Abstract
Objectives
The aim of this case-control study was to carry out an oral health assessment on a group of Alzheimer’s patients and to establish a hypothesis regarding the implication of the characteristics of the disease and the treatment of oral health.
Materials and methods
A total of 70 Alzheimer’s patients, residents at the Alzheimer Center Reina Sofia Foundation (Madrid, Spain) and at the Alzheimer State Reference Center (Salamanca, Spain), and 36 controls (companions/acquaintances), were studied by oral examination and saliva sampling. The oral health indices DMFT/DMFS, CPI, the prosthetic condition, oral hygiene, saliva volume, and pH, as well as the specific microbiological parameters governing the risk of developing caries were assessed.
Results
Alzheimer’s patients exhibited, as compared to the control group, (1) fewer teeth (10.9 ± 10.5 vs 23.7 ± 6.5), (2) fewer obturations (2.2 ± 3.4 vs 6.6 ± 5.6), (3) fewer periodontally healthy sextants (0.1 ± 0.4 vs 1.4 ± 2.2), (4) worse oral hygiene (43.1 vs 72.2% brushed), (5) greater use of removable prostheses (47.8 vs 8.4%), (6) higher incidence of candida infection (11.8 vs 0.0%) and cheilitis (15.9 vs 0.0%), (7) lower salivary flow (0.6 ± 0.6 vs 1.1 ± 0.6), and (8) lower buffering capacity (46 vs 80%).
Conclusions
After taking into account the influence of age, Alzheimer’s patients had worse oral health (caries and periodontal disease), more mucosal lesions (cheilitis and candidiasis), and worse saliva quantity and quality.
Clinical relevance
Clinicians should be aware of the implications of Alzheimer’s disease in oral health, in order to stablish the effective preventive measures and the optimal treatment plan.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Within progressively aging populations, the increase of the incidence of dementia, such as Alzheimer’s disease (AD), can be expected, and it is predicted that in 2050 more than 113 million people will suffer from the disease worldwide [1]. As a consequence, the World Health Organization (WHO) is raising the alarm about the possible repercussions regarding this reality, and is advising various governments and authorities to take the appropriate measures to reduce the social and health impact of this devastating disease.
AD frequently occurs after the age of 60 and often in women [1]. The most commonly used scale for defining the degree of severity of the disease is the Global Deterioration Scale (GDS), which is made up of 7 stages that measure its progression [2]. Persons with AD experience a decline in their ability to learn new information, carry out routine tasks, and remain situated in time and space [3]. The disease causes the loss of the ability to care for oneself, and in the final stage, can cause loss of motor function (motor difficulties in daily care and hygiene) [3]. The cardinal symptom of AD is the loss of episodic memory that is also accompanied by other types of cognitive impairment such as the aphasia-apraxia-agnosia triad (speech-language and movement disorders as well as the inability to recognize stimuli). Neuropsychiatric symptoms are common and early onset and include the following: apathy, depression anxiety, and delusions [3].
Given the cognitive and motor decline associated with the progression of AD, some authors have proposed that both the disease itself (symptoms-like cognitive deterioration, apathy, and apraxia cause lack of interest and the inability to carry out daily hygiene), as well as the pharmacological treatment (decrease of salivary flow), increase the risk of oral pathology (caries and periodontal disease, xerostomia, and candidiasis) [4]. Also, the changes in behavior that should be adopted by the dentist with respect to treating patients with Alzheimer’s disease have been described [4].
To our knowledge, there are very few studies that evaluate the implications of Alzheimer’s disease in oral health, although the most significate theoretical study was carried out by Friedlander et al. [4]. The clinical studies regarding Alzheimer’s patients that do exist only focus in part on the oral component without relating it to the disease as a whole [5,6,7,8,9,10,11].
