored process that comprises different phases, each overlapping and interacting with the others [1]. The inflammatory phase begins with the intrinsic and extrinsic pathway of the coagulation cascade, which is activated after injury. Neutrophils of the blood, after their migration into the fresh wound, remove foreign material, such as bacteria, dead cells, and damaged extracellular matrix (ECM) [2]. Multiple growth factors and cytokines, such as IL-1β, IL-6, IL-8, and TNFα, have been shown to be secreted by periodontal ligament (PDL) cells and gingival fibroblasts. They regulate the production of matrix metalloproteinases (MMP) such as MMP1 and influence the remodeling of the soft and hard tissue [3,4,5]. Incoming mast cells releasing their granula are responsible for the typical signs of inflammation: redness, swelling, pain, and heat [6]. Two to three days after injury, monocytes are attracted to the wound through chemotaxis to initiate the process of phagocytosis [7]. The phase of proliferation usually starts at the second up to the tenth day after injury with the migration of fibroblasts which proliferate and produce new ECM components such as collagen and to build up granulation tissue [2]. Epidermal growth factor (EGF) stimulates the epithelialization, while angiogenesis is induced by vascular endothelial cell growth factor (VEGF) [8, 9]. The final phase of remodeling starts 2–3 weeks after injury and lasts up to 2 years [10]. It is characterized by a reorganization of the collagen matrix and a continuous contraction of the wound regulated by growth factors like PDGF, TGF-β, and FGF [11, 12].
For the entire wound healing process, periodontal recovery is of major importance [13]. The wound healing processes in the oral cavity, such as periodontal wound healing, are influenced by various internal and external factors, in particular systemic diseases or states of immunodeficiency (e.g., diabetes, HIV, and steroid therapy) or environmental toxins like nicotine or alcohol [14,15,16].
The PDL, consisting mainly of PDL fibroblasts and collagen fibers, connects the teeth with the surrounding bone and absorbs mechanical strain, induced by mastication or trauma. This physically adapted stress makes PDL cells an important factor in oral adaption processes, which is also essential in periodontal homeostasis and local immune response [17]. The PDL fibroblasts regulate the synthesis and remodeling of the ECM [18]. For this reason, we chose PDL as a wound model in our investigation.
Periodontal healing can be induced by surgical or non-surgical debridement to reduce pathogens in periodontal pockets. Usually, periodontal healing results in regeneration of tissue structures and can be supported by using an enamel matrix derivative [19] as well as laser procedures [20], each of both with the potential to significantly improve attachment levels.
Cold atmospheric plasma (CAP), a room temperate ionized gas, has recently been identified as a possible way to augment tissue restauration. Plasma is described as the fourth state of matter, which can be reached by adding energy to a gaseous material. There are different plasma devices, which can either use inert gas like argon or helium to generate CAP or the ambient air, which contains oxygen and nitrogen [21,22,23,24]. Ambient air plasma devices are easier to use for clinical application because of the absence of any inert gas. Plasma application leads to an electric charge of the atomic parts between instrument probe and the treated surface creating reactive components with several effects. Numerous studies have demonstrated the capacity of CAP to enhance wound healing as well as a treatment for candida or skin diseases or for decreasing tumor growth [25,26,27,28,29,30]. Nevertheless, our understanding of the role of CAP in periodontal wound healing and remodeling as well as of the underlying mechanisms is still limited so far. A number of studies have shown different effects of CAP on gene expression in various cells, including an upregulation of inflammatory or apoptotic genes [31, 32]. Therefore, the main objective of the present study was to examine the regulatory effect of CAP exposition to PDL cells in vitro.
