Abstract
Objectives
The aim of this study is to improve the understanding of interleukin mechanisms during osseointegration to enhance the monitoring of implant failure and success. Clinical parameters, implant stability, and cytokine levels in peri-implant crevicular fluid (PICF) during early bone healing after implant placement were investigated.
Material and methods
Sixty narrow implants were placed in mandible anterior region of 30 edentulous patients (67.23 ± 7.66 years). Bone type, insertion torque, and primary stability were registered during surgery. Clinical measurements of peri-implant health and the secondary implant stability quotient (ISQ) were recorded. Samples from the PICF were collected 1, 2, 4, 8, and 12 weeks after surgery and analyzed for IL-1β, IL-6, IL-10, and TNF-α levels using ELISAs.
Results
The gingival index increased significantly during the first week (p = 0.05), while the plaque index increased significantly between 4 to 8 and 8 to 12 weeks (p < 0.05). The probing depth and the ISQ also reduced significantly (p < 0.05) over time. The TNF-α release increased significantly after the 2nd week for non-atrophic patients and 4th week for atrophic patients (p < 0.05). The IL-1β concentrations showed a short-lived peak after 1st week (p = 0.003), specially in atrophic patients and sites with bone type I (p = 0.034; p = 0.007). The IL-6 concentrations peaked during the 1st and 2nd weeks (p < 0.05; p = 0.005) in atrophic patients and in bone type II (p = 0.023; p = 0.003). The IL-10 concentrations increased gradually over time, showing the highest concentrations at the 12th week (p < 0.005). A total of 12 implants failed at different periods.
Conclusion
While the clinical measurements presented differences between the evaluation periods, these were not indicative of early dental implant failure or peri-implant diseases. Smoking, bone atrophy, and bone type can greatly influence the cytokines concentrations during the healing time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most widely used parameters to determine successful installation of dental implants are usually not based on direct information from the peri-implant site. Instead, most methods rely on peri-implant soft tissue characteristics, the implant level, prosthesis condition, or the patient’s subjective evaluation [1]. However, some of these parameters are not easy to assess and interpret and might not be sensitive or specific to distinguish disease onset, activity, and risk rate [2].
Osseointegration depends on the osteogenesis at the implant interface. This dynamic process results from a complex set of inflammation-related reactions, such as bone resorption and apposition, angiogenesis, and neurogenesis [3, 4]. Once the body detects the implant, an immune-mediated foreign body reaction (FBR) activates several key biological processes, such as signaling pathways and activation of transcription factors, cell growth and differentiation, cytokines, and secretion of growth factors [4].
The ensuing inflammation requires active and well-engaged biochemical processes to return tissues to homeostasis, which eventually leads to implant osseointegration. Most of the interaction between immune system cells is biomodulated by cytokines, growth factors, and hormones. Cytokines can be categorized into Th1- and Th2-type profiles. The Th1-type is involved in cell-mediated pro-inflammatory reactions and activates cytotoxic, inflammatory, and immediate repair functions. Members include interleukin (IL)-1beta (IL-1β), IL-6, IL-12, and tumor necrosis factor alpha (TNF-α). The Th2-type profile induces antibody production and regulates angiogenesis, tissue remodeling, and humoral responses. This Th2-type is mainly characterized by the anti-inflammatory role carried out by IL-10 and transforming growth factor beta (TGF-β), among others [5, 6].
Understanding interleukin mechanisms in biological processes will improve the monitoring of implant success rates. IL-1β is a pro-inflammatory cytokine involved in several biologic processes, including immune regulation, inflammation, connective tissue metabolism, the formation and maturation of osteoclasts from osteoprogenitors, and the activation of mature multinucleated osteoclasts to resorb bone and inhibit bone formation [7, 8]. IL-1β levels around peri-implantitis lesions were also positively correlated with the amount of gingival inflammation, indicating that it could be a reliable marker to detect early signs of peri-implant mucositis before it progresses to peri-implantitis [7, 9,10,11].
The TNF-α stimulates bone resorption, prostaglandin synthesis, and protease production by many cell types including fibroblasts and osteoblasts [7]. TNF-α’s rapid synthesis and early release directs other cells to sites of microbial invasion and infection. High levels of TNF-α have been detected in sites with peri-implantitis [12, 13] and periodontitis [14, 15]. These studies show that excessive secretion of TNF-α is a significant clinical problem for acute or chronic inflammatory diseases [16, 17].
Both IL-1β and TNF-α can regulate IL-6 levels [18]. IL-6 is a multifunctional cytokine that stimulates immune responses, hematopoiesis, and acute-phase reactions, including B cell growth, T cell activation, and platelet production. The de-regulation of IL-6 production has been implicated in the pathogenesis of a variety of several chronic inflammatory proliferative diseases [18, 19].
One crucial regulator of the pro-inflammatory process is interleukin-10 (IL-10). It downregulates synthesis of Th1 pro-inflammatory cytokines and prevents excessive inflammation by acting on macrophages [15]. IL-10 is a B cell stimulator, enhancing B cell proliferation and differentiation, suggesting that IL-10 can play important roles in the regulation of cellular and humoral immune responses [15, 20].
However, while cytokines are essential for the activation, differentiation, and control of the osseointegration process, they are currently not used to monitor peri-implant health status, because their baseline levels are still unknown. The only accurate and non-invasive diagnostic tool to assess osseointegration is the implant stability quotient (ISQ), which is determined by resonance frequency analysis (RFA). This parameter is solely based on changes in the rigidity and stability of the implant-tissue interface over the time and is extensively used to determine the implant success and diagnose early and late failures [21, 22]. However, as this measure gives the contact quotient between the bone-implant interface (i.e., increased stability is related to bone formation), it is difficult to identify a transition point that indicates a fault [23].
