Abstract
Objectives
The aim of this study was to investigate bone turnover alterations after alendronate (ALD) withdrawal and its influence on dental implants osseointegration.
Materials and methods
Seventy female Wistar rats were randomly divided in 2 groups that received on day 0 either placebo (control group—CTL; n = 10) or 1 mg/kg sodium alendronate (ALD; n = 60) once a week for 4 months. At day 120, ALD treatment was suspended for 50 animals. Then, a titanium implant was placed in the left tibia of each rat that were randomly allocated in five subgroups of ten animals each, according to the period of evaluation: day 0 (INT-0), day 7 (INT-7), day 14 (INT-14), day 28 (INT-28), and day 45 (INT-45) after ALD withdrawal. CTL group and a group that received ALD until the end of the experimental period (non-interrupted group—non-INT; n = 10) underwent implant placement on day 120. Animals were euthanized 28 days after implant surgery. Bone mineral density (BMD) of femur and lumbar vertebrae were evaluated by DXA, biochemical markers of bone turnover were analyzed by ELISA, and bone histomorphometry was performed to measure bone-to-implant contact (BIC) and bone area fraction occupancy (BAFO).
Results
All groups receiving ALD showed higher BMD values when compared to CTL group, which were maintained after its withdrawal. Decreased concentrations in all bone turnover markers were observed in the non-INT group, and in the groups in which ALD was discontinued compared to the CTL group. The non-INT group showed lower %BIC and notably changes in bone quality, which was persistent after drug withdrawal.
Conclusion
Collectively, the findings of this study demonstrated that ALD therapy decreased bone turnover and impaired bone quality and quantity around dental implants, and that its discontinuation did not reverse these findings.
Clinical relevance
The severe suppression of bone turnover caused by the prolonged use of ALD may alter the capacity of bone tissue to integrate with the implant threads impairing the osseointegration process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antiresorptive medications, such as nitrogen-containing bisphosphonates (BPs), are widely used for the management of bone diseases such as osteoporosis or bone malignancy. BPs, such as alendronate, acts by inhibiting the farnesyl diphosphate synthase in the cholesterol pathway, which prevents the prenylation of guanosine triphosphatase signaling proteins [1]. As a result, alendronate inhibits osteoclast activity by impairing differentiation, reducing intracellular transport, disrupting the cytoskeleton, and leading to cell apoptosis [2, 3]. These events translate to decreased bone turnover, improved BMD and structural bone properties, and reduced risk of fractures [4, 5].
Osteoporosis, a metabolic disease of bone, increases bone turnover leading to high bone remodeling rate. As a consequence of excessive bone resorption, osteoporosis reduces BMD and bone cancellous mass with generalized microarchitectural deterioration, and subsequent increased risk for bone fractures [6, 7]. Risk factors of osteoporosis include postmenopausal estrogen deficiency, excessive glucocorticoid intake, and gene interactions in bone metabolism [8, 9]. In this context, alendronate is among the most frequently prescribed medications for the treatment of osteopenia and osteoporosis [10].
Over the last decades, implant therapy to replace missing teeth has increased significantly. In addition, the number of patients under BPs therapy, and candidates for implants rehabilitation has been rising. The long-term clinical success of endosseous dental implants is critically related to the bone quality and to direct contact between bone and implant. In this regard, several pre-clinical studies [11,12,13,14,15,16,17] have shown decreased bone area, bone volume, and bone-to-implant contact (BIC) around dental implants placed in osteoporotic animals. However, BPs therapy may be able to reverse the negative effect of osteoporosis on the osseointegration of dental implants as demonstrated by increased %BIC around osseointegrated implants [16, 18,19,20,21] as well as increased torque needed for implant removal [20, 22]. On the other hand, previous studies [23, 24] in healthy rabbits and rats demonstrated that alendronate administration had no effect on dental implant torque removal values. Accordingly, lower BIC values and decreased torque for implant removal were observed in rabbits on alendronate without osteoporosis [25].
Alendronate withdrawal has been proposed as potentially beneficial in re-establishing bone turnover, which could improve bone healing [26]. However, experimental data supporting the benefit of alendronate discontinuation before implant placement are lacking. Here, we explored the effects of alendronate withdrawal in rats before implant placement by means of histomorphometric, densitometric, and biochemical analyses. Our results indicate that, within the methodology employed, alendronate therapy impaired bone quality and quantity around dental implants and these findings were sustained after its withdrawal.
Material and methods
Animal care
The experimental protocol was handled according to the guidelines of the local Ethical Committee for Animal Care and Use (protocol #12/2009), and all animals received humane care according to the recommendations of the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines for the execution and submission of studies in animals [27]. Rats were kept in the animal facilities in a humidity (55%) and temperature-controlled room (22 ± 2 °C) on a 12-h light/dark cycle (light on at 07:00 am). All rats were housed in standard cages in groups of two animals, and normal chow diet and water were provided ad libitum.
