Abstract
Objectives
Strong expression of survivin is associated with worse survival in many different tumours, and in cell culture, a correlation between radiation resistance and survivin expression can be seen. The potential of survivin expression as a prognostic/predictive marker or therapeutic target has not been examined in head and neck squamous cell carcinomas (HNSCC) yet.
Material and methods
Retrospective study of 452 tissue samples and clinical data from patients with squamous cell carcinomas of the larynx/hypopharynx (LSCC), oral cavity (OSCC) and oropharynx (OPSCC) treated in the University Medical Centre Hamburg-Eppendorf between 2002 and 2006. The expression patterns were detected by tissue microarray technique and correlated with clinical parameters (sex, age, tumour location, TNM 7th edition, grading, recurrence-free and overall survival).
Results
222 OSCC, 126 OPSCC and 105 LSCC tumours of 118 females and 335 males with a mean follow-up of 41.3 months were examined. Survivin expression correlates with pN, cM, pT and overall survival.
Conclusion and clinical relevance
The potential of survivin as a prognostic/predictive marker is very high. The findings have to be confirmed in a larger cohort of HNSCC esp. in those tumours treated primarily with radio/radiochemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the most common cancers, with a global incidence of 500,000 cases per year, and is the sixth most common malignant tumour worldwide [1]. Despite significant advancements in diagnosis, disease management and novel treatment strategies to improve quality of life (QoL) in patients, the 5-year survival rate for HNSCC has not improved appreciably over the last decade [2,3,4,5,6,7]. The treatment of HNSCC comprises multiple-modality therapy with cytostatic drugs and radiotherapy, and is often combined with sophisticated surgery. However, radio- and/or chemotherapy resistance and tumour recurrences are key clinical issues in the management of HNSCC [6]. Therefore, identifying predictive elements of treatment response is critically necessary. Intriguingly, survivin is expressed in embryonic as well as foetal tissues, but is undetectable in normal adult tissues [8]. Previous studies have indicated survivin expression and its evolving functional complexity in head and neck cancer carcinogenesis [9, 10]. Survivin overexpression has been noted in a wide range of clinical cancers including bladder [11], breast [12], colorectal [13], oesophageal [14], gastric [15], lung [16], nasopharyngeal [17], pancreatic [18], prostatic [19], ovarian [20], renal [21], skin [22] and haematological cancers [23]. The induction of a natural antisense of survivin, effector cell protease receptor-1 (EPR-1), in a human colon cancer cell line resulted in the downregulation of survivin expression, with a similar decrease in cell proliferation, an increase in apoptosis and an increase in the sensitivity to anticancer agents [24]. Higher expression of survivin as a critical factor for radioresistance in HNSCC cell lines has also been demonstrated [25]. Thus, for HNSCC, the identification of biological prognostic markers indicating an increased risk of treatment failure could prove beneficial in the treatment modalities as well as the intensity of post-therapeutic follow-up [9].
Survivin, also known as baculoviral inhibitor of apoptosis protein repeat-containing 5 (BIRC5) and a member of the inhibitor of apoptosis protein (IAP) family, is a 16.5 kDa protein with a single baculovirus IAP repeat (BIR) and no really interesting new gene (RING) finger domain [5, 26]. It is the smallest member of the IAP family that inhibits caspases and blocks cell death, is highly expressed in most cancers and is associated with a poor clinical outcome. At the molecular level, survivin could be a multifunctional suitable protein for targeted therapy, not only playing a vital role in cell division but also inhibiting apoptosis (antiapoptotic function) and enhancing angiogenesis [1, 27, 28].
The prognostic value of survivin for many human cancers is apparent: it is correlated with an unfavourable clinical outcome. These findings suggest that survivin expression has the potential for use as a predictive biomarker in identifying cancers [29]. The high expression of survivin in cancer cells, with little expression in most normal tissues, makes survivin a potential anticancer molecular therapeutic target with multiple anticancer activities. The purpose of this study was to show the expression of survivin in a large cohort of HNSCC. We hypothesise that the expression of survivin has a high potential as prognostic and /or predictive marker in the treatment of HNSCC.