Understanding the roles of biomarkers during healing will enable dentists to perform peri-implant fluid collection during their clinical practice in order to diagnose the status of dental implants during a routine check-up. The aim of this study is to compare five periodontal clinical parameters (plaque index, calculus, gingival index, pocket depth, and bleeding on probing) and implant stability with different cytokine levels in the peri-implant crevicular fluid (PICF) during the bone healing process after implant placement in edentulous patients. In addition, we will investigate the influence of bone atrophy, bone type, insertion torque, and smoking on implant osseointegration. This study hypothesizes that there will be an initial increase in pro-inflammatory cytokines during wound healing, followed by an increase in anti-inflammatory cytokine over time in order to balance the bone remodeling process. Finally, our results will enable to establish preliminary baseline levels for several cytokines in various clinical situations.
Materials and methods
The population of this prospective longitudinal clinical trial was recruited from the patients referred to the School of Dentistry of the Federal University of Pelotas, Brazil, between June 2014 and June 2015. All recruited patients were edentulous in both the upper and the lower jaw and were wearing conventional complete dentures in both arches. Clinical atrophy was diagnosed for at least 3 years, and the patients experienced reduced stability and insufficient retention of the mandibular denture. Subjects were excluded if they had severe diabetes (poor glycemic control and hyperglycemia), bleeding disorders (hemorrhagic diathesis, drug-induced anticoagulation), serious systemic diseases (rheumatoid arthritis, osteogenesis imperfecta), compromised immune systems (HIV, immunosuppressive medications), a history of radiotherapy in the head or neck region, and had to present no previously inserted oral implants or were treated with bisphosphonates in the last 12 months.
The study was approved by the institutional Committed Ethics board for human subjects (protocol 1.267.086). Forty subjects were invited to participate in the research. They were informed about the treatment and the associated risks. After recruitment, 30 patients accepted to participate and signed an informed consent form.
Digital panoramic radiographs were performed (Rotograph Plus, Del Medical Imaging Corp., USA) and linear measurements related to morphology and mandibular height were performed with the DBSWIN software 4.5 (Dürr Dental, Bietigheim-Bissingen, Germany) by a single calibrated examiner following the methodology describe by Xie and Ainamo [24].
The following mandibular bone parameters were measured: mandibular body length, height in the anterior (midline) and posterior (molar region), and superior height of the foramina (distance from the top edge of the mentonian foramen to the alveolar ridge). The patients were classified as atrophic when their ridge height was below 25 mm in the anterior region and below 16 mm in the posterior region, according to the classification described by Cawood and Howell [25]. Each patient subsequently received two dental implants in the interforaminal region (ø2.9–10 mm Facility- NeoPorossurface, Neodent Osseointegrated Implants, Curitiba, Brazil).
Surgical and postoperative protocol
Dental implants were placed using a traditional single-stage surgical protocol performed by an experienced surgeon. Anesthesia was administrated afterwards. Full-thickness flaps were reflected and osteotomies were prepared 5 mm anterior to the mental foramina. Two dental implants were inserted in the drilled sites, respecting an inter-implant distance of 20 mm. Surgical sequence followed the protocol described by the implant manufacturer. Bone type and implant insertion torque were recorded during osteotomies and implant insertion. The experienced surgeon recorded the bone strength during the preparation of the bone site and the implant placement based on subjective perception. The bone type was then classified according the perceived bone density (dense bone and extremely soft bone), according to the classification of Lekholm and Zarb [26]. All mandibles in our study were classified as bone type I or II. The insertion torque values were dichotomized into high torque (>32 Ncm) and low torque (<32 Ncm) because the implant system used in this study has a surgical wrench with a fixed torque of 32 Ncm. Finally, the mucoperiosteal flaps were adapted around implant neck to allow non-submerged healing cap. The mandibular prosthesis was adjusted and an intermediate liner (Trusoft, Bossworth Company, USA) was fitted to surface until the end of the bone healing period. Mandibular overdentures were conventionally loaded after 3 months.
Postoperative medication included amoxicillin 500 mg three times a day during 7 days, ibuprofen 600 mg three times a day during 3 days, and paracetamol 500 mg four times a day as needed. Silk sutures were removed 10 days after surgery. Complete denture care instructions were provided for all patients and reinforced in each follow-up.
Implant stability analysis
The implant stability quotient (ISQ) was determined by RFA using Osstell® (Integration Diagnostics AB, Göteborg, Sweden). A Smartpeg™ was inserted manually into the internal connection of the healing cap and the RFA value was measured from four different directions (mesial, distal, buccal, and lingual). Measurements were performed at implant placement (baseline) and 1, 2, 4, 8, and 12 weeks after implant placement. Analyses were made in triplicate by the same examiner (AMB).
Clinical monitoring and data sample collection
Patients were submitted to clinical assessments after implant installation and peri-implant crevicular fluid (PICF) sample collection. The clinical parameters of the dental implant sites were evaluated by assessing the plaque index (PI) score, the presence of calculus, the probing depth (PD), the bleeding on probing index (BOP) [27], and the gingival index (GI) score. The GI was scored as follows: 0—normal peri-implant mucosa; 1—mild inflammation, slight change in color, slight edema; 2—moderate inflammation, redness, edema, and glazing; and 3—severe inflammation, marked redness, edema, and ulceration [27]. All measurements were performed at four sites around each implant (mesial, distal, buccal, and lingual) using a periodontal Goldman/Fox Williams probe in millimeters at 1, 2, 4, 8, and 12 weeks post implantation. The PD and the BOP were not assessed before the first week. Panoramic radiographs with standardized settings were taken at the implant surgery and abutment connection. The digital images were analyzed by an independent investigator and a calibrated examiner (RMMM), to measure the bone-level changes [28].