A total of 70 3-month-old female Wistar rats (Rattus norvegicus albinus), with average body weight between 250 and 300 g were randomly (by means of drawing lots) divided into 2 groups that received on day 0 either subcutaneously injection of endotoxin-free saline (control group—CTL; n = 10) or 1 mg/kg sodium alendronate (test group; n = 60) once a week for 4 months, following previously published protocol [20]. At day 120, alendronate treatment was suspended for 50 animals (INT-0, INT-7, INT-14, INT-28, INT-45 groups; n = 10 for each group). CTL group, INT-0, and a group that received alendronate until the end of the experimental period (non-interrupted group, non-INT; n = 10) underwent surgery for implant placement on day 120. Furthermore, a titanium implant was placed in the rats left tibiae on days 7 (INT-7), 14 (INT-14), 28 (INT-28), and 45 (INT-45) after alendronate withdrawal (n = 10 for each group). The dosage of 1 mg/kg of sodium alendronate was based in our previous published manuscript [20]. A flowchart comprising the experimental design is presented in Fig. 1.
Implant surgery
Experimental surgery for implant placement was performed as described [28]. Briefly, the animals were fasted for 8 h prior to surgery, and general anesthesia was done by intramuscular (im) injection of a combination of ketamine hydrochloride (Cetamin®; Sintec Ltda, Cotia, SP, Brazil; 0.08 ml/100 g body weight) and xylazine hydrochloride (Xilazin®; Bayer S.A., Sao Paulo, SP, Brazil; 0.04 ml/100 g body weight). Then, rats underwent local anesthesia by infiltrative injection with mepivacaine hydrochloride (0.3 ml/kg, Scandicaine 2% with epinephrine 1:100,000, Septodont, France) to assist hemostasis of the wound area [29].
To facilitate implant placement, a 10-mm-length incision was made on the proximal tibia just below the knee, running up to the tibial diaphysis. A full-thickness flap was lifted exposing the lateral tibial bone for implant placement. Then, receptor beds were prepared using a progressive sequence of drills (lance drill and spiral 1.8 mm drill; Conexao Implant System, Sao Paulo, SP, Brazil) to a final diameter of 2 mm, under profuse sodium chloride irrigation (0.9% Darrow, Rio de Janeiro, Brazil). Subsequently, a titanium implant, 4 mm in length and 2.2 mm in diameter (Conexao Implant System, Sao Paulo, SP, Brazil), was installed in the tibia using both cortical bones (medial and lateral tibial metaphysis) with the aid of an electric motor (BLM 600, Driller Electric Equipment Ltda, Sao Paulo, SP, Brazil). The soft tissues were replaced and sutured in separate layers, using absorbable threads (Poligalactina 910—Vycril 4.0, Ethicon, Johnson Prod., Sao Jose dos Campos, SP, Brazil) and the skin was sutured with monofilament suture (Nylon 4.0, Ethicon, Johnson, Sao Jose dos Campos, SP, Brazil).
In the immediate postoperative period, the animals received a single intramuscular injection of pentabiotic (0.1 ml/kg, Fort Dodge Animal Health Ltd., Campinas, Sao Paulo, Brazil) and a single dose of Sodium Dipyrone (1 ml/kg/day Ariston Chemical and Pharmaceutical Industries Ltda, Sao Paulo, Brazil).
Animal euthanization and analyses
Ten rats per group were euthanized by anesthetic overdose at the end of the experimental periods, i.e., 28 days after implant placement. CTL, non-INT, and INT-0 were sacrificed at day 148. INT-7, INT-14, INT-28, and INT-45 were sacrificed at days 155, 162, 176, and 193, respectively. The vertebrae, femurs, and tibiae (bone-implant interface) from each group were removed and fixed in 10% paraformaldehyde for 72 h.
Bone mineral density evaluation
Bone mineral density (BMD) of lumbar vertebrae and femurs were analyzed by dual-energy X-ray absorptiometry (DXA) in order to evaluate the systemic effects of alendronate in each group. Using a densitometer (Discovery® A SN: 80999; Hologic Inc., Bedford, TX, USA) operating in high-resolution mode, the samples were analyzed employing specific software for small animals (DPX, Small Animal Software Version 1.0, Lunar Radiation Corp., Madison, WI). The DXA accuracy for BMD determination was evaluated by measuring the coefficient of variation after five consecutive measurements of the same sample, and was expressed as a percentage of the mean. The coefficient of variations of 1.8% for the femur and 2.1% for the lumbar vertebrae were obtained. For lumbar vertebrae were measured the whole vertebra (Global) and four independently vertebrae, as follow: L1, L2, L3, and L4. For the femurs, the following areas were measured: Global, R1 (proximal epiphysis), R2 (distal epiphysis), and R3 (diaphyseal).
Evaluation of bone turnover biochemical markers
In the moment of implant installation (T1) and before animal euthanization (T2), blood samples were collected from the rat caudal artery in order to assess bone turnover marker serum concentrations. Markers of bone formation, such as osteocalcin (OCN) and aminoterminal propeptide of procollagen type 1 (P1NP), were measured using a rat OCN enzyme-linked immunosorbent assay (ELISA) kit (OCN; RatMID Osteocalcin®; Immunodiagnostic Systems Inc., Boldon, United Kingdom) and rat P1NP kit (P1PN; Rat Mouse P1NP®; Immunodiagnostic Systems Inc., Boldon, United Kingdom), respectively. C-terminal telopeptide of type I collagen (CTX), a marker of bone resorption, was measured using rat CTX ELISA kit (CTX; RatLaps®; Immunodiagnostic Systems Inc., Boldon, United Kingdom). A urine sample comprising the last 24 h of the experimental period for each group was collected from each animal in metabolic cages. Urinary deoxypyridinoline (DPD) was measured by ELISA kit (Metra Biosystems, Palo Alto, CA, USA). DPD results were corrected for the urinary concentration of creatinine (DPyr/creatinine; nM/mM). All ELISA tests were performed according to the manufacturer instructions.
