Abstract
Objectives
The aim of this longitudinal study was to compare the oral health of chronic kidney disease patients at the predialysis (baseline) and post-transplantation (follow-up) stages and to investigate differences in oral health between diabetic nephropathy and other kidney disease patients at follow-up.
Materials and methods
Fifty-three kidney disease patients (34 men) aged 31–86 years were followed up to 157 months. Clinical and radiological oral examinations, salivary and laboratory analyses, and oral health behavior questionnaires were conducted at the predialysis and follow-up stages at Helsinki University Hospital, Finland. Oral inflammatory burden was estimated by calculating deep periodontal pockets, periodontal inflammatory burden (PIBI), decayed, missing, and filled teeth (DMFT), and total dental indices (TDI). Results were analyzed using cross-tabulation Pearson chi-square or Fisher’s exact test and the Mann-Whitney U test, and the McNemar and Wilcoxon signed-rank test.
Results
At the predialysis stage, patients more often had calculus and deep periodontal pockets; TDI, PIBI, number of teeth, and salivary flow rates were also statistically significantly higher compared to follow-up. At follow-up, diabetic nephropathy patients more often had Candida growth, more plaque, and used more drugs and had lower stimulated salivary flow than patients with other kidney diseases.
Conclusion
Oral health was better at follow-up than at the predialysis stage; however, attention should be given to the lower salivary flow rate and higher number of drugs used at that stage.
Clinical relevance
This study confirms the importance of treating oral infectious foci at the predialysis stage in order to prevent adverse outcomes after kidney transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplantation is the treatment of choice for end-stage kidney disease because it improves patient survival, is cost effective, and offers better life quality compared with dialysis treatment [1, 2]. Kidney transplant surgery is programmed after the candidate has been screened for malignancies and infections. Poor oral health is common among chronic kidney disease (CKD) patients worldwide [3]. In these lines, foci of oral infections should be treated before exposing the patient to immunosuppressive medications.
CKDs are an increasing health concern worldwide because of their increasing prevalence and the economic impact on public health care. Hypertension, diabetes, and obesity may irreversibly affect kidneys leading to insufficiency. Many systemic rheumatologic diseases and infections cause persistent inflammation that may also contribute to chronic kidney disease [4]. Periodontitis is a bacteria-induced infection of tooth supporting tissues causing not only local but also low-grade systemic inflammation, which has been suggested to be a risk factor for CKD [5].
Patients with an organ transplant need immunosuppressive drugs to prevent rejection for the rest of their lives. As a consequence of immunosuppression, these patients may develop oral lesions like gingival overgrowth induced by ciclosporin or by calcium channel blockers, or oral candidiasis, viral infections, and malignancies, such as lip cancer [6–8]. Since patients are susceptible to oral lesions after organ transplantation due to their immunosuppressive or antihypertensive medication, it is important to examine these patients regularly to allow early detection of oral diseases and facilitate proper treatment to avoid major complications. To our knowledge, only one longitudinal study has been published on clinical and/or self-reported oral health where CKD patients are followed up from predialysis to the post-transplantation stage, and this was by our group [9]. In the present study on the same patient material, the primary aim was to further investigate oral infections and mucosal lesions, and especially the prevalence of periodontitis, at these two stages of kidney disease. Another aim was to study the oral inflammatory burden at follow-up among diabetic nephropathy patients and to compare it with CKD patients with some other etiology of their kidney disease. A corresponding comparison of the patients at predialysis has been published by our group [10, 11]. Our third aim was to investigate whether different immunosuppressive drugs have different impacts on oral health. Finally, we wanted to study self-reported oral health behavior at the predialysis and post-transplantation stages using a questionnaire.
Our hypothesis was that oral health and oral health behavior are better after transplantation, since eradication of oral infection foci is routinely made in our hospital at the predialysis stage, before commencing dialysis or kidney transplantation. We have previously found that compared with non-diabetic patients, diabetic nephropathy associates with worse periodontal health and higher oral inflammatory burden at the predialysis stage [10, 11]. We further assumed that patients with CKD etiology other than diabetes would have better oral health after transplantation compared to the diabetic nephropathy patients.