PICF collection and analysis
The peri-implant crevicular fluid was collected with standardized paper strips (Periopaper™, Proflow, Amityville, NY, EUA) 1, 2, 4, 8, and 12 weeks post implantation. The supragingival plaque was removed and each implant was dried for 10 s with compressed air and isolated with a cotton roll. Two paper strips were inserted separately for 40 s at the mesial and the distal surface of each implant. The paper strips were then placed in a single Eppendorf vial containing 100 μl phosphate-buffered saline and stored at −80 °C. Interleukin (IL-)1 beta (IL-1β), IL-6, IL-10, and tumor necrosis factor alpha (TNF-α) were quantified by enzyme-linked immunosorbent assay (ELISA) kits following the procedures recommended by the manufacturer (Duoset kit; R&D, Minneapolis, MN, USA). The standard solution and samples were added to wells, which had been pre-coated with specific monoclonal capture antibodies. After 3 h, polyclonal antibodies conjugated with horseradish peroxidase were added to each well and incubated for 1 h. A substrate solution containing hydrogen peroxidase and chromogen was added and allowed to react for 20 min. The cytokine levels were assessed by a micro-ELISA reader (Ultramark, Bio-Rad, CA, USA) at 450 nm and normalized to the abundance of standard solution. All analyzes were performed by a blinded technician.
Statistical analyses
The main outcome of our study was the cytokine release from all markers. However, the sample size was determined according to data reported in the study of Emecen-Huja et al. [29]. Because of the heterogeneity of the follow-up times, our sample size calculation was based on the IL-6 data. This cytokine required the largest number of dental implants to obtain a high power of the statistics test and to detect small effect sizes and significant differences in a paired study, especially during the early and late healing stages. The following parameters were used for the sample size calculation: a minimum expected difference between means of 0.5, standard deviations on the difference between means of 0.7, an effect size of 0.71, a beta error of 10%, and one-tailed alpha error of 5%. This resulted in a required sample size of 14 patients. However, based on previous investigations used to assess the peri-implant health [30,31,32] and based on the anticipated individual variations in cytokine responses during wound healing, the specific study design (inclusion of additional variables and observation time points) and accounting for potential losses and refusals, the sample size was doubled. These calculations thus estimate a minimum of 28 patients or 56 implants. For all statistical analyses, the implant was considered as the experimental unit. To investigate the clinical performance of dental implants, data related to smoking habits, bone atrophy presence, bone type, insertion torque (high or low), and clinical status of the peri-implant tissue were dichotomized. The Kolmogorov-Smirnov test showed that the clinical data was normally distributed, while Bartlett’s test indicated equal variances. Analyses were performed using a one-way ANOVA. The differences in ISQ measurements at each time point were calculated using paired t tests. However, the cytokine data violated the assumption of a normal distribution. Therefore, we used Wilcoxon’s matched pairs signed-rank test to compare the cytokine levels at different times of sampling (1, 2, 4, 8, and 12 weeks).
Data was adjusted using a multilevel analysis using the first assessed data as reference category for comparison. Differences in clinical parameter or cytokine level outcomes were estimated using a two-level mixed effects linear regression model with time of assessment (1, 2, 4, 8, and 12 weeks). Analysis were carried out using STATA 14.1 (StataCorp, College Station, TX, USA).
Results
Sixty implants were installed in 30 fully edentulous patients (10 males and 20 females; age range 49 to 89 years), with a mean edentulism time of 24.53 ± 13.29 years. The bone height in the anterior region was 23.42 ± 3.78 mm, mandibular bone atrophy was diagnosed in 13 patients, and five subjects were smokers. A total of 16 patients indicated periodontitis as the cause of their complete edentulism. Detailed demographic information is presented in Table 1. Twenty implants were inserted in bone type I and 40 implants in bone type II; insertion torque was above 32 N for 33 implants. At least 2 mm of keratinized mucosa was present around the implants and no radiographic changes in bone levels were noted (data not shown). In this study, 12 implants failed at different time points: one implant at week 8, six implants between weeks 8 and 12, and five implants at week 12. Only one patient lost 2 implants (cf. supplementary material: Tables S2; S3; S4).
Peri-implant tissue and stability monitoring
The plaque index (PI) showed two successive, statistically significant reductions from weeks 4 to 8 and from weeks 8 to 12. The gingival index (GI) significantly reduced from the first to the last week. The probing depth (PD) significantly decreased between 4 and 8 weeks (p < 0.001). The bleeding on probing (BOP) was stable throughout the experiment (p > 0.05; Table 2). A significant decrease in ISQs was seen after 4 weeks compared to the baseline measurements (p ≤ 0.05). This effect was more pronounced around implants with signs of gingival inflammation after the 1st and 2nd weeks of healing (p = 0.002; p = 0.05). The multilevel adjusted results revealed that possible confounders such as age, gender, and smoking did not influence significantly the results (Table 2).
Cytokine analysis
The median and range of cytokine concentrations in the PICF at 1, 2, 4, 8, and 12 weeks after implant installation are shown in Table 3. Comparative statistics of clinical parameters and cytokine levels at various time points are shown in Table S1. The mean TNF-α concentration significantly increased by 26.6% in the second week (p = 0.005) and remained roughly at the same level after week 4, dropping back to the baseline level at week 8. High levels of IL-1β and IL-6 were observed during the 1st week. Those concentrations gradually decreased between weeks 2 and 8, followed by an increase before the 12th week where the peak concentrations of IL-1β and IL-6 were recorded. The multilevel adjusted results revealed that possible confounders such as age, gender, and smoking did not influence significantly the results (Table 3).
Cytokine levels and clinical parameters
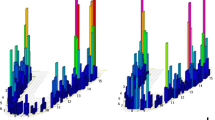
The influence of smoking habit, atrophy, bone type, and insertion torque on levels of TNF-α, IL-1β, IL-6, and IL-10 in the peri-implant fluid (pg/μl) during the osseointegration periods are shown in Figs. 1, 2, 3, and 4.
Smoking habits influenced the concentration of all cytokines. After week 2, statistically significant differences were found for IL-1β and IL-10 (p = 0.013; p = 0.020). Smokers had increased IL-1β release and decreased IL-10 release. After week 4, smokers had significantly lower TNF-α, IL-6, and IL-10 levels (p = 0.05; p = 0.022; and p = 0.002, respectively). After week 12, IL-1β release increased for smokers, while their IL-10 release decreased (p = 0.043; p = 0.004). Overall, the IL-10 levels were higher in non-smokers patients in all periods, except for the first week (p ≤ 0.05). Gingival inflammation was significantly associated with the peak in IL-1β and IL-6 concentrations after week 4 (p = 0.002; p = 0.033) and with high IL-6 and low TNF-α release after week 12 (p < 0.001; p = 0.042).