Materials and methods
PDL cell culture
PDL cells of 5 young, healthy individuals (age 11–19 years; 3 males/2 females) from premolar roots using the explant method [33] were used. The teeth of all patients had been extracted for orthodontic reasons and written informed consent and approval of the Ethics Committee of the University of Bonn were given (#111/17). PDL tissue explants were dissected from the middle third of the root surface with a sharp scalpel, washed with phosphate-buffered saline (PBS, Invitrogen, Karlsruhe, Germany), minced into small pieces, and cultured in Dulbecco’s modified essential medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 100 units penicillin, and 100 μg/ml streptomycin (Invitrogen) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air for 2 to 4 weeks. After passaging, cells were seeded into 35 × 10 mm Petri dishes and cultured to 80% confluence. One day prior to the experiments, the FBS concentration was reduced to 1%. Medium was changed every 2 days throughout the whole cultivation period. Seeding protocol was applied to analysis of gene expression, wound healing assay, and XTT assay.
Cold plasma application and preliminary experiments
A plasma device (Plasma ONE DENTAL/MEDICAL, Plasma MEDICAL SYSTEMS® GmbH, Nievern, Germany) was used for the application of CAP to PDL cells (Fig. 1). In this device, pulsed direct current (35 V) is transformed to high voltage that leads to an electric field at the tip of the probe. This electric field ionizes the ambient air and generates CAP thereby. The CAP can be used at five levels of intensity, modulating the high voltage (3–18 kV).
In preliminary experiments, PDL cells were exposed to CAP treatment with various intensities, distances, and for different durations. Optimal research conditions were identified after 30, 60, and 120 s of CAP treatment, with intensity levels 3 and 5 and at 1 and 2 cm distance to monolayer cells using the PS30 instrument probe, totally covering the 35 × 10 mm Petri dish. For each setting, the optimal time, intensity, and distance were selected after preliminary experiment.
Analysis of gene expression
After CAP treatment with intensity levels 3 and 5, 1 and 2 cm distance, and a duration of 30, 60, and 120 s, total RNA of PDL cells was extracted 24 h after CAP treatment using an RNA extraction kit (Qiagen, Hilden, Germany). First, 1 μg of RNA was reversely transcribed using iScript™ Select cDNA Synthesis Kit (Bio-Rad Laboratories, Munich, Germany) at 42 °C for 90 min followed by 85 °C for 5 min. mRNA expression of TNFα, COX2, MMP1, IL-1β, IL-6, IL-8, COL1α, Ki67, PCNA, Apaf1, and p53 was detected by real-time PCR using the iCycler iQ™ detection system (Bio-Rad Laboratories), SYBR Green (Bio-Rad Laboratories), and specific primers (QuantiTect Primer Assay, Qiagen). One microliter of cDNA was amplified as a template in a 25-μl reaction mixture containing 12.5 μl 2× QuantiFast SYBR Green PCR Master Mix (Qiagen), 2.5 μl of primers (0.5 μM each), and 9 μl deionized water. The mixture was heated at 95 °C for 5 min initially, followed by 40 cycles with denaturation at 95 °C for 10 s and combined annealing/extension at 60 °C for 30 s. GAPDH was used as an endogenous control. mRNA was quantified by the comparative threshold cycle method.
Wound healing assay
In the PDL cell monolayers, 3 mm wide defects (wounds) were created in a standardized manner, as previously described by Memmert et al. [34]. The wounded monolayers were treated with CAP for 60 s at a distance of 2 cm at high intensity (level 5). Filling of the wound and cell migration were evaluated over a period of 72 h using JuLI™ Br Live Cell Analyzer and a special JuLI™ Br PC software (both: NanoEnTek, Seoul, Korea). For this test module, the chosen time, intensity, and distance to the cell layer were shown by preliminary experiments.
XTT assay
Cell viability was determined using the cell proliferation kit XTT (Applichem, Darmstadt, Germany). After preliminary experiments to determine the appropriate study design, PDL cells (5.000/well) were seeded into 96-well plates and grown to 80% confluence. CAP stimulation was performed at a distance of 2 cm for 60 s at low intensity (level 3) and high intensity (level 5). After 24 h of cultivation, XTT reagent solution was added to the medium for 4 h followed by the measurement of absorbance at 475 nm with correction wavelength 630–690 nm in a microplate reader (PowerWave x, BioTek Instruments, Winooski, VT, USA). This study design was shown by preliminary experiments as the optimal treatment modality.