In Spain, a previous study was published involving elderly persons who required assistance with their oral hygiene, many of whom were suffering from dementia, and a direct association between dementia and poor oral hygiene was reported [12]. However, this study did not conduct a microbiological analysis or investigate factors such as pH or salivary flow. Furthermore, it would be interesting to explore the association between the degree of neuromotor deterioration with the severity of the main oral pathologies suffered by AD patients (caries and periodontal disease).
Our main objective was to conduct a case-control study regarding the oral health of a group of Alzheimer’s patients, studying caries levels, periodontal disease, and the prosthetic situation, as well as hygiene levels, the microbiological implications, and buffering capacity.
Materials and methods
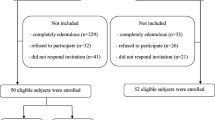
The case-control study took place between March 2012 and July 2013. The study, previously approved by the Ethical Committee of the Institute of Health Carlos III (Madrid, Spain), included 106 participants: 70 were individuals suffering from AD and residents at the Alzheimer Center Reina Sofia Foundation (Madrid, Spain) and at the Alzheimer State Reference Center (and other dementias) Salamanca (Spain). Thirty-six of the participants formed the healthy control group and were selected from among the patients’ caregivers (family members or friends having the same sociocultural level and from the same reference center, although mostly of them were younger than the patients).
All of the participants of the Alzheimer group fulfilled the criteria of dementia caused by Alzheimer’s disease based on McKhann et al. diagnosed criteria [13], regardless of their stage. They do not have any other neurological disease. When candidates for participation in the study were unable to collaborate in examinations, they have not been included. As is shown at the bottom of Table 3, if any participants were unable to collaborate properly in the saliva tests they have been excluded for these tests, and the saliva tests were only conducted with the rest of the group. The control group was healthy people who had no neurological disease and who were able to collaborate properly.
All participants and families members were informed about the study, and the caregivers or legal representatives signed an informed consent form in order to partake in the study. The patients did not have any other neurodegenerative disease except for AD.
Variables
AD patients were evaluated on the same day by a neurologist, neuropsychologist, and a dentist. The neurological and neuropsychological assessment was carried out first followed by the oral exploration.
The neurological evaluation protocol was designed to globally assess the neurological and neuropsychological functioning of the study participants using simple and easy scales. The protocol for neurological exploration used the following neurological scales: Severe MiniMental State Exam (SMMSE) [14], the Mini-Cog Test [14], the clock draw test [14], and the Functional Assessment Staging of Alzheimer’s Disease-FAST (alterations in daily functioning scale) [14]. Additionally, two-scored scales were included within the analysis that staged cognitive decline and dementia: the Clinical Dementia Rating (CDR) [15] and the Global Deterioration Scale (GDS) [2]. The CDR is a test that determines the overall degree of dementia (values from 0 to 3, the greater the degree of dementia the higher the score) [15], and the GDS is an complete characterization of the stages of decline that permit the actual clinical state of the patient to be determined (values from 0 to 7, the greater the degree of dementia the higher the score) [2].
All of the participants were later subjected to an oral, dental, and periodontal examination, according to the criteria established by the World Health Organization [16], to check for caries in teeth (DMFT index: sum of decayed, missed and filled teeth) and in surfaces (DMFS index: sum of the decayed, missed and filled teeth surfaces), as well as for periodontal disease (Community Periodontal Index) and the prosthetic status (fixed, implant-supported, removable partial, or complete prostheses). In addition, the presence of excessive wear facets (bruxism) and cheilitis were assessed via clinical inspection, and all patients were asked about the sensation of mouth dryness or xerostomia. We recorded also the presence of marked redness and edema in the attached gingiva supporting the dentures (presumably candidiasis). The presence of temporomandibular pain was determined using preauricular palpation and palpation of the masticatory muscles (temporal and masseters).
All participants were interviewed by a second examiner. In the case of the Alzheimer’s patients, their family members or caregivers were questioned, regarding their hygiene, brushing frequency, visits to the dentist, use of dental floss, and mouth rinse. Sociodemographic data were also obtained by interview: gender, age, place of residence, civil status, and social class were assessed. We follow the International Classification of Occupations [17].