Bone atrophy did not affect the IL-10 release significantly. During the first and the second weeks, IL-1β and IL-6 levels were significantly higher in patients with atrophic jaws (IL-1β: p = 0.034; p = 0.023; IL-6: p = 0.011; p = 0.034, respectively). After week 4, TNF-α and IL-6 were significantly higher in atrophic patients (p = 0.006; p = 0.027). The TNF-α levels were significantly higher than the baseline levels after week 8 for atrophic patients (p = 0.007) and after week 12 for non-atrophic patients (p = 0.008). The IL-1β and IL-6 levels were significantly different in bone type I and type II at various times. After the second week, the IL-1β concentrations were higher in bone type I (p = 0.007), while IL-6 levels were higher in bone type II at weeks 2 and 4 (p = 0.001; p = 0.019). High insertion torque significantly influenced the IL-6 release after week 8 (p = 0.002).
Discussion
Our study investigated clinical and biological parameters of soft and bony tissue around dental implants in order to investigate the inflammatory-driven processes during dental implants osseointegration in mandibular edentulous patients. In order to achieve optimal standardization, two implants with same size and diameter were placed in the intraforaminal anterior region of mandible in 30 patients with prolonged edentulism (24.53 ± 13.29 years).
The biological parameters related to the healing process have been previously investigated [33, 34]. These studies mainly describe differences during the healing process according to the type of implant or implant loading. Most of the investigated biological markers were bone formation markers (transforming growth factor beta, osteoprotegerin, osteocalcin, osteopontin, and parathyroid hormone) and only inflammation scores were reported [32, 35, 36]. Because of this, the present literature does not inform on the early healing during osseointegration, especially in edentulous patients who experienced a long period of edentulism. In such patients, bone microarchitecture, can interfere with the blood supply and consequently, with the quality and intensity of cellular responses [37].
A statistically significant decrease in ISQs was occurred 4 weeks after initial measurements. Similar results have been obtained in the studies from Güncü et al. [34] and Gokmenoglu et al. [38], who found significant differences in ISQ after 4 and 8 weeks of bone healing, respectively. The implant stability around implant sites that presented inflammation reduced significantly after the 1st and 2nd weeks of healing (p = 0.002; p = 0.05). The ISQ values reported in other studies usually range between 60 to 84 [29, 31, 38,39,40,41]. The inferior range between 45 and 63 observed in our study can partially be explained by the connection between the smartpeg and the healing abutment, instead of a direct connection with the implant. Another aspect that contributes to the inferior stability is the bone type and the bone quality of the sample population. Our study recruited only edentulous patients that showed prolonged edentulism and decreased bone volume in the anterior region of the mandible. It is therefore not entirely unexpected that this specific population could require more time to reach the acceptable secondary stability, implying a need for longer follow-up periods.
Early healing periods during the osseointegration were previously studied [29, 39, 42, 43]. The results from Slotte et al. [39] open a new window for longitudinal studies in the field of peri-implant osteoimmunology. In this study, the authors indirectly inferred cytokine release via the messenger RNA levels, which can be degraded before translation into protein. To the best of the authors’ knowledge, the present study is the first that directly observes the temporal changes in cytokine protein release.
A limited number of clinical studies reported TNF-α concentration and their experimental designs are diverse [10, 29, 39, 44, 45]. Therefore, TNF-α’s precise release mechanism and function during the osseointegration period remain unclear, mainly because of the high variability of the reported TNF-α levels [44]. Other studies have shown weak or non-significant release of TNF-α [29, 39]. TNF-α release showed no difference when different types of abutments (rough and smooth) or types of loading (immediate and conventional) were used [10, 39]. In our study, TNF-α levels varied significantly over time with higher concentrations up to the 4th week, followed by a decrease. Since TNF-α is a potent stimulator of bone resorption and extracellular matrix degradation [46], the observation of an initial transient peak corroborates the current knowledge about osteoimmunology [39]. Previously, TNF-α was described as an ephemeral cytokine found in the peri-implant microenvironment only in the first 3 days of healing [47].
Interestingly, the TNF-α levels were significantly higher values at weeks 4 and 8 in atrophic mandibular ridges and at week 12 in non-atrophic mandibles. It is well established that TNF-α is involved in the metabolism of normal bone remodeling and can also be stimulated by the activation of the inflammatory process, therefore inducing bone resorption by controlling the differentiation and activity of osteoblasts and osteoclasts [48]. This could suggests that the intensity of the cellular events required for bone remodeling process is different for patients with bone atrophy, mainly because of the positive correlation between TNF-α release and systemic bone loss (osteoporosis, menopause and arthritis) [48, 49]. In smoking patients, the TNF-α release is higher during the early inflammatory response in the first 2 weeks. The latter can be explained by a suppressed release of anti-inflammatory cytokines (IL-10). However, after week 4, non-smokers released significantly more TNF-α (p = 0.05). This increased progressively until week 12, showing that the bone remodeling is more intense for non-smokers and that this process is delayed in smokers [50].