Subjects and methods
The ethical committee of the Helsinki and Uusimaa Hospital District had approved the study, which was conducted according to the principles of the Declaration of Helsinki (Dnro 305/13/03/02/2012).
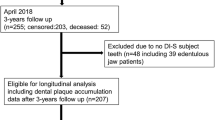
This is a longitudinal study with a total follow-up time of 157 months. Fifty-three kidney transplant patients aged 31 to 86 years were originally examined and treated at the predialysis stage between the years 2000 and 2005 at the Departments of Nephrology and Oral and Maxillofacial Diseases of Helsinki University Hospital, Helsinki, Finland (Table 1). Details of these baseline examinations have been published earlier [10, 11]. In brief, 144 CKD patients were examined at the predialysis stage with emphasis on periodontal inflammatory burden. The inclusion criterion was an estimated glomerular infiltration rate (eGFR) of <20 ml/min/1.73 m2, corresponding to mid-CKD stage 4 (eGFR <30 ml/min/1.73 m2) and stage 5 (eGFR <15 ml/min/1.73 m2). At the time of the present follow-up examination, 65 patients out of the 144 were deceased. Thus, 79 patients were invited to the re-examination between 2013 and 2015. Of these, 26 patients dropped out because they did not want to participate or for other reasons such as moving away from the Helsinki and Uusimaa Hospital district area or living in a remote location. The study profile is shown in Fig. 1.
The 53 CKD patients were clinically and radiologically examined, and unstimulated and stimulated salivary flow rates were measured. Candida growth was assessed using routine microbiological methods in the clinical microbiology unit of the university hospital laboratory.
Clinical oral examination comprised oral mucosal, periodontal, and cariological examination and was performed by the same periodontist (HR) as in the baseline. Mucosal disorders such as gingival overgrowth, coated tongue, ulcers, lichen planus, lichenoid reaction, and leukoplakia were recorded. Calculus index, gingival recessions, periodontal/implant pocket depths, and clinical attachment levels were also recorded. Number of teeth and implants, signs of attrition/erosion, diastemas, mobility of teeth, and furcation lesions were further recorded.
DMFT (decayed, missing, and filled teeth) index was calculated from 32 teeth following the WHO criteria except for “missing teeth” parameter, where wisdom teeth were also recorded. Prostheses (removable/fixed) were separately recorded. Total dental index (TDI, from 32 teeth) and periodontal inflammatory burden index (PIBI, from 28 teeth) were calculated to describe the total inflammatory burden of the mouth. TDI is the sum of scores from caries, periodontitis, periapical, and pericoronitis lesions; the index score may vary from 0 to 10 [12]. PIBI is calculated by adding the number of moderate periodontal pockets (4 to 5 mm) to the weighted number of advanced periodontal pockets ≥6 mm [13].
The Centers for Disease Control and Prevention and the American Academy of Periodontology (CDC/AAP) joint definition for periodontitis was used to classify patients with no, mild, moderate, or severe periodontitis [14]. In the present study, however, comparison between predialysis and follow-up stages could only be made with moderate periodontitis cases because clinical attachment levels had not been recorded at predialysis. Visible plaque index (VPI, four sites from 32 teeth) and bleeding on probing (BOP, six sites from 28 teeth) index were also recorded. These indices were not available from the predialysis stage records.
Panoramic jaw radiographs had been taken from all patients and were analyzed by a specialist in oral radiology in our hospital. Maximum bone loss in each sextant (cervical, middle, and apical bone loss of root lengths), periapical lesions, cysts, tumors, and signs of pericoronitis were recorded from the radiographs.