Sample preparations and histomorphometric procedures
Tissue samples for nondecalcified sections were dehydrated in increasing concentrations of ethanol (60–100%), and were then embedded in light-cured resin (Technovit 7200 VLC®, Heraeus Kultzer GmbH & Co., Wehrheim, Germany). The block samples were cut at the center of the implant diameter with the aid of a cutting-grinding unit (EXAKT Apparatebeau, Hamburg, Germany), as described [30]. A unique section was obtained from each sample and a final thickness of 30 μm was achieved after grinding and polishing. Then, samples were stained with Stevenel blue. Descriptive histologic analyses were performed by a blinded and experienced examiner (LCS). The mean values of both sides of the implant were considered.
Bone histomorphometry
The histological slides were analyzed using a light microscopy (DiaStar, Reichert & Jung products Leica, Germany) and images were captured by a digital camera Leica Microsystems DFC-300-FX (Leica Microsystems, Germany) with a resolution of eight megapixels, coupled to the optical microscope common light. The digital images of histological slides were imported and the histomorphometric analysis was performed using ImageJ analysis software (ImageJ®, Jandel Scientific, San Rafael, CA, USA) in a standardized manner by one blinded and calibrated examiner (MHAV), masked to the original treatment protocol. The results of the linear extension of BIC and BAFO between the threads occupied by bone tissue obtained from the square pixels and pixels, respectively, were converted to percentage values, as described [28, 31]. To evaluate the bone near the implant, two histomorphometric variables were analyzed as stated:
-
1.
The implant linear extension including bone tissue in contact with the implant surface, between the initial three threads in the mesial and distal region (BIC);
-
2.
The area between the threads, occupied by bone tissue in the first three implant threads in the mesial and distal region (BAFO). The values of BIC and BAFO for each group were compared with each other, as described previously [18, 28, 32, 33].
Statistics
Analyses were performed using GraphPad Prism Software 7.0 (GraphPad® Software, Inc., La Jolla, CA, USA). Group measures were expressed as mean ± the standard error of the mean (SEM). The data obtained in each type of comparison were tested using the D’Agostino and Pearson normality test. For BMD, CTX, and BIC analyses, statistical significance was assessed using one-way analysis of variance (ANOVA) followed by post-hoc Tukey’s test for multiple comparisons among groups. For OCN, P1NP, and BAFO analyses, statistical significance was assessed using Kruskal-Wallis followed by post-hoc Dunn test for multiple comparisons among groups. Differences were considered significant at p < 0.05.
Results
Clinical evaluation
Alendronate treatment did not influence implant healing and all implants presented without complication or infection. None of the rats were lost during the experiment. There were no intercurrences in the follow-up periods and the rat’s weight did not change during alendronate treatment or after its discontinuation (data not shown).
Bone mineral density
BMD levels of vertebrae and femurs were measured at the end of the experimental periods. For lumbar vertebrae, the whole vertebra (global, Fig. 2a), and vertebrae L1, L2, L3, and L4 were measured (Supplemental Fig. 1). For the femurs, global sub-regions (Fig. 2b), R1 (proximal epiphysis), R2 (distal epiphysis), and R3 (diaphyseal) areas were also measured (Supplemental Fig. 2). For the CTL group, BMD levels remained relatively steady in all the regions evaluated for femurs and vertebrae. On the other hand, non-INT group demonstrated increased BMD in all the regions evaluated, which was statistically significant compared to the CTL group in the femurs and vertebrae. Alendronate withdrawal did not influence BMD levels that remained unchanged in all groups evaluated compared to the non-INT group, but it was statistically significant compared to CTL group. BMD levels of R2 sub-region in the femurs after 45 days (INT-45) were statistically significantly higher compared to INT-0 group (Supplemental Fig. 2). BMD levels in the L4 vertebrae of INT-14 group was statistically significantly higher compared to INT-0, as well as between INT-45 compared to INT-0 for L3 and L4 vertebrae (Supplemental Fig. 1).
Bone turnover biochemical markers
At the moment of implant installation (T1) and before animal euthanization (T2), blood samples were collected in order to assess bone formation and resorption markers serum concentrations. On T1, the CTL group demonstrated increased concentration of P1NP and OCN compared to all other groups but the differences were statistically significant only between CTL and INT-7 for P1NP and OCN, and between CTL and INT-45 group for P1NP (Fig. 3a, c). CTX and DPD in the treatment groups were decreased compared to the CTL group. However, CTX concentration was statistically significantly lower only between INT-28 group compared to non-INT and INT-0 groups. DPD concentrations were statistically significant lower between CTL group compared to non-INT, INT-0, and INT-28 (Fig. 3e, g).