IL-1β was explored as another biomarker inducing remodeling of bone and connective tissue. A higher IL-1β concentration was observed during the first week, especially in response to the bone surgical trauma. This was not unexpected, and the effect of the antibiotic prescription on the cytokine release is thought to be minor at this early stage [42, 43]. Up to 8 weeks after implantation, no significant changes were found in the IL-1β levels, indicating that this cytokine is unable to discriminate changes in osseointegration during the healing process. Gokmenoglu et al. [38] also reported that IL-1β levels remained unchanged, even after treatment with light-emitting diode (LED) photomodulation in an attempt to increase osseointegration. Previous studies also suggests that IL-1β might enhance the healing process by protecting the open wound from bacterial colonization and invasion, in the postsurgical site and in inflamed site with plaque accumulation [51]. The protective role of this cytokine is illustrated in our trial. Increases in the plaque scores were found at week 8 along with contemporaneous increases IL-1β levels. The prolonged production of IL-1β was also found in other studies, where it may reflect the extent of tissue trauma and delayed wound healing [43, 51]. In this particular microenvironment, IL-1β initiates the inflammation cascade and can have a signaling and protective effect in the presence of bacterial biofilms mainly by having direct action in the inflammatory process and in the immune process of infections [52]. Petkovit el al. [53] reported that patients with clinically healthy peri-implant tissue with visible plaque had higher concentrations of IL-1β when the amount of local biofilm was higher. The latter further supports the hypothesis that this interleukin responds to the presence of local microorganisms and may generate a response of tissue destruction. Finally, Dogan et al. [31] also suggested that an overproduction of this cytokine may be involved in the modulation of periodontal destruction induced by biofilm, especially in diabetics.
IL-6 was included in our study, to explore cytokines related with the acute and chronic healing processes. Only two studies monitored its production during osseointegration [29, 44]. IL-6 has been associated with cases involving infection, trauma, and inflammatory states, rising within minutes of an traumatic event and remaining elevated for days. In addition, great tissue trauma has been associated with greater IL-6 production and inflammatory response. Therefore, IL-6 is a reliable marker of injury severity in the acute inflammatory response to surgery and trauma [54, 55]. Our study presents results that agree with these physiological functions. The increase in IL-6 levels at weeks 1 and 2 is likely connected to acute inflammatory response to implantation. The high values at week 12 were likely related to mucosa trauma caused by prosthesis settlement, since the PD values indicate that peri-implant tissue healing was completed. IL-6 production was significantly higher for atrophic patients during the first month, and higher concentrations were also found in patients with bone type II. These findings suggest that higher metabolic bone activity can be observed in edentulous patients with bone type II, probably due to increased blood supply. Currently, it is well established that the host’s response to implants is altered by smoking. For instance, pro-inflammatory cytokine release can be increased. Tatli et al. [56] reported an increased release of inflammatory markers in smoking patients in a population with osseointegrated implants. The latter may be in response to toxic by-products of smoking and the hot smoke itself. Our results suggest a lower IL-6 release in smoking patients until week 8, but this difference was only significant at week 4 (p = 0.022). After week 12, IL-6 release was similar in both smokers and non-smokers. However, the IL-6 levels of smokers were 73.4% higher than after week 8. This increase in IL-6 might be related to the longer interval between the follow-ups and a decreased concern of the patient regarding the implants, since the peri-implant soft tissues are already healed at this stage. Furthermore, IL-1β release was also significantly higher in smokers at week 2 and week 12 (p = 0.013; p = 0.043). This could also be in response to the toxic products generated by smoking and the hot smoke itself [56].
The role of IL-10 in during the osseointegration as an inhibitor of the pro-inflammatory process has not been explored in detail. Only two studies quantified this cytokine in the PICF, without conclusive results [29, 44]. Our study found a progressive increase in IL-10 release in the first 12 weeks, interpreted as an attempt of the host to resolve the inflammation process. IL-10 is an important endogenous suppressor of infection and bone resorption by suppressing osteoclastic differentiation [15]. So it can be suggested that IL-10 is an important regulator of bone homeostasis and inflammatory conditions [15], since low IL-10 levels cause insufficient inhibition of pro-inflammatory cytokines and collagenases activity [20]. In accordance with findings from other studies, IL-10 levels were significantly higher in non-smoking patients compared to smokers, which is potentially related to modification of macrophages activity [57, 58].
Narrow diameter implants (NDI) allow to rehabilitate edentulous jaws without the need for surgical interventions to increase bone availability, allowing a more conservative surgical intervention [59]. Therefore, we opted to use NDI treatment for our study population, because our sample population has a low mandibular bone availability and a high average age of 67.3 ± 7.3 years. Studies show that overdentures retained by small diameter implants have a survival rate of 82–100% [60,61,62]. These studies also point out that there is greater marginal bone loss in women, in patients with low bone height, in sites with low bone density, and around implants that received lower insertion torque [60]. Our study showed a survival rate of 80%, in line with previous studies, and 12 implants failed at different time points during this study and one patient lost both implants. The mean age of the patients who presented failures was 69 ± 8.74 years and their mean edentulism time was 24.41 ± 9.74 years. The majority of these patients were atrophic (66.7%), and most of the failures occurred in women (66.7%). Among these women, 62.5% were atrophic and had implant insertion torques below 32 N. The latter results are consistent with the results from Emami et al. [63], where in patients with a mean age of 62.39 ± 7.65 years rehabilitated with NDI-retained mandibular overdentures obtained a survival rate of 91.7%. These authors emphasize that bone quality and primary stability of the implant are the primary predictors of success of and that failures may be related to characteristics of the recipient bone bed and to the mechanical stress generated by the implants.
Finally, it is important to note that the clinical parameters showed no critical index values in these cases. Only mucositis was diagnosed and associated with lost implants, but this was not present in all lost implants. The ISQ values documented the loss of implant stability. After removal of the lost implants, these were replaced by 9-mm-long implants with 3.5 mm diameter (Titamax Cone Morse Implant - Neodent Implants Osseointegrated, Curitiba, Brazil). Three months after replacement, these patients underwent occlusal loading to install the mandibular overdentures. The success and survival rate of these implants was 100%. These data cannot be integrated with the data from the initial implants, because the new implants are not representative, but they may allow future studies to further investigate bone metabolism for these peri-implant sites.