Two saliva samples per participant were taken in sterile containers (Duerolab, Salamanca, Spain) by a third examiner, who was the same person on all occasions.
The saliva is collected by having the patient sitting in a relaxed position, with their elbows resting on their knees. In this position, the patient tilts their head forward and all the secreted saliva is allowed to drip into a sterile recipient for 5 min. The resulting volume is measured in milliliters per minute. Once the non-stimulated saliva has been collected, the studied subject is asked to repeat the process in order that the stimulated saliva may be obtained. The difference being that the individual should gently chew a 1-g (approx) capsule of sterile paraffin in order to stimulate the saliva glands.
The samples were transported, in refrigeration, to the laboratory (Department of Microbiology and Genetics at the University of Salamanca), at which time the pH (micropH2000, Crison) and saliva volume of all samples were measured. The commercial kit CRTBacteria® (Ivoclar; Vivadent, Liechtenstein) was used to evaluate the colony-forming units (CFU) of the Streptococcus mutans (including the S. mutans, S. rattus, S. cricetus, S. sobrinus, S. ferus, S. downei, and S. macacae species) and lactobacillus count in saliva samples, and therefore for determining the microbiological risk of caries. The analysis was carried out according to the manufacturer’s instructions. In a microbiological safety cabinet, a NaHCO3 tablet was placed at the bottom of each vial, and the selective agar surfaces were inoculated with five drops of the saliva samples. Vials were incubated for 48 ± 2 h at 37 ± 1 °C. After the incubation period, the results were recorded, and counts equal to or greater than 105 CFU/mL of S. mutans or lactobacillus were classified as having a high risk of caries.
All participants in the control group were subjected to the same tests, except for the neurological exploration.
Statistical analysis
The sample distribution (n, %) of the categorical variables and the mean and standard deviations (mean ± SD) of the quantitative variables were used for describing the relevant variables in the sample. The differences between groups were analyzed using the unpaired Student’s t test for quantitative variables or chi-square tests for categorical variables. In situations where the sample size of each subgroup was reduced, this information appears in the Table footnotes. Spearman’s correlation coefficients (rs) were calculated to assess the linear relationship between gds and cdr with several oral health-related clinical variables. furthermore, a forward stepwise linear regression analysis for predicting several clinical parameters was calculated (DMFT, CPI, standing teeth, pH, salivary flow rate, and buffer effect) after including all the potentially related variables. All analyses were carried out with the SPSS Windows v-20 (SPSS Inc., Chicago, IL). The level of significance was set at 0.05.
Results
The analysis of Table 1 shows that the average age of the Alzheimer’s patients (77.4 ± 10.6 years) was significantly higher than that of the controls (62.6 ± 7.1 years). Women comprised 54% of the AD group and 63% of the control group, with no significant differences. Regarding the comparison of oral hygiene habits between Alzheimer’s patients and the control group, the AD patients had a lower brushing frequency (43.1 vs 72.2%), did not use dental floss (96.9 vs 69.4%), and visited the dentist less frequently (33.3 vs 58.3%), with significant differences. However, more than 50% of the participants of both groups used mouthrinse daily, with no significant differences.
The Alzheimer’s patients analyzed obtained the following assessment with respect to the CDR: 26% had mild dementia (CDR 1), 31.1% had moderate dementia (CDR 2), and 42.2% had severe dementia (CDR 3). With respect to the assessment using the GDS, stages 4 and 7 were identified: 30.2% had moderate cognitive decline (GDS 4), 37.2% had moderately severe cognitive decline (GDS 5), 27.9% had severe cognitive decline (GDS 6), and 4.7% had very severe cognitive decline (GDS 7).
There was correlation between the CDR and GDS values (rs = 0.76; p < 0.001); however, there was no statistical correlation between the state of oral health (DMFT, CPI, ect.) and the degree of the deterioration of the dementia; that is to say, differences were found between the participants that had AD and those that did not, but there was not an increase of poor oral health as the severity of the disease progressed.