Statistical analysis
All experiments were performed in triplicates and repeated at least twice. Mean values and standard errors of the mean (SEM) were calculated. Parametric (ANOVA and Dunnett’s tests) and non-parametric (Mann-Whitney U) tests were applied for statistical analysis using the IBM SPSS Statistics 24 software (IBM Corporation, Armonk, NY, USA). Differences between groups were considered significant at p < 0.05.
Results
Upregulation of proinflammatory cytokines and MMP1 in CAP-treated PDL cells
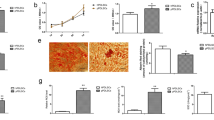
CAP on PDL cells increased significantly mRNA expression of IL-1β, IL-6, and IL-8 as compared to untreated cells after 1 day, which is shown in Fig. 2a. In addition, we observed a significant increase in mRNA expression of TNFα in CAP-treated PDL cells as compared to control cells as shown in Fig. 2b. A significant in upregulation of COX2, which also plays an important role in the inflammation phase, was also observed after CAP application (Fig. 2b). Moreover, since matrix remodeling is another also essential process during wound healing, we also analyzed MMP1 regulation after CAP treatment and observed a significant increase of MMP1 gene expression after 1 day as shown in Fig. 2b. The gene expression of CAP in PDL cells was time dependent as shown in Fig. 2c, d. After 30 s of CAP exposition, a significant increase in gene regulation of IL-8 and COX2 was observed with the highest RNA expression after 120 s of CAP application (Fig. 2c, d). Other observed genes showed similar results (data not shown).
mRNA expression of PDL cells after CAP treatment at a distance of 2 cm, level 5 of plasma device at 1 day. a mRNA expression of IL-1β, IL-6, and IL-8 after 60 s of CAP treatment (n = 6). b mRNA expression of TNFα, COX2, and MMP1 after 60 s of CAP treatment (n = 6). c Time-dependent mRNA expression of IL-8 after CAP treatment for 30, 60, and 120 s (n = 6). *statistical significance. d Time-dependent mRNA expression of COX2 after CAP treatment for 30, 60, and 120 s (n = 6). *statistical significance
Regulation of proliferation and type I collagen formation in CAP-treated PDL cells
According to the important role of PDL cell proliferation in periodontal wound healing, we studied the effects of CAP on the proliferation markers, PCNA, and Ki67, and also on the expression of a component for extracellular matrix synthesis, COL1α. After 60 s of CAP treatment, the mRNA expression of these genes—especially PCNA—increased significantly as compared to untreated cells (Fig. 3a, b). Additionally, in order to study the stimulation of cell proliferation effects employing CAP, we used a commercial XTT assay. As shown in Fig. 3c, CAP treatment of PDL cells for 60 s significantly improved cell viability after 24 h (Fig. 3c). The increase in proliferation rate was intensity dependent, resulting in different responses to CAP exposition at levels 3 and 5 of the plasma device. Furthermore, the increase in proliferation rate was more pronounced for cells covered by medium during CAP exposition, as shown in Fig. 3c.
CAP exposition on PDL cell culture at a distance of 2 cm for 60 s at 1 day. a mRNA expression of Ki67 and PCNA (intensity: level 5 of plasma device) (n = 6). b mRNA expression of COL1α (intensity: level 5 of plasma device) (n = 6). c XTT assay (cell proliferation assay): intensity-dependent (low = level 3 of plasma device, high = level 5 of plasma device) CAP exposition on medium-covered PDL cells and PDL cells without medium after 24 h (n = 9). *statistical significance
Effects of CAP on periodontal wound healing
In addition, we used an established assay to study the wound healing. Using live microscopy, we demonstrated the influence of CAP treatment on PDL cells which, interestingly, resulted in a remarkable higher wound closure rate as compared to the non-plasma-treated group after 72 h. Stimulation of wound closure by plasma confirmed and expanded our previous results (Fig. 4a, b).