On T2, increased levels of P1NP in CTL and INT-7 groups were statistically significant compared to INT-14, INT-28, and INT-45 groups, which demonstrated lower levels of P1NP after alendronate withdrawal (Fig. 3b). Decreased concentration of OCN was observed in all the treatment groups compared to the CTL group, but the differences were statistically significant only between CTL and non-INT group and between CTL and INT-45 group (Fig. 3d). In relation to the levels of bone resorption markers, CTX concentration was significantly decreased in the non-INT and in the INT-45 groups compared to the CTL, INT-0, and INT-14 group (Fig. 3f). DPD concentration was lower in all the treatment groups compared to the CTL group, but differences were found between INT-28 and INT-45 groups when compared to the CTL group (Fig. 3h).
Histomorphometric analysis
BIC
The linear extension of bone-to-implant contact was evaluated among groups. Animals from CTL group showed more bone-to-implant contact between the implant threads compared to all the other groups. CTL group demonstrated statistical differences when compared to the all the other groups. The lowest bone fill between the implant threads was observed in the INT-45 group (Fig. 4a).
BAFO
Bone area fraction occupancy between implant threads was evaluated for all groups. The average percentages of bone area between the initial three mesial and distal threads of the implants were recorded. Non-INT and INT-0 group presented higher percentage of BAFO compared to the INT-45 group, which was statistically significant. No differences were found among CTL and alendronate treatment groups (Fig. 4b).
Histologic descriptive analysis
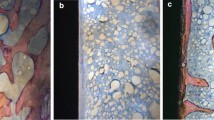
The effect of alendronate treatment and drug discontinuation on implant osseointegration was also assessed by descriptive histological analysis (Fig. 5a–g). All implants installed in the different groups showed successful implant osseointegration due to the observed direct contact, partial or total, between bone and implant. In the CTL group (Fig. 5), the bone tissue around osseointegrated implants showed normal and healthy characteristics with compact bone, presenting osteons with concentric lamellar organization formed in the area around the implant threads. Concentric lamellar forming haversian channels in the newly formed bone were also observed in the CTL group. Interestingly, higher percentage of bone-to-implant contact was observed in the CTL group compared to the groups that received alendronate (Fig. 5a).
In the groups treated with alendronate (non-INT), and after its discontinuation (INT-0, INT-7, INT-14, INT-28, and INT-45), the bone around the implant threads showed the presence of amorphous matrix and apparently little calcified tissue deposited between the implant threads. Loss of continuity between the resident bone tissue and the newly formed bone was also observed in these groups, suggesting the fragility of the bone tissue, which could result in a higher rate of long-term failure of these implants. It was not possible to observe the presence of collagen fibers organized in lamellae that is characteristic of secondary bone formation. Osteocytic lacunae were absent in the bone formed around the implant threads (Figs. 5b–g).
Discussion
This study examined differences in the BMD, biochemical markers of bone formation and resorption, as well as in the percentage of BIC and BAFO in response to the discontinuation of alendronate in rats. Our data showed that BMD was significantly increased in animals under alendronate treatment and that its discontinuation did not reversed these findings. Accordingly, bone turnover markers tend to not be re-established in rats treated with alendronate. Specifically, BMD remained significantly higher in the trabecular bone of the femurs and vertebrae in all groups evaluated compared to the CTL rats. Bone formation rates (P1NP and OCN) remained lower compared to the CTL group during implant placement (T1) and before animal sacrifice (T2). Biological markers of bone resorption (CTX and DPD) also remained lower especially in the INT-45 group compared to CTL rats in T2. Moreover, BIC was lower in all groups on alendronate especially after 73 days of drug discontinuation (INT-45 group) compared to the CTL group, which might suggest the impairment of bone healing around osseointegrated implants.
The findings of this current investigation demonstrated that BPs administration increased BMD compared to the CTL animals. This expected result occurs because BPs inhibits bone resorption, allowing higher matrix deposition and mineralization, which reflects the increased bone density in the long bones and vertebrae [34]. These findings are consistent with our previous studies [18,19,20, 22] in normal or in ovariectomized rats, in which the increase in BMD in femurs and vertebrae after treatment with alendronate could be observed. Some clinical and pre-clinical studies [35,36,37,38] point to decreased BMD after antiresorptive discontinuation. Our findings did not reveal any change in the BMD values in both femurs and vertebrae even after 73 days of alendronate withdrawal. This fact could be explained due to the short period of follow-up in these animals, which closely resemble findings from previous reports that showed the maintenance of BMD values in patients after BPs removal [26, 39,40,41]. These variations in BMD indices after BP discontinuation could reflect differences in BP biodistribution, mode of administration, and bone mineral affinity.