Dental status
Table 2 shows the state of dental periodontal health and the prosthetic status of the study participants. The DMFT and DMFS indices showed very significant differences between both groups, and identified worse dental health among the group of Alzheimer’s patients. There were highly significant differences regarding tooth loss, where the AD group had, on average, lost 2.5-fold more teeth than the controls (21.0 ± 10.4 vs 8.3 ± 6.5). Also, the indices of both groups showed highly significant differences with respect to filled teeth; the AD group had 3 times more filled teeth than the control group (2.2 ± 3.4 vs 6.6 ± 5.6). The distribution per surface was according to that observed in teeth. No significant differences were found as regards to the number of decayed teeth between the AD and control groups. However, there was a highly significant difference in the ratio between caries (DMFT or decayed teeth) and standing teeth, being 24.5 ± 28.4 in the Alzheimer’s group versus 7.1 ± 10.0 in the control group. Similarly, significant differences were found in the number and status of posterior and anterior groups of teeth.
Periodontal status
The CPI showed worse periodontal health in AD patients. In addition, highly significant differences were observed between AD and control groups regarding healthy sextants, coded as CPI = 0 (0.1 ± 0.4 vs 1.4 ± 2.1) and sextants with bleeding on probing, coded as CPI = 1 (0.0 ± 0.3 vs 1.0 ± 1.4).
Prosthetic status
Highly significant differences were found in the use of different types of dental prostheses, where a higher percentage of the Alzheimer’s patients were denture wearers, as compared to the control group (65.2 vs 58.3%). AD patients predominantly used complete dentures (33.3 vs 2.8%) and fewer fixed implant-supported prostheses (17.4 v 36.1%).
Oral clinical assessment
The incidence of both cheilitis and candidiasis were found to be significantly higher in the AD patients compared to the controls (15.9 vs 11.8%) (Table 3). No significant differences were found regarding the perception of mouth dryness or xerostomia, or problems with TMJ or bruxism.
Salivary laboratory test and buffer effect
Table 3 shows the results of the saliva volume and pH tests, as well as the results of the microbiological analysis. It was found that AD patients secrete a significantly less amount of basal or stimulated saliva per minute (1.0 ± 1.3 vs 1.5 ± 1.1; 3.0 ± 3.0 vs 5.2 ± 3.2). Additionally, the basal saliva was more acidic in the Alzheimer’s group (7.0 ± 0.8 vs 7.4 ± 0.4) and presented a lower buffering capacity (46 vs 80%).
Microbiological assay (CRT_bacteria ivoclar)
The CFU counts of the S. mutans group and Lactobacillus ssp. revealed that a large percentage of the individuals analyzed in the AD group had a high risk to developing caries (between 76 and 93%), as compared to control group (67–86%), although this difference was not statistically significant.
Linear regression
Table 4 shows the results of the linear regression analysis, which confirmed that age and the presence of the disease were predictive factors of the state of patient oral health (DMFT, standing teeth, and CPI), as well as salivary characteristics (pH, volume of stimulated saliva, and buffering capacity). Age and the presence of AD were the major predictors of DMFT and the number of standing teeth. Moreover, the salivary flow rate, pH, and buffering capacity of non-stimulated saliva, and the number of sextants coded as CPI = 0 and 1, decreased as a result of AD.
Discussion
Our main findings, in this case and control study regardless oral health in a group of Alzheimer’s disease participants, show a worse dental, periodontal, prosthetic, and salivary (quantitatively and qualitatively) status for the AD group in comparison to the control group. The presence of AD is shown as a predictive factor of the deterioration of the oral health, independently of the effect of age in the regression analyses (See Table 4).