A structured questionnaire recorded patients’ self-reported oral health behavior. Health habits such as smoking (current smoker and non-smoker; former smoker was not recorded), alcohol use (none or monthly/weekly/daily), oral hygiene habits (tooth brushing alone or combined with interdental cleaning), last visit to dental office, and consideration of the importance of oral health for kidney disease were recorded from an oral health questionnaire for the present study. Tooth brushing and alcohol consumption were not included in questionnaires at the predialysis stage. Other results from the questionnaires concerning quality of life will be published in further study.
Statistical analyses
This study focuses on clinical oral health between the baseline (predialysis stage) and follow-up (post-transplantation).
IBM SPSS statistics for Windows, version 22 (IBM Corp., Armonk, NY, USA), was used to conduct the statistical analyses. Cross-tabulation with Pearson chi-square test and risk test for odds ratios were used to analyze the results of demographic and clinical data comparing diabetic nephropathy patients with the other CKD patients. The Mann-Whitney U test was used when comparing continuous variables between diabetic nephropathy and other CDK patients and the Pearson chi-square or Fisher test when comparing categorical variables. When comparing within-subject changes between predialysis and follow-up, p values were calculated using the McNemar test for categorical variables and the Wilcoxon signed-rank test for continuous variables. The non-parametric Mann-Whitney U test, McNemar test, and Wilcoxon signed-rank test were chosen due to the relatively small sample size and non-normal distribution of the continuous variables. The Shapiro-Wilk test was used to assess the normality of the distribution of the continuous variables.
Results
Demographic and oral health data at the follow-up stage
Of the 53 participants, 11 had diabetic nephropathy (8 DM1 nephropathy, 3 DM2 nephropathy) while 42 had some other CKD etiology (15 cases with polycystic kidney disease, 10 IgA nephropathy, 4 focal segmental glomerulosclerosis, 4 unspecified glomerulosclerosis, 2 interstitial nephritis, 1 chronic pyelonephritis, 1 nephroptosis, 1 SLE nephritis, and 4 kidney failure of unknown etiology). Fifty-one patients had received a kidney transplant, while 2 patients had never received a transplant because of their poor physical health secondary to generalized severe atherosclerosis. Follow-up time ranged from 20 to 157 months. During the median follow-up of 128 months, (interquartile range 115 to 140) five kidney transplants failed and these patients returned to dialysis. Reasons for transplant failures were one post-transplant lymphoproliferative disease (PTLD), one chronic rejection, and three unknown reasons for graft function decline. These patients were not considered candidates for re-transplantation because of their serious general condition. After follow-up, 46 kidney transplants were functioning.
The total number of drugs used daily varied from 3 to 19 (median 9). Diabetic patients took more drugs compared to the other CKD patients (p = 0.001). Also, diabetic patients were more often treated with a calcium channel blocker than the other CKD patients (81.8 vs. 40.5%, p = 0.015). The most common combination of immunosuppressive drugs used to prevent rejection was ciclosporin combined with mycophenolate. No statistically significant difference was found between different drug combinations. CKD patients were mostly non-smokers (Table 1).
Oral mucosal lesions were found in 28% of CKD patients. Diabetic patients had oral Candida infection (N = 8, 72.7%) more often than the other CKD patients (N = 10, 25%) (p = 0.001). The odds ratio of Candida infection in diabetic nephropathy patients versus other CKD patients was 8.0 (95% CI, 1.722 to 36.127). Gingival overgrowth was detected in three (27.3%) of the diabetic nephropathy patients and in three (7.1%) of the other CKD patients (n.s.). Other oral lesions were found in 15 patients, with coated tongue the most common (N = 12, 22.6%) finding followed by lip herpes (N = 2, 3.8%), hemangioma-like lesion (N = 1, 1.9%), and fibroma-like lesion (N = 1, 1.9%). Lip herpes was presumably reactivation of latent Herpes simplex virus 1. One patient had both coated tongue and fibroma-like lesion.