The specific aims of the study were (1) to detect the expression of survivin in HNSCC and (2) to correlate the findings with clinical parameters (age, sex, TNM, grading and tumour location) and survival data (RFS/OS).
Material and methods
Study design and samples
To address the research purpose, we designed a retrospective analyses of all patients treated with an HNSCC in the University Medical Centre Hamburg, Department for Head and Neck Surgery and Oncology from the years 2002 to 2006. Inclusion criteria were a primary tumour treatment either by surgery or radio (chemo) therapy alone or treatment in a combined surgical and risk based adjuvant setting. Patients not being suitable for therapy or presenting with recurrent disease were not included in the study.
The study was approved through the local ethics commission.
Variables
Cytoplasmic staining was evaluated by staining intensity (0, 1+, 2+, 3+) and the fraction of positive tumour cells was scored for each tissue spot.
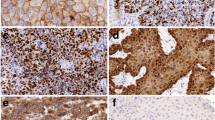
An overall score was derived from these two parameters. Negative scores had a staining intensity of 0 and 1+ in ≤ 10% of tumour cells; weak scores had a staining intensity of 1+ in > 10% and ≤ 70% of tumour cells or a staining intensity of 2+ in ≤ 30% of tumour cells; moderate scores had a staining intensity of 1+ in > 70% of tumour cells, staining intensity of 2+ in > 30% and ≤ 70% of tumour cells or a staining intensity of 3+ in ≤ 30% of tumour cells; and strong scores had a staining intensity of 2+ in > 70% of tumour cells or a staining intensity of 3+ in > 30% of tumour cells. Examples of IHC stainings can be seen in Fig. 1. Additionally, for dichotomic analysis, all negative and weak cases were grouped as low-expressing tumours, whereas moderate and strong cases were grouped as high-expressing tumours.
Immunohistochemical (IHC) staining of head and neck squamous cell carcinoma (HNSCC) tissue. a Sample with negative staining intensity for survivin (score 0). b Sample with a weak staining intensity for survivin (score 1). c Sample with a medium staining for survivin (score 2). d Sample with a strong staining for survivin (score 3)
Clinical parameters
As predictor variable, the TNM Classification of Malignant Tumours 7th edition UICC was used. We used the pathological staging (pT/pN/pM) for surgically resected tumours and the clinical staging (cT/cN/cM) for those treated primarily by R(C)T.
Staging was obtained by neck ultrasound, neck MRI, chest, abdominal CT and panendoscopy. Survival data was recorded as overall survival (OS) and recurrence-free survival (RFS). Recurrence-free survival was defined as time till tumour relapse after initial therapy (operation, radio- and/or chemotherapy) later than 3 months after end of therapy. Overall survival was defined as time till death or end of follow-up. Patients were censored for RFS without evidence of tumour recurrence at last follow-up or censored for OS at last documented follow-up. Tumour localisations were described according to the typical HNSCC subtypes oral cavity, oropharynx and larynx.
Patient data
The study retrospectively evaluated 453 cases of HNSCC including 222 oral (49%), 126 pharyngeal (27.8%) and 105 laryngeal (23.2%) tumours. The tumours were obtained from 118 females (26.1%) and 335 males (73.9%). The cohort consisted of 103 pT1 (23.1%), 133 pT2 (29.9%), 79 pT3 (17.8%) and 130 pT4 tumours (29.2%). Of these cases, 219 were in pN 0-stage (49.2%), 68 were pN1 (15.3%), 134 were pN2 (30.1%) and 24 were pN3 (5.6%). Thirty cases showed distant metastatic disease (pM1/cM1; 6.8%), whereas all other patients were defined as cM0 status. Follow-up data were available for 441 patients, ranging from 1 to 306 months (mean = 41.3 months).
Detailed clinical and pathological data is shown in Table 1 in the Results section.