The influence of antibiotics and anti-inflammatory medicines on biomarker release is not well-known. Most studies prescribe antibiotics for postsurgery protocols, but they do not discuss if influence on the biomarkers release. Khoury et al. [43], reported that systemic amoxicillin prescription may have a modest effect on clinical parameters during the first postoperative week and may have a negligible or limited effect on biomarkers. The authors also state that the amoxicillin may not be beneficial and that it may not be the correct antibiotic to prescribe for pre-surgical prophylaxis. In addition, Gomes et al. [64] report that short-term administration of non-steroidal anti-inflammatory drugs does not affect osseointegration, but may affect this process with long-term administration [64].
This study is the first one designed to prospectively evaluate osseointegration in totally edentulous patients taking into account bone atrophy, bone type, and smoking habits, and some limitations need to be addressed. In depth analysis of the mediation potential of different cytokines using larger sample populations could provide useful information. In addition, further stratification of the sample according to smoking habits, nutritional status, and gender, among others, would also be interesting. Understanding how different habits and behaviors interact with cytokine release may assist the dental practitioner in choosing the most efficient parameters for a more accurate diagnosis of dental implants’ success during the follow-ups. Our study evaluated only four types of cytokines; other inflammatory markers definitely play diverse roles during the healing process and may synergistically interact with the analyzed cytokines. Further investigation of IL-10 as a marker of bone metabolism and consolidation of osseointegration seems promising. We found some interesting points for future studies to investigate in more detail, such as the role of IL-10 and the high IL-10 release in sites with implant failure and the influence of bone atrophy or bone quality on pro-inflammatory cytokine release during the early stages of healing.
Conclusion
Our data provides new insights regarding cytokine release during the first 3 months following implant placement, and this was compared with results from clinical parameters. The first 2 weeks were critical for the plaque index and the gingival index. The probing depth stabilized at 8th week, while the ISQ values decreased after 4 weeks of healing. The variations in the cytokine concentrations and their predominant role can be attributed to the healing balance at different time periods. The cytokines likely interact synergistically with each other and show an association with clinical parameters and population characteristics. The bone type, smoking habits, and atrophy all affect the cytokine levels released during osseointegration of implant. Our study showed that not all evaluated cytokines have the potential to be markers for peri-implant monitoring health. In this respect, IL-10 seems to be the most promising cytokine. Our results provide a preliminary baseline for normal cytokine levels for patients with prolonged edentulism. While these first results are promising, future studies that expand this dataset are required in order to explore stratified data and accurately predict disease outcomes.
Change history
04 September 2017
An erratum to this article has been published.
References
Papaspyridakos P, Chen CJ, Singh M, Weber HP, Gallucci GO (2012) Success criteria in implant dentistry: a systematic review. J Dent Res 91(3):242–248
Duarte PM, de Mendonca AC, Maximo MB, Santos VR, Bastos MF, Nociti FH (2009) Effect of anti-infective mechanical therapy on clinical parameters and cytokine levels in human peri-implant diseases. J Periodontol 80(2):234–243
Trindade R, Albrektsson T, Tengvall P, Wennerberg A (2016) Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clin Implant Dent Relat Res 18(1):192–203
Shanbhag S, Shanbhag V, Stavropoulos A (2015) Genomic analyses of early peri-implant bone healing in humans: a systematic review. Int J Implant Dent 1(1):5
Javed F, Al-Hezaimi K, Salameh Z, Almas K, Romanos GE (2011) Proinflammatory cytokines in the crevicular fluid of patients with peri-implantitis. Cytokine 53(1):8–12
Mosmman T, Sad S (1996) The expanding universe of T-cell subset: Th1, Th2 and more. Immunol Today 17(3):138–146
Hall J, Pehrson N-G, Ekestubbe A, Jemt T, Friberg B (2015) A controlled, cross-sectional exploratory study on markers for the plasminogen system and inflammation in crevicular fluid samples from healthy, mucositis and peri-implantitis sites. Eur J Oral Implantol 8(2):153–166
Mundy GR (1991) Inflammatory mediators and the destruction of bone. J Periodontal Res 26(3 Pt 2):213–217
Li JY, Wang HL (2014) Biomarkers associated with periimplant diseases. Implant Dent 23(5):607–611
Boynuegri AD, Yalim M, Nemli SK, Erguder BI, Gokalp P (2012) Effect of different localizations of microgap on clinical parameters and inflammatory cytokines in peri-implant crevicular fluid: a prospective comparative study. Clin Oral Investig 16(2):353–361
Ataoglu H, Alptekin NO, Haliloglu S, Gursel M, Ataoglu T, Serpek B, Durmus E (2002) Interleukin-Iβ, tumor necrosis factor-α levels and neutrophil elastase activity in peri-implant crevicular fluid: correlation with clinical parameters and effect of smoking. Clin Oral Implants Res 13(5):470–476
Duarte PM, Serrao CR, Miranda TS, Zanatta LC, Bastos MF, Faveri M, Figueiredo LC, Feres M (2016) Could cytokine levels in the peri-implant crevicular fluid be used to distinguish between healthy implants and implants with peri-implantitis? A systematic review. J Periodontal Res 51(6):689–698
Faot F, Nascimento GG, Bielemann AM, Campao TD, Leite FR, Quirynen M (2015) Can peri-implant crevicular fluid assist in the diagnosis of peri-implantitis? A systematic review and meta-analysis. J Periodontol 86(5):631–645