In order to evaluate bone turnover markers after alendronate withdrawal, P1NP, OCN, CTX, and DMD were measured during implant installation (T1) and before animal’s sacrifice (T2). Bone formation markers, P1NP and OCN, had their levels significantly decreased in T1 and T2 for all groups on alendronate and after its interruption compared to the CTL group. Significant differences were found for both markers in T1 and T2 especially for INT-45 group compared to the CTL group. Related to P1NP, which is a type 1 collagen marker, the results of the present study were similar to the OCN marker where BPs withdrawal did not increase its values in the later groups, which corroborates previous report [42, 43]. These results suggest that BPs has an effect on osteoblasts and in the process of bone remodeling [44]. Since OCN is released during the bone mineralization process, the lower values for OCN and P1NP in T1 and T2 might explain the deleterious effect of alendronate therapy in the histomorphometric and histomorphological findings even after its discontinuation [45, 46].
Relative to the markers of bone resorption, CTX values were significantly lower in the non-INT group when compared to the CTL group during T1 and T2. These findings indicate the effectiveness of alendronate in the inhibition of bone resorption. After drug discontinuation, CTX values were significantly higher in the INT-28 group followed by a decrease in the CTX values in the INT-45 group during T1. In T2, similar results were observed but after 73 days of drug discontinuation (INT-45 group), significantly lower CTX values compared to the CTL group and similar with the non-INT group were noted. These findings suggest that alendronate withdrawal did not influence bone resorption markers, which result in the maintenance of osteoclastic activity inhibition, which are in agreement with previous studies [47, 48] where CTX values remained unaltered even after 3 years of BPs discontinuation. DPD values in the non-INT group were statistically significantly lower compared to the CTL group. Drug discontinuation did not influence the levels of DPD that was sustained after its removal. These findings corroborate previous studies [41, 49] where DPD values remained unaltered after BPs discontinuation.
Our findings that BIC in animals on alendronate (non-INT) and after drug withdrawal remained lower compared to the CTL animals differ from the findings of previous study [50]. Duarte et al. [50] evaluated whether alendronate influences bone healing around endosseous implants inserted in healthy and ovariectomized (OVX) rats and also evaluated the effect of alendronate discontinuation in these animals. The results showed that alendronate therapy immediately after OVX inhibited bone loss, and that BIC and bone area presented with similar values to the sham-operated rats. Moreover, alendronate discontinuation revealed that BIC, bone area, and bone density in cancellous and cortical bone were similar to the control group. These contradictory results could potentially be attributed due to the high alendronate dose used (5 mg/kg/w), time intervals (four times/week), and period of evaluation (40 days of treatment followed by 40 days of discontinuation). It is important to note that our study used 1 mg/kg once per week for 120 days, which might have an impact on the BIC values around titanium implants. Our results parallel observations made by Mardas et al. [51], in which alendronate administration impaired bone healing around osseointegrated implants. Similar findings were encountered when alendronate gel was applied to the titanium implant and installed in the rabbit tibia [25]. Taken together, the results of the present study and others [25, 51] could be explained because alendronate has the capacity to exert their effect on osteoblasts, altering their physiology and diminishing bone formation by inhibiting their differentiation and maturation [44]. This may explain the detrimental effects of alendronate on bone remodeling for the compromised osseointegration results found in the present investigation.
BPs-suppressed remodeling allows microdamage to accumulate in the bone tissue and this fact could increase bone microdamage over time so long as bone remodeling has being suppressed [52]. According to Berglundh et al. [53], the placement of an implant in the receptor bed lead to a sequence of healing events involving bone micro-fractures and bone necrosis (due to damage caused by the drilling during implant bed preparation) followed by a subsequent resorption of the damaged bone with concomitant bone neo-formation. Due to its mechanism of action, alendronate administration strongly suppresses the osteoclast activity, which could harm the bone remodeling impairing the osseointegration process, and triggering the development of osteonecrosis after surgery for implant placement [54]. This fact could also explain the lower percentage of BIC in animals treated with alendronate and in animals after alendronate withdrawal compared to the CTL group.
The suppressing of bone remodeling that occurs after ALD treatment might impair bone-to-implant contact around osseointegrated implants [51]. Importantly, previous studies showed that the prolonged use of BPs, especially at high doses, has been clinically associated with osteonecrosis of the jaws (ONJ) after implant surgery, and increased rate of implant failures [55,56,57,58]. Periodontal and periapical diseases, dental extraction, and surgery for dental implant placement play a pivotal role as triggers for BRONJ development [3, 59,60,61]. Thus, clinicians should be aware in relation to the incorporation of BPs in the bone mineral before implant installation because BPs can exert their anti-osteoclastic effects long after their administration [2]. In this regard, from a practical standpoint, clinicians should avoid dental implant placement in patients on BPs therapy to prevent ONJ development.
An interesting caveat that our studies did not address was whether a control group with geranylgeraniol would have excluded nonspecific factors. This fact should be evaluated in further pre-clinical studies to strengthen the findings of the current investigation. Another important consideration that should be mentioned is the animal model utilized. The chosen rat tibiae model for implant installation does not resemble the situation at mandible and maxilla. With the tibia being surrounded by thick and well-perfused muscles, it does not mimic to the conditions at the jaws. Additionally, experimental rat model possess some limitations compared to the humans, such as faster skeletal change and maturity, faster bone turnover, and different healing behavior, i.e., rat synthesizes ascorbic acid, which is important for the collagen synthesis. However, rats were selected as the experimental model because they are easy to handle, due to low maintenance care, and because they are the smallest animal that can accept implants in the long bones in the proximal region of the tibia, far from the growth plate. Finally, further studies utilizing different animal models, such as the minipig with commercially available implants installed in the maxilla and/or mandible, should be performed before definitive conclusions can be drawn.