Tissue microarray construction
All samples were stored and analysed in the Department of Pathology in the University Medical Centre. Tissue samples were fixed in buffered 4% formalin, embedded in paraffin and used for tissue microarray (TMA) construction. Haematoxylin-eosin stained sections were made from each selected primary tumour block to define the representative tumour region. One tissue cylinder (0.6 mm in diameter) was then punched from each tumour of that region of the block, using of a homemade semi-automated tissue arrayer [30]. The control samples included larynx (n = 11), hypopharynx (n = 3), tonsil (n = 3), tongue (n = 2), epiglottis (n = 1), lymph node (n = 4), lung (n = 4), heart muscle (n = 4), endometrium (n = 2), skin (n = 2), skeletal muscle (n = 2), colon mucosa (n = 2), stomach (n = 2), prostate (n = 2), liver (n = 2) and kidney (n = 2). Three micrometre sections were made using the Paraffin Sectioning Aid System (Instrumentics, Hackensack, NJ) and used for immunohistochemical (IHC) staining.
Immunohistochemistry
Freshly cut 3-μm TMA sections were analysed on the same day in a single experiment. Survivin (Abcam, rabbit monoclonal, 1:900) after preoxidase blocking with H2O2 (DAKO S2023) for 10 min. High-temperature pretreatment of slides was carried out in an autoclave with a citrate buffer at pH 7.8 for 5 min. The Envision™ system (DAKO 5007) was used to visualise the IHC staining.
Statistical analysis
For the statistical analysis, JMP 11.0 software (SAS institute Inc., Cary, NC, USA) was used. All p values were 2-sided and p values < 0.05 were considered significant. To study the relationship between survivin expression and clinicopathological parameters, a contingency table analysis and Chi-square test (likelihood) was used. Analysis on recurrence-free and overall survival rates were evaluated by using the Kaplan–Meier method and compared via log-rank test.
Results
Survivin expression in HNSCC
Evaluation of 453 cases of primary HNSCC (oral cavity (OSCC), oropharynx (OPCC) and larynx (LSCC)) revealed that 299 tissue samples were interpretable for IHC cytoplasmic and/or membranous survivin expression (66%). The remaining 154 samples were noninformative. The decrease in sample size was due to absence of tissue on the TMA or a lack of unequivocal tumour cells in the arrayed samples. Table 1 represents an overview of clinical and pathological data.
Positive survivin expression was found in 209 of 299 cases of HNSCC (69.90%). Staining was negative in 90 (30.10%) cases, weak in 168 (56.19%), moderate in 40 (13.38%) and strong in 1 (0.33%) case. A homogeneous staining pattern was seen in all specimens (nuclear staining with percentage of stained cells > 50% for positively stained specimen). Survivin was significantly overexpressed in tumour tissue whereas no survivin expression was detected in normal tissue (control).
Representative images showing survivin expression in samples of HNSCC are shown in Fig. 1.
Significant differences in expression could not be detected between the HNSCC subsites OSCC, OPSC and LSCC.
Regarding the results of the whole series of HNSCC cases examined, statistical analysis revealed significant correlations between survivin expression and pT stage (pT1, pT2 versus pT3, pT4) (p = 0.018) and pN stage (pN0/pN1 versus pN2, pN3) (p = 0.030).
A strong correlation between pM stage (p = 0.0266) and survivin expression was also found.
Influence on overall and recurrence-free survival rates
The data from all cases, including all subsites of the head and neck, showed that recurrence-free survival was independent from survivin expression levels.
Survivin expression in HNSCC (at all tumour subsites) was significantly correlated with overall survival (p = 0.0068) (Fig. 2). In the HNSCC subsites (oral cavity, oropharynx, larynx), no correlations between overall survival and recurrence-free survival were noticed.
Correlations in HNSCC subsites
There was a significant correlation between survivin expression (survivin pos. versus survivin neg.) and the pM status (p = 0.025) in HNSCCs of the oral cavity.
In the oropharyngeal subsite, the tumours showed a higher survivin expression in locally advanced tumours (pT3/pT4 versus pT1/pT2) with a significance level of p = 0.018.
Most of the clinicopathological correlations were found within the subsite of LSCCs. In these tumours, statistically significant correlations could be found between pN staging (pN 0–3) and the survivin expression patterns (moderate, weak and negative expression) (p = 0.0163). Furthermore, there was a highly significant correlation between pN positive LSCC and survivin expression patterns (p = 0.008). Similar to the results over all HNSCC subsites, the pT status correlated with positive/negative survivin expression in LSCCs when comparing locally advanced tumours (pT3/pT4) with local tumours (pT1/pT2) (p = 0.030).