Wu Y, Zhang C, Squarize CH, Zou D (2015) Oral rehabilitation of adult edentulous siblings severely lacking alveolar bone due to ectodermal dysplasia: a report of 2 clinical cases and a literature review. J Oral Maxillofac Surg 73(9):1733):e1–12
Zhang Q, Chen B, Yan F, Guo J, Zhu X, Ma S and Yang W (2014) Interleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseases. Biomed Res Int 2014 (284836)
Stow JL, Murray RZ (2013) Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev 24(3):227–239
Stow JL, Low PC, Offenhauser C, Sangermani D (2009) Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology 214(7):601–612
Kishimoto T (2010) IL-6: from its discovery to clinical applications. Int Immunol 22(5):347–352
Shrum JP (1996) Cytokines. Clin Dermatol 14:331–336
Guncu GN, Akman AC, Gunday S, Yamalik N, Berker E (2012) Effect of inflammation on cytokine levels and bone remodelling markers in peri-implant sulcus fluid: a preliminary report. Cytokine 59(2):313–316
Javed F, Ahmed HB, Crespi R, Romanos GE (2013) Role of primary stability for successful osseointegration of dental implants: factors of influence and evaluation. Interv Med Appl Sci 5(4):162–167
Cavallaro J, Greenstein B, Greenstein G (2009) Clinical methodologies for achieving primary dental implant stability. J Am Dent Assoc 140(11):1366–1372
Oates TW, Valderrama P, Bischof M, Nedir R, Jones A, Simpson J, Toutenburg H, Cochran DL (2007) Enhanced implant stability with a chemically modified SLA surface a randomized pilot study. Int J Oral Maxillofac Implants 22(5):755–760
Xie Q, Wolf J, Ainamo A (1997) Quantitative assessment of vertical heights of maxillary and mandibular bones in panoramic radiographs of elderly dentate and edentulous subjects. Acta Odontol Scand 55(3):155–161
Cawood JL, Howell RA (1988) A classification of the edentulous jaws. Int J Oral Maxillofac Surg 17(4):232–236
Molly L (2006) Bone density and primary stability in implant therapy. Clin Oral Implants Res 17(Suppl 2):124–135
Mombelli A, van Oosten MA, Schurch E Jr, Land NP (1987) The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol 2(4):145–151
Al-Nawas B, Bragger U, Meijer HJ, Naert I, Persson R, Perucchi A, Quirynen M, Raghoebar GM, Reichert TE, Romeo E, Santing HJ, Schimmel M, Storelli S, ten Bruggenkate C, Vandekerckhove B, Wagner W, Wismeijer D, Muller F (2012) A double-blind randomized controlled trial (RCT) of titanium-13Zirconium versus titanium grade IV small-diameter bone level implants in edentulous mandibles—results from a 1-year observation period. Clin Implant Dent Relat Res 14(6):896–904
Emecen-Huja P, Eubank TD, Shapiro V, Yildiz V, Tatakis DN, Leblebicioglu B (2013) Peri-implant versus periodontal wound healing. J Clin Periodontol 40(8):816–824
Onuma T, Aquiar K, Duarte PM, Feres M, Giro G, Coelho PG, Cassoni A, Shibli JA (2015) Levels of osteodastogenesis-related factors in the peri-implant crevicular fluid and clinical parameters of immediately loaded implants in patients with osteopenia: a short-term report. Int J Oral Maxillofac Implants 30(6):1431–1436
Dogan SB, Kurtis MB, Tuter G, Serdar M, Watanabe K, Karakis S (2015) Evaluation of clinical parameters and levels of proinflammatory cytokines in the crevicular fluid around dental implants in patients with type 2 diabetes mellitus. Int J Oral Maxillofac Implants 30(5):1119–1127
Tsoukaki M, Kalpidis CD, Sakellari D, Tsalikis L, Mikrogiorgis G, Konstantinidis A (2013) Clinical, radiographic, microbiological, and immunological outcomes of flapped vs. flapless dental implants: a prospective randomized controlled clinical trial. Clin Oral Implants Res 24(9):969–976
Taxel P, Ortiz D, Shafer D, Pendrys D, Reisine S, Rengasamy K, Freilich M (2014) The relationship between implant stability and bone health markers in post-menopausal women with bisphosphonate exposure. Clin Oral Investig 18(1):49–57
Güncü GN, Tözüm TF, Güncü MB, Yamalik N (2008) Relationships between implant stability, image-based measures and nitric oxide levels. J Oral Rehabil 35(10):745–753
Prati AJ, Casati MZ, Ribeiro FV, Cirano FR, Pastore GP, Pimentel SP, Casarin RCV, Author A, Division of Maxillofacial Surgery P, University SPB, Division of Periodontics PUS, Paulo B, Correspondence A, R.C.V. Casarin DoPPUSP and Brazil. Email cycb (2013) Release of bone markers in immediately loaded and nonloaded dental implants: a randomized clinical trial. J Dental Res 92(12):161S–167S
Basegmez C, Yalcin S, Yalcin F, Ersanli S, Mijiritsky E (2012) Evaluation of periimplant crevicular fluid prostaglandin E2 and matrix metalloproteinase-8 levels from health to periimplant disease status: a prospective study. Implant Dent 21(4):306–310
Raggatt LJ, Partridge NC (2010) Cellular and molecular mechanisms of bone remodeling. J Biol Chem 285(33):25103–25108
Gokmenoglu C, Ozmeric N, Erguder I, Elgun S (2014) The effect of light-emitting diode photobiomodulation on implant stability and biochemical markers in peri-implant crevicular fluid. Photomed Laser Surg 32(3):138–145
Slotte C, Lenneras M, Gothberg C, Suska F, Zoric N, Thomsen P, Nannmark U (2012) Gene expression of inflammation and bone healing in peri-implant crevicular fluid after placement and loading of dental implants. A kinetic clinical pilot study using quantitative real-time PCR. Clin Implant Dent Relat Res 14(5):723–736
Güncü MB, Aslan Y, Tümer C, Güncü GN, Uysal S (2008) In-patient comparison of immediate and conventional loaded implants in mandibular molar sites within 12 months. Clin Oral Implants Res 19(4):335–341
Tozum TF, Turkyilmaz I, Yamalik N, Tumer C, Kilinc A, Kilinc K, Karabulut E, Eratalay K (2005) Analysis of thte possible impact of inflammation severity and early and delayed loading on nitric oxide metabolism around dental implants. Int J Oral Maxillofac Implants 20(4):547–556
Gruber R, Nadir J, Haas R (2010) Neutrophil elastase activity and concentrations of interleukin 1-beta in crevicular fluid after immediate replacement and immediate loading of implants. Br J Oral Maxillofac Surg 48(3):228–231