Evaluation of wound closure with JuLi Br Live Cell Analyzer in wounded PDL cell cultures after CAP expression for 60 s at a distance of 2 cm, level 5 of plasma device over a period of 72 h. a Microscopical evaluation before wounding, immediately after wounding, and after 72 h past CAP treatment. b Quantitative assay of wound closure (n = 14). *statistical significance
Regulation of apoptosis markers in CAP-treated PDL cells
Finally, we aimed to investigate apoptotic effects of CAP on PDL cells. For this reason, we measured gene regulation of Apaf1 and p53. The application of CAP on PDL cells at both intensity levels (3 and 5) significantly decreased mRNA expressions of Apaf1 and especially p53 as compared with untreated cells after 1 day, as shown in Fig. 5. Although both intensity levels downregulated apoptotic markers, the inhibitory effect of CAP was more effective at the low-intensity level. Specifically, the downregulation of Apaf1 gene was almost 90% and approximately 40% for p53 (Fig. 5a, b).
Discussion
The present study provides evidence that CAP has a positive effect on PDL cells, evidenced by a higher cell proliferation. The upregulation of matrix, proliferation, inflammation, and degradation genes as well as a downregulation of apoptotic genes underlines these findings.
In the literature, many in vivo studies describe the positive effects of cold atmospheric plasma on various cells. The plasma device we used is not based on inert gas like argon or helium, which is often described [21, 35]. We used ambient air as working gas, like other authors [22, 23]. The Plasma ONE uses direct current to create a high frequency, which causes an electric field at the tip of the probe. Finally, the plasma is produced by ionization of the ambient air, allowing for an easier clinical use. In comparison to plasma devices using inert gas, however, the ambient air has variable temperature and pressure instead of a consistent inert gas. In fact, Haertel et al. showed differences of multiple plasma devices on cell viability of HaCaT keratinocytes [24]. Further studies are needed to show the difference between the effects of ambient air-generated CAP and argon/helium-generated CAP on human PDL cells.
Different ways of CAP application were discussed—either applying the CAP directly on cells or exposing medium to CAP in order to generate plasma-activated medium with cell-influencing effects [36,37,38]. In our study, a higher level of 60 s CAP application in a distance of 2 cm to the cells resulted in higher gene expression of critical inflammation, matrix, proliferation, and degradation markers with downregulated apoptotic genes. A higher mRNA expression was also achieved by with longer times of CAP exposure (data not shown). Interestingly, recent studies demonstrated a time- and distance-dependent stimulatory effect by application of CAP, attributed to the generation of reactive oxygen species [21, 39]. Further experiments are needed to disclose both the duration of CAP application and different levels of intensity to find the most effective way of application for wound healing effects.
In the present study, TNFα, COX2, MMP1, IL-1β, IL-6, IL-8, COL1α, Ki67, and PCNA gene expression increased significantly. The cytokines IL-1β, IL-6, IL-8, and TNFα mediate key roles in inflammation, and play an important role in operating the extracellular matrix, which is essential for the normal healing process in case of injury. An upregulation of these inflammatory genes after CAP treatment was also shown by Arndt and Zhong [32, 40]. In this context, the upregulation of COX2, which plays an important role in regulating the production of prostanoids associated with cytokines and inflammation, was demonstrated in our examination (Fig. 2b, d). MMP1 being part of the extracellular matrix plays an important role in morphogenesis, inflammation, and wound healing, by regulating cell migration, differentiation, angiogenesis, and degradation. An upregulation of MMP1 in PDL cells after CAP treatment could be shown, which underlines the proliferation process of matrix molecules (Fig. 2b).