Discussion
Head and neck squamous cell carcinoma is a devastating disease, affecting 500,000 new patients per year globally and is the sixth most common malignancy worldwide, accounting for more than 90% of head and neck cancers [31]. It involves the upper aerodigestive tract and can affect the structure and function of organs involved in voice, speech, taste, smell and hearing, as well as vital structures necessary for survival [32, 33]. HNSCC constitutes a noteworthy growing public health problem and is a major cause of mortality [34].
Despite modern disease management with strategically designed clinical trials, diagnostic and innovative approaches, the 5-year survival rate for HNSCC has not improved significantly over the past decades [35]. Furthermore, loco-regional relapse following therapy is a major cause of death [7]. The prognostication of patients with HNSCC is extremely variable but essential for reducing deaths due to head and neck cancer [1]. Therefore, validating distinct biological prognostic markers that signal an increased risk of treatment failure, which will have a significant impact on the treatment modalities as well as the intensity of post-treatment follow-up, should be a high priority for improving quality of life in HNSCC patients.
Interestingly, survivin is strongly expressed in HNSCC and its multifaceted oncological role in various cellular pathways of different cancers, including head and neck cancer, has placed this IAP agent as a safe and ideal target for oncological research [10, 35]. Herein, we demonstrate its mechanistic action in HNSCC. The aim of the present study was to show survivin expression in HNSCC. Further we followed the hypothesis survivin expression to be of prognostic/predictive importance. Significant positive correlations with tumour stage, regional and distant metastases were observed. Furthermore, we could also show local differences in the expression patterns. The subgroups of OSCC and LSCC showed especially significant correlations between survivin expression and tumour stage. Additionally, LSCCs showed a correlation between survivin and nodal stage. Reports of survivin and its relationship with clinicopathological stages are sparse. Our findings support the results of another group which analysed a much smaller cohort of HNSCCs [5]. Compared with the report by Pickhard et al. [5] our analysis demonstrated a significant relationship with survival in the whole cohort. However, our subgroup analysis did not show any significant differences regarding survival for overall or recurrence-free survival. Unfortunately, Pickhard et al.[5] did not give any detailed information about the survival data (e.g. which specimens were included/excluded) and did not mention a subgroup analysis. Our findings do support the investigations of other groups looking at the function of survivin and its effect on therapy response [36].
It was recently reported that survivin facilitates HNSCC growth, which is supported by our results regarding the association with higher tumour stages, which are directly affiliated with tumour size [37]. In addition to its prognostic relevance, survivin may also be an important therapeutic target [38]. Zhang et al. looked at sepantronium bromide (YM155), a selective survivin suppressant that induces apoptosis and autophagy. They showed that YM155 in combination with docetaxel promoted tumour regression in a xenograft model [38]. Increased levels of survivin in malignant tissues, therefore, indicate the existence of a therapeutic platform for potential antisurvivin therapies, facilitating the diagnosis and treatment of cancer. Thus, it can be concluded that survivin is a potentially useful prognostic tumour biomarker for current treatment regimes, and its expression may play a key role in predicting the long-term clinical outcome and quality of life in HNSCC patient; it may preclude unnecessary treatments. In addition, survivin is potentially a new target for improving the outcome of chemotherapy. Further research is needed for clinical management and prognosis to understand and corroborate the novel targets, i.e. prognostic markers for individualising preventive therapeutic management and predicting disease progression in patients with HNSCC. This may lead to a more favourable anticancer therapeutic strategy and survival outcome. The big advantage of our study is the first presentation of a large cohort of HNSCC examined for survivin expression and compared to clinical data. In the last years, HNSCC oncologist had to learn the inter-tumour differences. OPSCC, not only regarding the HPV presence, seem to have different pathways in tumour development. It is of importance to approach the HNSCC subtypes as different entities. This study could show the importance of survivin. With our data collection, we were not able to look at the subtypes and different therapies.
Conclusion
Survivin expression correlates with survival and clinical parameters in HNSCCs. On the cellular level, these results are very promising and worth concentrating our efforts on the molecular level. Additionally, a bigger cohort of patients will be examined and hopefully we will be able to address the prognostic value of survivin expression related to the various treatment approaches and different tumour localisations in HNSCCs.