In summary, we report that BMD values from femurs and vertebrae were significantly increased in rats on alendronate when compared to the CTL group, which were maintained after drug withdrawal. Moreover, alendronate therapy decreased bone turnover markers and impaired bone quality and quantity around dental implants, and that its discontinuation did not reversed these findings. Indeed, to the best of the author’s knowledge, this is the first animal study to illustrate and quantify such findings.
References
Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ (1998) Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 13:581–589
Kimmel DB (2007) Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res 86:1022–1033
Soundia A, Hadaya D, Esfandi N, de Molon RS, Bezouglaia O, Dry SM, Pirih FQ, Aghaloo T, Tetradis S (2016) Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease. Bone 90:133–141
Tella SH, Gallagher JC (2014) Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 142:155–170
Tolia M, Zygogianni A, Kouvaris JR, Meristoudis C, Margari N, Karakitsos P, Kokakis I, Kardamakis D, Papadimitriou C, Mystakidou K, Tsoukalas N, Kyrgias G, Armonis B, Filippiadis DK, Kelekis AD, Kelekis N, Kouloulias V (2014) The key role of bisphosphonates in the supportive care of cancer patients. Anticancer Res 34:23–37
Russell G, Mueller G, Shipman C, Croucher P (2001) Clinical disorders of bone resorption. Novartis Found Symp 232:251–267 discussion 267-271
Wang HL, Weber D, McCauley LK (2007) Effect of long-term oral bisphosphonates on implant wound healing: literature review and a case report. J Periodontol 78:584–594
Abraham A, Cohen A, Shane E (2013) Premenopausal bone health: osteoporosis in premenopausal women. Clin Obstet Gynecol 56:722–729
Vohra F, Al-Rifaiy MQ, Almas K, Javed F (2014) Efficacy of systemic bisphosphonate delivery on osseointegration of implants under osteoporotic conditions: lessons from animal studies. Arch Oral Biol 59:912–920
Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, Compston JE, Drake MT, Edwards BJ, Favus MJ, Greenspan SL, McKinney R Jr, Pignolo RJ, Sellmeyer DE (2016) Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 31:16–35
Shirota T, Tashiro M, Ohno K, Yamaguchi A (2003) Effect of intermittent parathyroid hormone (1-34) treatment on the bone response after placement of titanium implants into the tibia of ovariectomized rats. J Oral Maxillofac Surg 61:471–480
Tokugawa Y, Shirota T, Ohno K, Yamaguchi A (2003) Effects of bisphosphonate on bone reaction after placement of titanium implants in tibiae of ovariectomized rats. Int J Oral Maxillofac Implants 18:66–74
Yamazaki M, Shirota T, Tokugawa Y, Motohashi M, Ohno K, Michi K, Yamaguchi A (1999) Bone reactions to titanium screw implants in ovariectomized animals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 87:411–418
Pan J, Shirota T, Ohno K, Michi K (2000) Effect of ovariectomy on bone remodeling adjacent to hydroxyapatite-coated implants in the tibia of mature rats. J Oral Maxillofac Surg 58:877–882
Carvalho MD, Benatti BB, Cesar-Neto JB, Nociti FH, Jr., da Rocha Nogueira Filho G, Casati MZ, Sallum EA (2006) Effect of cigarette smoke inhalation and estrogen deficiency on bone healing around titanium implants: a histometric study in rats. J Periodontol 77:599–605
Duarte PM, Cesar Neto JB, Goncalves PF, Sallum EA, Nociti j F (2003) Estrogen deficiency affects bone healing around titanium implants: a histometric study in rats. Implant Dent 12:340–346
Nociti FH Jr, Sallum AW, Sallum EA, Duarte PM (2002) Effect of estrogen replacement and calcitonin therapies on bone around titanium implants placed in ovariectomized rats: a histometric study. Int J Oral Maxillofac Implants 17:786–792
Giro G, Coelho PG, Pereira RM, Jorgetti V, Marcantonio E Jr, Orrico SR (2011) The effect of oestrogen and alendronate therapies on postmenopausal bone loss around osseointegrated titanium implants. Clin Oral Implants Res 22:259–264
Giro G, Goncalves D, Sakakura CE, Pereira RM, Marcantonio Junior E, Orrico SR (2008) Influence of estrogen deficiency and its treatment with alendronate and estrogen on bone density around osseointegrated implants: radiographic study in female rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:162–167
Verzola MH, Frizzera F, de Oliveira GJ, Pereira RM, Rodrigues-Filho UP, Nonaka KO, Orrico SR (2015) Effects of the long-term administration of alendronate on the mechanical properties of the basal bone and on osseointegration. Clin Oral Implants Res 26:1466–1475
Viera-Negron YE, Ruan WH, Winger JN, Hou X, Sharawy MM, Borke JL (2008) Effect of ovariectomy and alendronate on implant osseointegration in rat maxillary bone. J Oral Implantol 34:76–82