The aim of this study was to assess the impact of AD on oral health using a case-control design. There are very few studies directed at this specific objective, and those that do exist only approach the subject from a theoretical point of view in an attempt to explain the possible oral manifestations and modes of behavior, providing some clinical guidelines to follow [4, 18,19,20,21]. Among the clinical studies, few utilize the different clinical indices to illustrate the clinical situation of AD sufferers [22,23,24,25], and none incorporate as many different parameters as those included within this study. Some studies only focus on the number of teeth [5] or the number of caries [6, 7] or the type of dental prosthesis used [8], where other reports have focused on the disease in relation to periodontal health [9,10,11]. Among the studies that included saliva, only one article, dealing with the effects of medication in the elderly, concluded that drugs were the cause of a reduction in salivary flow rate [26, 27]. Not one article approached the assessment of AD patients from a multidisciplinary perspective (neurological, neuropsychological, odontological).
In consonance with other authors that established the presence of a greater oral health risk in Alzheimer’s patients [20, 21], our study also showed worse dental health (higher rates of caries and periodontal disease among the standing teeth) among the group of AD patients. The results of the studies found in the literature coincide with our results in terms of the lower number of standing and filled teeth [5, 7, 23, 24], together with the greater number of decayed teeth [7, 23, 24]. However, the total number of caries in the patient sample population was not significantly higher than that of the controls, given that the significantly greater loss of teeth in the AD patients left fewer teeth susceptible to decay. Although, when the ratio of caries per standing teeth was calculated, this difference became significative. By contrast, in a paper published by Hatipoglu et al., no significant differences were found regarding the DMFT and the number of standing teeth, possibly due to a smaller sample size (n = 31) [8]. Other authors relate the risk of dementia with the number of standing teeth, establishing that dental loss could be an early marker for cognitive and physical decline [28, 29]. In our study, after carrying out the linear regression analysis, we found that the number of standing teeth was significantly associated with age and the presence of AD (Table 4). The fact that in the Alzheimer’s group there had 2.5 times fewer teeth and 3 times fewer fillings, compared to the control group, might suggest that the decayed teeth present in the AD patients were extracted as opposed to receiving a possible tooth conservation treatment. The reasons for this could be that (1) the conduct of the AD patient in the dental chair could be very difficult for the dentist to control [4], making extraction versus filling more feasible; (2) the poorer periodontal state of some of the decayed teeth could tip the risk/benefit scale towards extraction; and (3) larger size caries that may require elaborate reconstructions can bring about unpredictable outcomes in the medium to long-term.
In agreement with already published works [23,24,25], we also observed poor periodontal health in the Alzheimer’s group, compared to the control, which might be due to reduced salivary flow or to inadequate oral hygiene, as proposed by other authors [4, 23, 24]. Also, our results show that mouthrinse is used by more than 50% of the participants, with no significant differences with controls. Quite often in care facilities, the use of toothbrushes is replaced by mouthwash, which that is easier for the patient to use. This of course does not carry out the same function and leads to poor oral health maintenance. Brushing efficiency is directly related to the progression of AD, in the first stages apathy and depression generate lack of interest to carry out proper daily hygiene, and at moderate stages where the cognitive decline and dyspraxia associated with the disease leads to lack of interest or difficulty/inability to carry out proper daily hygiene [4], which in turn makes the Alzheimer’s patients dependent on the help of the caregiver and can cause disruptive situations and protests from the patients [30]. In addition, it was observed during the course of the study that oral hygiene did not tend to be a priority for the caregivers assisting in the patient’s daily hygiene routine [31].