References
Hunter KD, Parkinson EK, Harrison PR (2005) Profiling early head and neck cancer. Nat Rev Cancer 5(2):127–135
Parkin DM, Lӓӓrӓ E, Muir CS (1988) Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer 41(2):184–197
Ragin CC, Modugno F, Gollin SM (2007) The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res 86(2):104–114
Sharma H, Sen S, Mathur M, Bahadur S, Singh N (2004) Combined evaluation of expression of telomerase, survivin, and anti-apoptotic Bcl-2 family members in relation to loss of differentiation and apoptosis in human head and neck cancers. Head Neck 26(8):733–740
Pickhard A, Grӧber S, Haug AK et al (2014) Survivin and pAkt as potential prognostic markers in squamous cell carcinoma of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol 117(6):733–742
Farnebo L, Tiefenbӧck K, Ansell A, Thunell LK, Garvin S, Roberg K (2013) Strong expression of survivin is associated with positive response to radiotherapy and improved overall survival in head and neck squamous cell carcinoma patients. Int J Cancer 133(8):1994–2003
Lippert BM, Knauer SK, Fetz V, Mann W, Stauber RH (2007) Dynamic survivin in head and neck cancer: molecular mechanism and therapeutic potential. Int J Cancer 121(6):1169–1174
Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3(8):917–921
Marioni G, D’Alessandro E, Bertolin A, Staffieri A (2010) Survivin multifaceted activity in head and neck carcinoma: current evidence and future therapeutic challenges. Acta Otolaryngol 130(1):4–9
Scheper MA, Nikitakis NG, Sauk JJ (2007) Survivin is a downstream target and effector of sulindac-sensitive oncogenic Stat3 signalling in head and neck cancer. Int J Oral Maxillofac Surg 36(7):632–639
Cui M, Au JL, Wientjes MG, O’Donnell MA, Loughlin KR, Lu Z (2015) Intravenous siRNA silencing of survivin enhances activity of mitomycin C in human bladder RT4 xenografts. J Urol 194(1):230–237
Gibbons JA, Kanwar JR, Kanwar RK (2015) Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer 15:425
Adamkov M, Výbohová D, Tupá V, Chylíková J, Horáček J, Benčat M (2015) Expression and significance of survivin in colorectal high grade and low grade adenomas. Acta Histochem 117(6):590–594
Phatak P, Byrnes KA, Mansour D, Liu L, Cao S, Li R, Rao JN, Turner DJ, Wang JY, Donahue JM (2016) Overexpression of miR-214-3p in esophageal squamous cancer cells enhances sensitivity to cisplatin by targeting survivin directly and indirectly through CUG-BP1. Oncogene 35(16):2087–2097
Lu CD, Altieri DC, Tanigawa N (1998) Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res 58(9):1808–1812
Ezponda T, Pajares MJ, Agorreta J, Echeveste JI, Lopez-Picazo JM, Torre W, Pio R, Montuenga LM (2010) The oncoprotein SF2/ASF promotes non-small cell lung cancer survival by enhancing survivin expression. Clin Cancer Res 16(16):4113–4125
Yip KW, Shi W, Pintilie M, Martin JD, Mocanu JD, Wong D, MacMillan C, Gullane P, O’Sullivan B, Bastianutto C, Liu FF (2006) Prognostic significance of the Epstein-Barr virus, p53, Bcl-2, and survivin in nasopharyngeal cancer. Clin Cancer Res 12(19):5726–5732
Yi XP, Han T, Li YX, Long XY, Li WZ (2015) Simultaneous silencing of XIAP and survivin causes partial mesenchymal-epithelial transition of human pancreatic cancer cells via the PTEN/PI3K/Akt pathway. Mol Med Rep 12(1):601–608
Cavalieri F, Beretta GL, Cui J, Braunger JA, Yan Y, Richardson JJ, Tinelli S, Folini M, Zaffaroni N, Caruso F (2015) Redox-sensitive PEG-polypeptide nanoporous particles for survivin silencing in prostate cancer cells. Biomacromolecules 16(7):2168–2178
Lin CK, Chao TK, Yu CP, Yu MH, Jin JS (2009) The expression of six biomarkers in the four most common ovarian cancers: correlation with clinicopathological parameters. APMIS 117(3):162–175