COL1α is a part of the extracellular matrix as well. An increase in gene regulation of Col1α after CAP treatment signifies an upregulated proliferation function of the PDL cell. The upregulation of COL1α was also demonstrated by Kwon et al., who also found an increase in COL1 gene of human gingival fibroblasts after CAP application [41]. Ki67 and PCNA are molecules which are expressed in proliferating cells. CAP treatment of fibroblasts leads to an upregulation of Ki67 and PCNA, implicating an active cell proliferation process. Delben et al. showed similar results in an in vitro model, using CAP-treated oral keratinocytes [42]. p53 plays a central role in apoptosis and senescence and is an important factor in cellular stress response. The applications of CAP on PDL cells lead to a significant decrease in gene expression of p53 and Apaf1. We mainly focused on these genes, which are known to be a major part of the apoptotic pathway. p53 induces Apaf-1 gene expression, which results in an activation of CASP9, -3, -6, and -7, leading to apoptosis. Whether CAP application affects additional molecules of the apoptotic/survival pathways, such as BCL2, BAD, and CASP9, has to be examined in future studies. The CAP-associated reduction in expression of apoptotic genes underlines a positive effect of CAP treatment on PDL cells. Further experiments should clarify whether additional apoptotic genes are downregulated by CAP treatment. However, Turrini et al. showed an upregulation of p53 genes in leukemia cells after cultivation in CAP-treated medium [31]. An upregulation of p53 genes after CAP treatment was also shown by Arndt et al. for melanoma cells [22] and Gümbel et al. for osteosarcoma cells [43]. There seems to be a difference from the extent of p53 gene expression in various cell types after CAP treatment, suggesting that CAP treatment leads to proapoptotic properties of malignant cells. The fact that both our apoptotic markers (p53 and Apaf1) were downregulated by CAP treatment underlines a positive effect on oral tissues. Further studies will have to examine, if there are differences in other oral cell types.
In the in vitro scratch assay, a higher cell migration of 60 s CAP-treated PDL cells could be observed. Schmidt et al. demonstrated an improved healing in an in vitro wound model of fibroblasts and keratinocytes in CAP-treated medium [44]. The improved cell migration rate of fibroblasts was also shown by Arndt et al. [40], but was not observed in keratinocytes [45]. A higher wound closure of cells was also found by Lendeckel et al., who examined a time-dependent effect of plasma-treated epithelial cells up to an application time of 120 s. A longer duration of plasma application (240 s, 360 s) led to a lower regeneration efficiency and blocking of the regeneration process [46]. A further examination for longer application of CAP on PDL cells is necessary to confirm these results.
In this study, CAP treatment of 60 s resulted in a higher intensity-dependent cell viability of PDL cells, measured by an XTT proliferation assay. Medium-covered PDL cells showed higher cell viability in contrast to cells deprived of medium, maybe resulting in positive effects of an activation of the medium, as often described in literature [31]. However, Lee et al. described a viability loss of fibroblasts and oral squamous cell carcinoma cell lines after 1 min of CAP treatment [47]. Maish et al. also observed a decreasing viability of fibroblasts after 1 and 2 min of CAP treatment, while CAP treatment of keratinocytes leads to an increasing viability [48]. Arndt et al. did not see any influence in cell proliferation of fibroblasts and keratinocytes after 2 min of CAP treatment [40, 45]. In regard to the different outcomes of the cited studies and our experiments, varying cell lines seem to react differently towards CAP application further research has to show, whether there is an influence of CAP treatment for cell viability in other gingival cells.
Several studies reported a wound healing and anti-cancer capacity of CAP treatment: for example, a treatment of glioblastoma and breast cancer cells with so-called plasma-activated medium resulted in an intensity-dependent viability [36, 38]. This anti-cancer effect of plasma-activated medium was also supported by Shi et al., who used ambient air as working gas for creating CAP for cultivating oral carcinoma cells [23].