The study also found significant differences in the salivary flow rate and pH, which were in accordance with differences found in the literature [32]. However, when the AD patients were asked about xerostomia, paradoxically, a lower percentage of patients gave an affirmative answer, compared to the control group. This result could suggest that in spite of the objective evidence obtained, the AD patients were unable to recognize a lower salivary flow (anosognosia) (Table 3). These results are logical since Alzheimer’s sufferers are given multiple drugs, such as acetylcholinesterase inhibitors and neuroleptics [27], to treat the symptoms of the disease, as well as other age-related diseases, many of which cause hyposalivation [26]. Low salivary flow together with a low pH imply a greater risk of developing caries and a lower remineralization capacity [33]; also, increased oral dryness can promote the appearance of mouth lesions such as ulcers and cheilitis (Table 3). Moreover, the high incidence of candida infection (11.8%) observed within the AD group (Table 3) can be explained by the presence of hyposalivation and tanking neuroleptics as haloperidol (often prescribed to Alzheimer’s patients) which can produce a certain level of leucopenia [4].
As the number of lost teeth in the Alzheimer’s group was greater, the use of dentures was also more prevalent (mostly removable complete or partial dentures). This, together with oral dryness, often produced mouth lesions due to the continuous rubbing of the removable prosthesis. Also, the decline in memory and the possible neglected hygiene of patients in the moderate to severe stage could explain the lack of denture care (cleaning, removal etc.), as already reported by Hatipoglu et al. [8], leading to the accumulation of food and plaque that can harm soft and hard tissues [4]. On the other hand, given the special characteristics of the dental care of these patients, it should not be surprising that osseointegrated implants were not common.
We consider the analysis of the stages of Alzheimer’s disease (GDS and CDR) and the degree of deterioration in oral health (DMFT, CPI, etc.) to be fundamental for establishing adequate protocols of action for each situation. However, in this study, no statistically significant correlation was found between the state of oral health and the degree of deterioration caused by dementia; in other words, differences were found between the AD and control groups; however, oral health did not become worse as the severity of the disease progressed. This result might be explained by the variability of the onset of AD and the progression of the disease, since we have shown that there is no correlation between age and degree of severity according to the CDR (r = − 0.01, p = 0.93) and the GDS (r = − 0.12; p = 0.47).
The main strength of this study stems from the fact that it is a multidisciplinary assessment, encompassing the state of oral health and hygiene and neurological state, carried out following internationally standardized procedures. Each test was performed by the same examiner previously trained in the used methodology.
The main limitation of the present study lies in the selection of the participants for the control group. Despite being from the same socio-economic level as the AD patients, they were significantly younger, as has frequently occurred in similar studies [5, 8, 24]. However, this fact unfortunately lowers the scientific level of evidence of the findings provided by the current study. This shortcoming cannot fully be compensated by the statistical analysis. Nevertheless, in a try to minimize the effect of age on our findings, a multiple linear regression analysis was carried out to control, at least partially, such confounding factor. Afterwards, we have confirmed that within the majority of the clinical and salivary variables, the presence of the disease does have a predictive capacity, independent of age. The presence of AD alone seems to be able to predict the outcome of variables such as non-stimulated saliva, pH, and non-stimulated buffering capacity, CPI; while for other variable such as DMFT and standing teeth, it is both the presence of the disease and the influence of the patients’ age that is able to predict certain outcomes, although the presence of AD was found to be the most predictive factor.
Conclusions
After taking into account the influence of age, Alzheimer’s patients had worse oral health (caries and periodontal disease), more mucosal lesions (cheilitis and candidiasis), and worse saliva quantity and quality.