Khoury SB, Thomas L, Walters JD, Sheridan JF, Leblebicioglu B (2008) Early wound healing following one-stage dental implant placement with and without antibiotic prophylaxis: a pilot study. J Periodontol 79(10):1904–1912
Nogueira-Filho G, Pesun I, Isaak-Ploegman C, Wijegunasinghe M, Wierzbicki T, McCulloch CA (2014) Longitudinal comparison of cytokines in peri-implant fluid and gingival crevicular fluid in healthy mouths. J Periodontol 85(11):1582–1588
Nowzari H, Botero JE, DeGiacomo M, Villacres MC, Rich SK (2008) Microbiology and cytokine levels around healthy dental implants and teeth. Clin Implant Dent Relat Res 10(3):166–173
Gurol C, Kazazoglu E, Dabakoglu B, Korachi M (2011) A comparative study of the role of cytokine polymorphisms interleukin-10 and tumor necrosis factor alpha in susceptibility to implant failure and chronic periodontitis. Int J Oral Maxillofac Implants 26(5):955–960
Dimitriou R, Tsiridis E, Giannoudis PV (2005) Current concepts of molecular aspects of bone healing. Injury 36(12):1392–1404
Wei L, Sun Y, Kong XF, Zhang C, Yue T, Zhu Q, He DY, Jiang LD (2016) The effects of dopamine receptor 2 expression on B cells on bone metabolism and TNF-alpha levels in rheumatoid arthritis. BMC Musculoskelet Disord 19(17):352
Lim HS, Park YH, Kim SK (2016) Relationship between serum inflammatory marker and bone mineral density in healthy adults. J Bone Metab 23(1):27–33
Lang NP, Salvi GE, Huynh-Ba G, Ivanovski S, Donos N, Bosshardt DD (2011) Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res 22(4):349–356
Graves DT, Nooh N, Gillen T, Davey M, Patel S, Cottrell D, Amar S (2001) IL-1 plays a critical role in oral, but not dermal, wound healing. J Immunol 167(9):5316–5320
Carta S, Tassi S, Semino C, Fossati G, Mascagni P, Dinarello CA, Rubartelli A (2006) Histone deacetylase inhibitors prevent exocytosis of interleukin-1beta-containing secretory lysosomes: role of microtubules. Blood 108(5):1618–1626
Petkovic AB, Matic SM, Stamatovic NV, Vojvodic DV, Todorovic TM, Lazic ZR, Kozomara RJ (2010) Proinflammatory cytokines (IL-1beta and TNF-alpha) and chemokines (IL-8 and MIP-1alpha) as markers of peri-implant tissue condition. Int J Oral Maxillofac Surg 39(5):478–485
Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M (2011) Analytic review: interleukin-6 in surgery, trauma, and critical care: part I: basic science. J Intensive Care Med 26(1):3–12
Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M (2011) Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med 26(2):73–87
Tatli U, Damlar I, Erdogan O, Esen E (2013) Effects of smoking on periimplant health status and IL-1beta, TNF-alpha, and PGE2 levels in periimplant crevicular fluid: a cross-sectional study on well-maintained implant recall patients. Implant Dent 22(5):519–524
Rioux N, Castonguay A (2001) 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone modulation of cytokine release in U937 human macrophages. Cancer Immunol Immunother 49(12):663–670
Takanashi S, Hasegawa Y, Kanehira Y, Yamamoto K, Fujimoto K, Satoh K, Okamura K (1999) Interleukin-10 level in sputum is reduced in bronchial asthma, COPD and in smokers. Eur Respir J 4:309–314
Zweers J, van Doornik A, Hogendorf EA, Quirynen M, Van der Weijden GA (2015) Clinical and radiographic evaluation of narrow- vs. regular-diameter dental implants: a 3-year follow-up. A retrospective study. Clin Oral Implants Res 26(2):149–156
Preoteasa E, Imre M, Preoteasa CT (2014) A 3-year follow-up study of overdentures retained by mini-dental implants. Int J Oral Maxillofac Implants 29(5):1170–1176
de Souza RF, Ribeiro AB, Della Vecchia MP, Costa L, Cunha TR, Reis AC, Albuquerque RF Jr (2015) Mini vs. standard implants for mandibular overdentures: a randomized trial. J Dental Res 94(10):1376–1384
Catalan A, Martinez A, Marchesani F, Gonzalez U (2016) Mandibular overdentures retained by two mini-implants: a seven-year retention and satisfaction study. J Prosthodont 25(5):364–370
Emami E, Cerutti-Kopplin D, Menassa M, Audy N, Kodama N, Durand R, Rompre P, de Grandmont P (2016) Does immediate loading affect clinical and patient-centered outcomes of mandibular 2-unsplinted-implant overdenture? A 2-year within-case analysis. J Dent 50:30–36
Gomes FI, Aragao MG, de Paulo Teixeira Pinto V, Gondim DV, Barroso FC, Silva AA, Bezerra MM, Chaves HV (2015) Effects of nonsteroidal anti-inflammatory drugs on osseointegration: a review. J Oral Implantol 41(2):219–230
Acknowledgments
The authors thank Neodent for supplying the dental implants used in the study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
There was no grant support or funding for this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The research protocol was approved by the Ethics Committee of School of Dentistry (Number 1.267.086/2015), Federal University of Pelotas. Patients and their families or legal representatives provided signed written informed consent.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
The original version of this article was revised: the results of the cytokine analyses (page 5 and Table 3) should be referred to as “median” instead of “means”. In addition, the significant differences between the categorized groups in the Figs. 1–4 were indicated by asterisks, but these asterisks disappeared during the conversion of the figures’ source files.
An erratum to this article is available at https://doi.org/10.1007/s00784-017-2191-2.
Rights and permissions
About this article
Cite this article
Bielemann, A.M., Marcello-Machado, R.M., Leite, F.R.M. et al. Comparison between inflammation-related markers in peri-implant crevicular fluid and clinical parameters during osseointegration in edentulous jaws. Clin Oral Invest 22, 531–543 (2018). https://doi.org/10.1007/s00784-017-2169-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2169-0