Finally, further experiments will need to be confirmed in vivo. Schmidt et al. showed an accelerated wound closure in immunocompetent mice [44]. This is supported by a study of Arndt et al., who also observed an increased accumulation of inflammatory macrophages and neutrophils in the early phase of wound healing in CAP-treated wounded mice as suggested reason [40]. The same authors also observed an enhanced angiogenesis in mice after CAP treatment [49].
Conclusion
In conclusion, we hereby provide substantial evidence that the application of CAP on PDL cells has a positive influence on cell activity. An upregulation of matrix, proliferation, inflammation, and degradation genes, a downregulation of apoptotic genes, a higher cell proliferation, and wound closure were observed after the application of CAP. Although a standardized technique of CAP treatment still needs to be developed, with respect to standardization concerning the use of different plasma devices and treatment modalities, our results underline the practical use of CAP as a benefited treatment strategy.
References
Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA (2012) Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 1(2):142–149
Velnar T, Bailey T, Smrkoli V (2009) The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 37:1528–1542
Kondo T, Ohshima T (1996) The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int J Legal Med 108(5):231–236
Noh MK, Jung M, Kim SH, Lee SR, Park KH, Kim DH, Kim HH, Park YG (2013) Assessment of IL6, IL8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp Ther Med 6(3):847–851
Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mäntylä P (2006) Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 38(5):306–321
Degreef HJ (1998) How to heal a wound fast. Dermatol Clin 16:365–375
Hunt TK (1988) The physiology of wound healing. Ann Emerg Med 17:1265–1273
Schultz G, Rotatori DS, Clark W (1991) EGF and TGF-alpha in wound healing and repair. J Cell Biochem 45:346–352
Tonnesen MG, Feng XD, Clark RAF (2000) Angiogenesis in wound healing. J Invest Dermatol Symp Proc 5:40–46
Ramasastry SS (2005) Acute wounds. Clin Plast Surg 32:195–208
Clark RAF (1993) Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci 306:42–48
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453(7193):314–321
Dahlin C, Linde A, Gottlow J, Nyman S (1988) Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg 81(5):672–676
Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA (2017) Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol 199(1):17–24. https://doi.org/10.4049/jimmunol.1700223
Eisenbeis J, Peisker H, Backes CS, Bur S, Hölters S, Thewes N, Greiner M, Junker C, Schwarz EC, Hoth M, Junker K, Preissner KT, Jacobs K, Herrmann M, Bischoff M (2017) The extracellular adherence protein (Eap) of Staphylococcus aureus acts as a proliferation and migration repressing factor that alters the cell morphology of keratinocytes. Int J Med Microbiol 307(2):116–125
Swift ME, Kleinman HK, DiPietro LA (1999) Impaired wound repair and delayed angiogenesis in aged mice. Lab Investig 79(12):1479–1487
Zhang L, Liu W, Zhao J, Ma X, Shen L, Zhang Y, Jin F, Jin Y (2016) Mechanical stress regulates osteogenic differentiation and RANKL/OPG ratio in periodontal ligament stem cells by the Wnt/β-catenin pathway. Biochim Biophys Acta 1860(10):2211–2219
Marchesan JT, Scanlon CS, Soehren S, Matsuo M, Kapila YL (2011) Implications of cultured periodontal ligament cells for the clinical and experimental setting: a review. Arch Oral Biol 56(10):933–943