References
Jellinger KA, Attems J (2010) Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol 119:421–433. https://doi.org/10.1007/s00401-010-0654-5
Kim JS, Won CW, Kim BS, Choi HR (2013) Predictability of various serial subtractions on global deterioration scale according to education level. Korean J Fam Med 34:327–333. https://doi.org/10.4082/kjfm.2013.34.5.327
Cummings J (2004) Alzheimer’s disease. N Engl J Med 351:56–67. https://doi.org/10.1056/NEJMra040223
Friedlander AH, Norman DC, Mahler ME, Norman KM, Yagiela JA (2006) Alzheimer’s disease: psychopathology, medical management and dental implications. J Am Dent Assoc 137:1240–1251
Ribeiro GR, Costa JLR, Bovi Ambrosano GM, Rodrigues Garcia RCM (2012) Oral health of the elderly with Alzheimer’s disease. Oral Surg Oral Med Oral Pathol Oral Radiol 114:338–343. https://doi.org/10.1016/j.oooo.2012.03.028
Ellefsen BS, Morse DE, Waldemar G, Holm-Pedersen P (2012) Indicators for root caries in Danish persons with recently diagnosed Alzheimer’s disease: root caries indicators in Alzheimer’s disease. Gerodontology 29:194–202. https://doi.org/10.1111/j.1741-2358.2011.00560.x
Ellefsen B, Holm-Pedersen P, Morse DE, Schroll M, Andersen BB, Waldemar G (2009) Assessing caries increments in elderly patients with and without dementia: a one-year follow-up study. J Am Dent Assoc 140:1392–1400
Hatipoglu MG, Kabay SC, Güven G (2011) The clinical evaluation of the oral status in Alzheimer-type dementia patients: oral health in AD. Gerodontology 28:302–306. https://doi.org/10.1111/j.1741-2358.2010.00401.x
Singhrao SK, Harding A, Simmons T, Robinson S, Kesavalu L, Crean S (2014) Oral inflammation, tooth loss, risk factors, and association with progression of Alzheimer’s disease. J Alzheimers Dis 42:723–737. https://doi.org/10.3233/JAD-140387
Uppoor AS, Lohi HS, Nayak D (2013) Periodontitis and Alzheimer’s disease: oral systemic link still on the rise? Gerodontology 30:239–242. https://doi.org/10.1111/j.1741-2358.2012.00660.x
Abbayya K, Chidambar Y, Naduwinmani S, Puthanakar N (2015) Association between periodontitis and alzheimer’s disease. North Am J Med Sci 7(6):241–246. https://doi.org/10.4103/1947-2714.159325
Ruiz-Medina P, Bravo M, Gil-Montoya JA, Montero J (2005) Discrimination of functional capacity for oral hygiene in elderly Spanish people by the Barthel General Index. Community Dent Oral Epidemiol 33:363–369. https://doi.org/10.1111/j.1600-0528.2005.00222.x
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Sano M, Egelko S, Jin S, Cummings J, Clark CM, Pawluczyk S, Thomas RJ, Schittini M, Thal LJ, Alzheimer's Disease Cooperative Study Group (2006) Spanish instrument protocol: new treatment efficacy instruments for Spanish-speaking patients in Alzheimer disease clinical trials. Alzheimer Dis Assoc Disord 20:232–241. https://doi.org/10.1097/01.wad.0000213862.20108.f5
Han H-R, Park S-Y, Song H, Kim M, Kim KB, Lee HB (2013) Feasibility and validity of dementia assessment by trained community health workers based on clinical dementia rating. J Am Geriatr Soc 61:1141–1145. https://doi.org/10.1111/jgs.12309
World Health Organization (1987) Oral health surveys: basic methods, 3rd edn. World Health Organization, Geneva
Domingo A, Marcos J (1989) Proposal of an indicator of “social class” based on the occupation. Gac Sanit 3:320–326
Brennan LJ, Strauss J (2014) Cognitive impairment in older adults and oral health considerations. Dent Clin N Am 58:815–828. https://doi.org/10.1016/j.cden.2014.07.001
Henry RG, Smith BJ (2009) Managing older patients who have neurologic disease: Alzheimer disease and cerebrovascular accident. Dent Clin N Am 53:269–294. https://doi.org/10.1016/j.cden.2008.12.011
Kocaelli H, Yaltirik M, Yargic LI, Özbas H (2002) Alzheimer’s disease and dental management. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology 93:521–524. https://doi.org/10.1067/moe.2002.123538