Krambeck AE, Dong H, Thompson RH, Kuntz SM, Lohse CM, Leibovich BC, Blute ML, Sebo TJ, Cheville JC, Parker AS, Kwon ED (2007) Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res 13(6):1749–1756
Labarrade F, Bergeron L, Serre C, Lebleu A, Busuttil V, Botto JM, Domloge N (2015) Modulating the expression of survivin and other basal epidermal proteins protects human skin from UVB damage and oxidative stress. J Cosmet Dermatol 14(3):191–203
Fukuda S, Pelus LM (2006) Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther 5(5):1087–1098
Yamamoto T, Manome Y, Nakamura M, Tanigawa N (2002) Downregulation of survivin expression by induction of the effector cell protease receptor-1 reduces tumor growth potential and results in an increased sensitivity to anticancer agents in human colon cancer. Eur J Cancer 38(17):2316–2324
Khan Z, Khan N, Tiwari RP, Patro IK, Prasad GB, Bisen PS (2010) Down-regulation of survivin by oxaliplatin diminishes radioresistance of head and neck squamous carcinoma cells. Radiother Oncol 96(2):267–273
Khan Z, Tiwari RP, Mulherkar R, Sah NK, Prasad GBKS, Shrivastava BR, Bisen PS (2009) Detection of survivin and p53 in human oral cancer: correlation with clinicopathologic findings. Head Neck 31(8):1039–1048
Mita AC, Mita MM, Nawrocki ST, Giles FJ (2008) Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 14(16):5000–5005
Jaiswal PK, Goel A, Mittal RD (2015) Survivin: a molecular biomarker in cancer. Indian J Med Res 141(4):389–397
Waligórska-Stachura J, Jankowska A, Waśko R, Liebert W, Biczysko M, Czarnywojtek A, Baszko-Błaszyk D, Shimek V, Ruchała M (2012) Survivin—prognostic tumor biomarker in human neoplasms—review. Ginekol Pol 83(7):537–540
Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, Willi N, Mihatsch MJ, Sauter G, Kallioniemi OP (1999) Survey of gene amplifications during prostate cancer progression by high-throughput fluorescence in situ hybridization on tissue microarrays. Cancer Res 59(4):803–806
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45(4–5):309–316
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Thomas GR, Nadiminti H, Regalado J (2005) Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int J Exp Pathol 86(6):347–363
Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT (2002) Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck 24(2):165–180
Marioni G, Bertolin A, Giacomelli L, Marchese-Ragona R, Savastano M, Calgaro N, Marino F, de Filippis C, Staffieri A (2006) Expression of the apoptosis inhibitor protein survivin in primary laryngeal carcinoma and cervical lymph node metastasis. Anticancer Res 26(5B):3813–3817
Khan Z, Khan AA, Prasad G, Khan N, Tiwari RP, Bisen PS (2016) Growth inhibition and chemo-radiosensitization of head and neck squamous cell carcinoma (HNSCC) by survivin-siRNA lentivirus. Radiother Oncol 118(2):359–368
Sobin LH, Fleming ID (1997) TNM classification of malignant tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 80(9):1803–1804
Zhang L, Zhang W, Wang Y-F, Liu B, Zhang WF, Zhao YF, Kulkarni AB, Sun ZJ (2015) Dual induction of apoptotic and autophagic cell death by targeting survivin in head and neck squamous cell carcinoma. Cell Death Dis 6:e1771
Funding
The work was supported by the Institute of Pathology and the Department of Otolaryngology, Head and Neck Surgery at the University Medical Centre Hamburg-Eppendorf.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
No author has a financial or proprietary interest in any material or method mentioned.
Rights and permissions
About this article
Cite this article
Münscher, A., Prochnow, S., Gulati, A. et al. Survivin expression in head and neck squamous cell carcinomas is frequent and correlates with clinical parameters and treatment outcomes. Clin Oral Invest 23, 361–367 (2019). https://doi.org/10.1007/s00784-018-2444-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2444-8