Bosshardt DD (2008) Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol 35(8):87–105
Birang R, Shahaboui M, Kiani S, Shadmehr E, Naghsh N (2015) Effect of nonsurgical periodontal treatment combined with diode laser or photodynamic therapy on chronic periodontitis: a randomized controlled split-mouth clinical trial. J Lasers Med Sci 6(3):112–119
Brun P, Pathak S, Castagliuolo I, Palù G, Brun P, Zuin M, Cavazzana R, Martines E (2014) Helium generated cold plasma finely regulates activation of human fibroblast-like primary cells. PLoS One 9(8):e104397
Arndt S, Wacker E, Li YF, Shimizu T, Thomas HM, Morfill GE, Karrer S, Zimmermann JL, Bosserhoff AK (2013) Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp Dermatol 22(10):284–289. https://doi.org/10.1111/exd.12127
Shi L, Yu L, Zou F, Hu H, Liu K, Lin Z (2017) Gene expression profiling and functional analysis reveals that p53 pathway-related gene expression is highly activated in cancer cells treated by cold atmospheric plasma-activated medium. PeerJ 25(5):e3751. https://doi.org/10.7717/peerj.3751 eCollection 2017
Haertel B, von Woedtke T, Weltmann KD, Lindequist U (2014) Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol Ther (Seoul) 22(6):477–490
Daeschlein G, Scholz S, Ahmed R, von Woedtke T, Haase H, Niggemeier M, Kindel E, Brandenburg R, Weltmann KD, Juenger M (2012) Skin decontamination by low-temperature atmospheric pressure plasma jet and dielectric barrier discharge plasma. J Hosp Infect 81(3):177–183
Heinlin J, Isbary G, Stolz W, Morfill G, Landthaler M, Shimizu T, Steffes B, Nosenko T, Zimmermann J, Karrer S (2011) Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol 25(1):1–11
Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, Karrer S, Landthaler M, Shimizu T, Steffes B, Bunk W, Monetti R, Zimmermann JL, Pompl R, Stolz W (2010) A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol 163(1):78–82
Isbary G, Heinlin J, Shimizu T, Zimmermann JL, Morfill G, Schmidt HU, Monetti R, Steffes B, Bunk W, Li Y, Klaempfl T, Karrer S, Landthaler M, Stolz W (2012) Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol 167(2):404–410
Keidar M, Walk R, Shashurin A, Srinivasan P, Sandler A, Dasgupta S, Ravi R, Guerrero-Preston R, Trink B (2011) Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br J Cancer c105(9):1295–1301
Seyfarth K, Blum C, Plank A, Sommer C, Mergner M, Thomaschewski B (2015) Behandlung von Aphthen im Mundraum mit Plasma. Plasma Kurier 2(1):23–26
Turrini E, Laurita R, Stancampiano A, Catanzaro E, Calcabrini C, Maffei F, Gherardi M, Colombo V, Fimognari C (2017) Cold atmospheric plasma induces apoptosis and oxidative stress pathway regulation in T-lymphoblastoid leukemia cells. Oxidative Med Cell Longev 2017:4271065
Zhong SY, Dong YY, Liu DX, Xu DH, Xiao SX, Chen HL, Kong MG (2016) Surface air plasma-induced cell death and cytokine release of human keratinocytes in the context of psoriasis. Br J Dermatol 174(3):542–552
Mariotti A, Cochran DL (1990) Characterization of fibroblasts derived from human periodontal ligament and gingiva. J Periodontol 61(2):103–111
Memmert S, Nokhbehsaim M, Damanaki A, Nogueira AVB, Papadopoulou AK, Piperi C, Basdra EK, Rath-Deschner B, Götz W, Cirelli JA, Jäger A, Deschner J (2018) Role of cathepsin S in periodontal wound healing—an in vitro study on human PDL cells. BMC Oral Health 18(1):60
Bekeschus S, Schmidt A, Bethge L, Masur K, von Woedtke T, Hasse S, Wende K (2016) Redox stimulation of human THP-1 monocytes in response to cold physical plasma. Oxidative Med Cell Longev 2016:5910695