There was no statistically significant difference in the number of teeth, TDI, PIBI, DMFT index values, or in periodontal parameters of these two groups of patients. VPI median was higher among diabetic patients compared to the other CKD patients (p = 0.037). The odds ratio over median VPI values in diabetic nephropathy patients versus other CKD patients was 4.8 (95% CI, 1034 to 22,293). The stimulated salivary flow rate was significantly lower among diabetic nephropathy patients versus other CKD patients; the odds ratio for less than median stimulated salivary flow rate was 15.6 (95% CI 1.821–134.040). However, oral self-care worked well in both groups, since 60% of diabetic nephropathy and 65% of the other CKD patients used to brush their teeth with fluoride toothpaste and interdental cleaning aids.
Of the diabetic patients, 55% and, of the other CKD group, 76% had visited a dentist in the past year. Oral health care was considered to be very important for kidney disease by 55% of diabetic nephropathy patients compared to 88% of the other CKD patients (p = 0.017). More details are given in Table 2.
Oral health comparison between predialysis and follow-up stages
At the predialysis stage, all 53 patients had calculus, compared with 77.4% at follow-up (p < 0.001). Periodontal pockets (≥1 site with 4 mm or deeper periodontal pocket) were more prevalent at the predialysis stage than at follow-up (83 vs. 36.2%, p < 0.001). Deep periodontal pockets (≥2 sites with 6 mm or deeper) were also more frequent at the predialysis stage compared with follow-up (20.8 vs. 5.7%, p = 0.021). TDI, PIBI, number of teeth, and salivary flow rates in terms of medians were statistically significantly higher at predialysis than at follow-up. DMFT index median was the same in both groups, while the prevalence of gingival overgrowth was lower at follow-up.
However, the number of drugs was higher at the follow-up stage. Table 3 compares predialysis stage examination results with those at follow-up.
No statistically significant difference was found in the number of partial or overdenture prostheses, maximum alveolar bone loss, mobility of teeth, signs of diastemas, attrition and erosion, or furcation lesions between predialysis and follow-up (data not given). No statistically significant association could be seen between the use of calcium channel blockers and ciclosporin, alone or in combination, and the prevalence of gingival overgrowth, when comparing the predialysis results with follow-up.
There was no statistically significant difference in the frequency of dental visits, although 72% of patients at the follow-up stage and 52% at the predialysis stage had visited a dentist in the last year (Table 3).
Discussion
This longitudinal clinical study confirmed our hypothesis that patients at the follow-up examination (when most of them had a functioning kidney transplant) had better clinical oral health compared to baseline findings at the predialysis stage. Statistically significantly fewer deep periodontal pockets, lower TDI and PIBI scores, and lower salivary flow rates were found among the patients at follow-up. Our results emphasize the importance of the practice (as used in our hospital) of eradicating oral infectious foci at the predialysis stage in order to prevent adverse outcomes after kidney transplantation.
When comparing diabetic patients with other CKD patients at follow-up, diabetic patients more often had visible plaque, more oral candidiasis, and lower stimulated salivary flow rate. However, more than half of the diabetic patients claimed to use interdental cleaning aids along with brushing with fluoride toothpaste. Notably, according to the questionnaires, diabetic patients did not consider oral health care to be as meaningful for kidney disease as the other CKD patients.
The current study is important since, to the best of our knowledge, only one longitudinal clinical study has been published following CKD patients all the way from predialysis stage to post-transplantation stage, and this is the study from the same patient material as reported here [9]. Most earlier studies describe findings only at the dialysis and post-transplantation stages [6, 15, 16]. In our previous study, we followed nine of our CKD patients for 10 years through predialysis and dialysis to post-transplantation but found no statistically significant differences in clinical oral symptoms or signs, although salivary proteins (IgG, IgA, IgM, albumin) and urea decreased during the follow-up [9]. In these patients, the mean salivary flow rate was the lowest post-transplantation, probably due to the immunosuppressive and cardiovascular medication [9]. But by the time of those analyses, only a few patients had gone through the whole treatment scale of kidney disease. Hence, the present findings on 53 patients are more reliable.