Giro G, Sakakura CE, Goncalves D, Pereira RM, Marcantonio E Jr, Orrico SR (2007) Effect of 17beta-estradiol and alendronate on the removal torque of osseointegrated titanium implants in ovariectomized rats. J Periodontol 78:1316–1321
Chacon GE, Stine EA, Larsen PE, Beck FM, McGlumphy EA (2006) Effect of alendronate on endosseous implant integration: an in vivo study in rabbits. J Oral Maxillofac Surg 64:1005–1009
Narai S, Nagahata S (2003) Effects of alendronate on the removal torque of implants in rats with induced osteoporosis. Int J Oral Maxillofac Implants 18:218–223
Guimaraes MB, Bueno RS, Blaya MB, Shinkai RS, Marques LM (2015) Influence of the local application of sodium alendronate gel on osseointegration of titanium implants. Int J Oral Maxillofac Surg 44:1423–1429
Fuchs RK, Phipps RJ, Burr DB (2008) Recovery of trabecular and cortical bone turnover after discontinuation of risedronate and alendronate therapy in ovariectomized rats. J Bone Miner Res 23:1689–1697
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2012) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthr Cartil 20:256–260
de Molon RS, Morais-Camilo JA, Verzola MH, Faeda RS, Pepato MT, Marcantonio E Jr (2013) Impact of diabetes mellitus and metabolic control on bone healing around osseointegrated implants: removal torque and histomorphometric analysis in rats. Clin Oral Implants Res 24:831–837
Dos Santos PL, de Molon RS, Queiroz TP, Okamoto R, de Souza Faloni AP, Gulinelli JL, Luvizuto ER, Garcia IR Jr (2016) Evaluation of bone substitutes for treatment of peri-implant bone defects: biomechanical, histological, and immunohistochemical analyses in the rabbit tibia. J Periodontal Implant Sci 46:176–196
Kim YJ, de Molon RR, Horiguti FR, Contador GP, Coelho MA, Mascarenhas VI, de Souza Faloni AP, Cirelli JA, Sendyk WR (2018) Vertical bone augmentation using Deproteinized bovine bone mineral, absorbable collagen sponge, and recombinant human bone morphogenetic Protein-2: an in vivo study in rabbits. Int J Oral Maxillofac Implants, 33, 512, 522
Queiroz TP, de Molon RS, Souza FA, Margonar R, Thomazini AH, Guastaldi AC, Hochuli-Vieira E (2017) In vivo evaluation of cp Ti implants with modified surfaces by laser beam with and without hydroxyapatite chemical deposition and without and with thermal treatment: topographic characterization and histomorphometric analysis in rabbits. Clin Oral Investig 21:685–699
Vidigal GM Jr, Aragones LC, Campos A Jr, Groisman M (1999) Histomorphometric analyses of hydroxyapatite-coated and uncoated titanium dental implants in rabbit cortical bone. Implant Dent 8:295–302
Zagury R, Harari ND, Conz MB, Soares Gde A, Vidigal GM Jr (2007) Histomorphometric analyses of bone interface with titanium-aluminum-vanadium and hydroxyapatite-coated implants by biomimetic process. Implant Dent 16:290–296
Russell RG (2011) Bisphosphonates: the first 40 years. Bone 49:2–19
Yildiz A, Esen E, Kurkcu M, Damlar I, Daglioglu K, Akova T (2010) Effect of zoledronic acid on osseointegration of titanium implants: an experimental study in an ovariectomized rabbit model. J Oral Maxillofac Surg 68:515–523
Silva AG, Vieira JG, Kunii IS, Lana JM, Lazaretti-Castro M (2011) The effects of discontinuing long term alendronate therapy in a clinical practice setting. Arq Bras Endocrinol Metabol 55:272–278
Wasnich RD, Bagger YZ, Hosking DJ, McClung MR, Wu M, Mantz AM, Yates JJ, Ross PD, Alexandersen P, Ravn P, Christiansen C, Santora AC, 2nd, Early Postmenopausal Intervention Cohort Study G (2004) Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause 11:622–630
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, Cummings SR, Hue TF, Lippuner K, Lakatos P, Leung PC, Man Z, Martinez RL, Tan M, Ruzycky ME, Su G, Eastell R (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27:243–254
Black DM, Reid IR, Cauley JA, Cosman F, Leung PC, Lakatos P, Lippuner K, Cummings SR, Hue TF, Mukhopadhyay A, Tan M, Aftring RP, Eastell R (2015) The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 30:934–944
Shahnazari M, Yao W, Wang B, Panganiban B, Ritchie RO, Hagar Y, Lane NE (2011) Differential maintenance of cortical and cancellous bone strength following discontinuation of bone-active agents. J Bone Miner Res 26:569–581
Bagger YZ, Tanko LB, Alexandersen P, Ravn P, Christiansen C (2003) Alendronate has a residual effect on bone mass in postmenopausal Danish women up to 7 years after treatment withdrawal. Bone 33:301–307
Grey A, Bolland M, Wattie D, Horne A, Gamble G, Reid IR (2010) Prolonged antiresorptive activity of zoledronate: a randomized controlled trial. J Bone Miner Res 25:2251–2255
Manzano-Moreno FJ, Ramos-Torrecillas J, De Luna-Bertos E, Reyes-Botella C, Ruiz C, Garcia-Martinez O (2015) Nitrogen-containing bisphosphonates modulate the antigenic profile and inhibit the maturation and biomineralization potential of osteoblast-like cells. Clin Oral Investig 19:895–902