Tanaka H, Mizuno M, Ishikawa K, Nakamura K, Kajiyama H, Kano H, Kikkawa F, Hori M (2011) Plasma-activated medium selectively kills glioblastoma brain tumor cells by down-regulating a survival signaling molecule, AKT kinase. Plasma Medicine 1:265–277
Virard F, Cousty S, Cambus JP, Valentin A, Kémoun P, Clément F (2015) Cold atmospheric plasma induces a predominantly necrotic cell death via the microenvironment. PLoS One 10(8):e0133120
Yan DY, Talbot A, Nourmohammadi N, Cheng XQ, Canady J, Sherman J, Keidar M (2015) Principles of using cold atmospheric plasma stimulated media for cancer treatment. Sci Rep 5:18339
Bundscherer L, Nagel S, Hasse S, Tresp H, Wende K, Walther R, Reuter S, Weltmann K-D, Masur K, Lindequist U (2015) Non-thermal plasma treatment induces MAPK signaling in human monocytes. Open Chem 13:606–613
Arndt S, Unger P, Wacker E, Shimizu T, Heinlin J, Li YF, Thomas HM, Morfill GE, Zimmermann JL, Bosserhoff AK, Karrer S (2013) Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS One 8(11):e79325 2013b
Kwon JS, Kim YH, Choi EH, Kim CK, Kim KN, Kim KM (2016) Non-thermal atmospheric pressure plasma increased mRNA expression of growth factors in human gingival fibroblasts. Clin Oral Investig 20(7):1801–1808
Delben JA, Zago CE, Tyhovych N, Duarte S, Vergani CE (2016) Effect of atmospheric-pressure cold plasma on pathogenic oral biofilms and in vitro reconstituted oral epithelium. PLoS One 11(5):e0155427
Gümbel D, Gelbrich N, Weiss M, Napp M, Daeschlein G, Sckell A, Ender SA, Kramer A, Burchardt M, Ekkernkamp A, Stope MB (2016) New treatment options for osteosarcoma-inactivation of osteosarcoma cells by cold atmospheric plasma. Anticancer Res 36(11):5915–5922
Schmidt A, Bekeschus S, Wende K, Vollmar B, von Woedtke T (2017) A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp Dermatol 26(2):156–162
Arndt S, Landthaler M, Zimmermann JL, Unger P, Wacker E, Shimizu T, Li YF, Morfill GE, Bosserhoff AK, Karrer S (2015) Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS One 10(3):e0120041
Lendeckel D, Eymann C, Emicke P, Daeschlein G, Darm K, O'Neil S, Beule AG, von Woedtke T, Völker U, Weltmann KD, Jünger M, Hosemann W, Scharf C (2015) Proteomic changes of tissue-tolerable plasma treated airway epithelial cells and their relation to wound healing. Biomed Res Int 2015:506059
Lee JH, Om JY, Kim YH, Kim KM, Choi EH, Kim KN (2016) Selective killing effects of cold atmospheric pressure plasma with NO induced dysfunction of epidermal growth factor receptor in oral squamous cell carcinoma. PLoS One 11(2):e0150279
Maisch T, Bosserhoff AK, Unger P, Heider J, Shimizu T, Zimmermann JL, Morfill GE, Landthaler M, Karrer S (2017) Investigation of toxicity and mutagenicity of cold atmospheric argon plasma. Environ Mol Mutagen 58(3):172–177
Arndt S, Unger P, Berneburg M, Bosserhoff AK, Karrer S (2018) Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J Dermatol Sci 89(2):181–190
Acknowledgements
The authors would like to thank Ms. Ramona Menden and Ms. Silke van Dyck for their valuable support.
Funding
This study was supported by the research funds of the Department of Oral Surgery of the University of Bonn.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. A vote of approval by the ethics committee of the Faculty of Medicine at the University of Bonn was obtained (Lfd.Nr. 111/17).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kleineidam, B., Nokhbehsaim, M., Deschner, J. et al. Effect of cold plasma on periodontal wound healing—an in vitro study. Clin Oral Invest 23, 1941–1950 (2019). https://doi.org/10.1007/s00784-018-2643-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2643-3