Gitto CA, Moroni MJ, Terezhalmy GT, Sandu S (2001) The patient with Alzheimer’s disease. Quintessence Int 32:221–231
Arrivé E, Letenneur L, Matharan F, Laporte C, Helmer C, Barberger-Gateau P, Miquel JL, Dartigues JF (2012) Oral health condition of French elderly and risk of dementia: a longitudinal cohort study: Elderly’s oral health and risk of dementia. Community Dent Oral Epidemiol 40:230–238. https://doi.org/10.1111/j.1600-0528.2011.00650.x
Chen X, Shuman SK, Hodges JS, Gatewood LC, Xu J (2010) Patterns of tooth loss in older adults with and without dementia: a retrospective study based on a Minnesota cohort: tooth loss patterns in older adults. J Am Geriatr Soc 58:2300–2307. https://doi.org/10.1111/j.1532-5415.2010.03192.x
Syrjälä A-MH, Ylöstalo P, Ruoppi P, Komulainen K, Hartikainen S, Sulkava R, Knuuttila M (2012) Dementia and oral health among subjects aged 75 years or older: dementia and oral health. Gerodontology 29:36–42. https://doi.org/10.1111/j.1741-2358.2010.00396.x
Srisilapanan P, Jai-Ua C (2013) Oral health status of dementia patients in Chiang Mai Neurological Hospital. J Med Assoc Thail 96:351–357
Leal SC, Bittar J, Portugal A, Falcão DP, Faber J, Zanotta P (2010) Medication in elderly people: its influence on salivary pattern, signs and symptoms of dry mouth: medication in elderly people. Gerodontology 27:129–133. https://doi.org/10.1111/j.1741-2358.2009.00293.x
Turner LN, Balasubramaniam R, Hersh EV, Stoopler ET (2008) Drug therapy in Alzheimer disease: an update for the oral health care provider. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 106:467–476. https://doi.org/10.1016/j.tripleo.2008.06.009
Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ (2007) Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc 138:1314–1322. https://doi.org/10.14219/jada.archive.2007.0046
Tsakos G, Watt RG, Rouxel PL, de Oliveira C, Demakakos P (2015) Tooth loss associated with physical and cognitive decline in older adults. J Am Geriatr Soc 63:91–99. https://doi.org/10.1111/jgs.13190
Hugo FN, Hilgert JB, Bertuzzi D, Padilha DM, De Marchi RJ (2007) Oral health behaviour and socio-demographic profile of subjects with Alzheimer’s disease as reported by their family caregivers. Gerodontology 24:36–40. https://doi.org/10.1111/j.1741-2358.2007.00149.x
Vergona KD (2005) A self-reported survey of Alzheimer’s centers in Southwestern Pennsylvania. Spec Care Dentist 25:164–170
Ship J, Puckett S (1994) Longitudinal study on oral health in subjects with Alzheimer’s disease. J Am Geriatr Soc 42:57–63. https://doi.org/10.1111/j.1532-5415.1994.tb06074.x
Zickert I, Emilson CG, Krasse B (1983) Correlation of level and duration of Streptococcus mutans infection with incidence of dental caries. Infect Immun 39:982–985
Acknowledgements
The authors would like to thank the Alzheimer Center Reina Sofia Foundation (Madrid, Spain), the Alzheimer and other Dementias State Reference Center (Salamanca, Spain) and their staff, and all of the participants who took part in the study for their collaboration and willingness to help.
Funding
This work was partially supported by the project number BIO/SA72/13 of the Regional Government of Castilla y León (Spain) and the Research Group (Avances en Salud Oral; GIR_USAL_2015) of the University of Salamanca (Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed involving human participants were in accordance with the ethical standards of the Ethical Committee of the Institute of Health Carlos III (Madrid, Spain) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individuals participants included in the study.
Rights and permissions
About this article
Cite this article
Aragón, F., Zea-Sevilla, M.A., Montero, J. et al. Oral health in Alzheimer’s disease: a multicenter case-control study. Clin Oral Invest 22, 3061–3070 (2018). https://doi.org/10.1007/s00784-018-2396-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2396-z