Chen JT, Hosoda K, Hasumi K, Ogata E, Shiraki M (1996) Serum N-terminal osteocalcin is a good indicator for estimating responders to hormone replacement therapy in postmenopausal women. J Bone Miner Res 11:1784–1792
Wolf G (1996) Function of the bone protein osteocalcin: definitive evidence. Nutr Rev 54:332–333
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, Group FR (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the fracture intervention trial long-term extension (FLEX): a randomized trial. JAMA 296:2927–2938
Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, Black DM, Fracture Intervention Trial Long-Term Extension Research G (2004) Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the fracture intervention trial long-term extension. J Bone Miner Res 19:1259–1269
Tamura Y, Miyakoshi N, Itoi E, Abe T, Kudo T, Tsuchida T, Kasukawa Y, Sato K (2001) Long-term effects of withdrawal of bisphosphonate incadronate disodium (YM175) on bone mineral density, mass, structure, and turnover in the lumbar vertebrae of ovariectomized rats. J Bone Miner Res 16:541–549
Duarte PM, de Vasconcelos Gurgel BC, Sallum AW, Filho GR, Sallum EA, Nociti FH Jr (2005) Alendronate therapy may be effective in the prevention of bone loss around titanium implants inserted in estrogen-deficient rats. J Periodontol 76:107–114
Mardas N, Schwarz F, Petrie A, Hakimi AR, Donos N (2011) The effect of SLActive surface in guided bone formation in osteoporotic-like conditions. Clin Oral Implants Res 22:406–415
Komatsubara S, Mori S, Mashiba T, Li J, Nonaka K, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H (2004) Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res 19:999–1005
Berglundh T, Abrahamsson I, Lang NP, Lindhe J (2003) De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res 14:251–262
Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19:733–759
Yip JK, Borrell LN, Cho SC, Francisco H, Tarnow DP (2012) Association between oral bisphosphonate use and dental implant failure among middle-aged women. J Clin Periodontol 39:408–414
Bedogni A, Bettini G, Totola A, Saia G, Nocini PF (2010) Oral bisphosphonate-associated osteonecrosis of the jaw after implant surgery: a case report and literature review. J Oral Maxillofac Surg 68:1662–1666
Starck WJ, Epker BN (1995) Failure of osseointegrated dental implants after diphosphonate therapy for osteoporosis: a case report. Int J Oral Maxillofac Implants 10:74–78
Yuan K, Chen KC, Chan YJ, Tsai CC, Chen HH, Shih CC (2012) Dental implant failure associated with bacterial infection and long-term bisphosphonate usage: a case report. Implant Dent 21:3–7
de Molon RS, Cheong S, Bezouglaia O, Dry SM, Pirih F, Cirelli JA, Aghaloo TL, Tetradis S (2014) Spontaneous osteonecrosis of the jaws in the maxilla of mice on antiresorptive treatment: a novel ONJ mouse model. Bone 68:11–19
de Molon RS, Hsu C, Bezouglaia O, Dry SM, Pirih FQ, Soundia A, Cunha FQ, Cirelli JA, Aghaloo TL, Tetradis S (2016) Rheumatoid arthritis exacerbates the severity of osteonecrosis of the jaws (ONJ) in mice. A randomized, prospective, controlled animal study. J Bone Miner Res 31:1596–1607
de Molon RS, Shimamoto H, Bezouglaia O, Pirih FQ, Dry SM, Kostenuik P, Boyce RW, Dwyer D, Aghaloo TL, Tetradis S (2015) OPG-fc but not Zoledronic acid discontinuation reverses osteonecrosis of the jaws (ONJ) in mice. J Bone Miner Res 30:1627–1640
Acknowledgements
This work was supported by grant support from the State of Sao Paulo Research Foundation FAPESP (#2012/16939-1).
Funding
This work was supported by grant support from the State of Sao Paulo Research Foundation FAPESP (#2012/16939-1).
Author information
Authors and Affiliations
Contributions
Study conception and design: FF, MHAV, RSM, GJPLO, GG, LCS, RMRP, ST, JAC, and SRPO. Data acquisition: FF, MHAV, RSM, GJPLO, GG, RMRP, ST, JAC, and SRPO. Data analysis and interpretation: FF, MHAV, RSM, GJPLO, GG, LCS, RMRP, ST, JAC, and SRPO. Drafting of manuscript: RSM, ST, and SRPO. Edition of manuscript: FF, MHAV, RSM, GJPLO, GG, LCS, RMRP, ST, JAC, and SRPO. All authors were involved in revising the paper critically for important intellectual content, and all authors approved the final version to be published. FF, MHAV, and SRPO had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Frizzera, F., Verzola, M.H.A., de Molon, R.S. et al. Evaluation of bone turnover after bisphosphonate withdrawal and its influence on implant osseointegration: an in vivo study in rats. Clin Oral Invest 23, 1733–1744 (2019). https://doi.org/10.1007/s00784-018-2612-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2612-x