Bots and co-workers (2007) followed 43 patients up to 2 years from dialysis to kidney transplantation. They found that salivary flow rate increased after transplantation and BOP decreased. The authors reported that oral dryness, xerostomia, and thirst are continuing problems in patients on dialysis and that reduced salivary flow rate is reversible and is restored after transplantation [15]. We found that salivary flow rate decreased while the number of daily drugs increased from predialysis to follow-up. This confirms earlier results from elderly patients that the more drugs used daily, the less saliva [17].
In the cross-sectional study by Thorman et al. (2009), uremic patients in predialysis and dialysis had a worse DMFT index score, periodontal loss of attachment, and more periapical lesions compared to age- and gender-matched healthy controls [16]. In our patients, scores of oral inflammatory burden (TDI, PIBI values) and the number of deep periodontal pockets decreased from the uremic predialysis stage to follow-up; at which time, most of our patients had received a new kidney and had also had proper dental treatment right from the predialysis stage. After transplantation, patients are advised to take care of their oral health with regular oral examinations and maintenance. Even though the p value is statistically significant when comparing DMFT between baseline and follow-up, this has no clinical importance since there is no actual difference in the median numbers and is merely explained by the sensitivity of the Wilcoxon signed-rank test due to the transgression of a few random values. The range between minimum and maximum values shifts from 4–32 to 8–33 during follow-up.
Kidney transplantation is the preferred treatment for end-stage renal disease in many regards. It brings better life quality to the patient, it is cost effective, and it increases survival compared with other kidney function replacement therapies [2, 18–21]. According to the European Renal Association and the European Dialysis and Transplant Association (ERA, EDTA), 2-year and 5-year survival rates for all dialysis patients were recently 77.5 and 52.5%, respectively, compared with survival rates of 96 and 91.5% after the first kidney transplantation [21]. Of our 144 patients originally examined, 65 died during the follow-up of 157 months. Among those who had died, diabetic nephropathy diagnosis was more common than the other CKD diagnoses. Although no causality can be drawn from the present results, oral infection may affect graft function [22–24]. For example, Helenius-Hietala et al. (2013) have shown in liver transplant patients that poor oral health does indeed affect prognosis [25]. Furthermore, according to Zwiech et al. (2013), poor oral health was associated with increased risk of acute 1-year rejection after kidney transplantation [24]. In the present study, only five kidney transplants failed during the follow-up. Although no oral infections were found to have caused graft losses, we again emphasize the importance of proper diagnosis and treatment of oral foci right at the predialysis stage.
CKD patients at the follow-up stage had better oral health and less oral inflammation than uremic patients at the predialysis stage. However, the present results clearly show that diabetic nephropathy is the disease of particular concern in relation to oral health. Diabetic nephropathy patients had lower stimulated salivary flow rate, more candidiasis, and more dental plaque and did not consider oral health care to be of the same importance as the other CKD patients; thus, clinicians should be alert in this respect, properly diagnose and treat oral infections, and keep patients in regular maintenance therapy.
The strength of our study was the longitudinal design: patients were followed from predialysis through dialysis up to the post-transplant stage. The study was carried out in Finland, which has an ethnically homogeneous Caucasian population. Our university hospital is the tertiary referral center for kidney disease patients and the only national center for organ transplant operations. Thus, the patients presented here represent the whole population in this respect.
One limitation is the fairly small number of patients who during the follow-up of up to 157 months had gone through the whole treatment panorama. Another weakness is the fact that when commencing the study, not all parameters were recorded, unlike the case later in the follow-up examination. This prevented a comparison of certain periodontal parameters. Although oral health showed improvements after eradication of oral foci at the predialysis stage, oral maintenance therapy might have played a significant role since all the transplant patients are referred to a maintenance program. Furthermore, for practical reasons, no healthy controls could be investigated, and thus, the results are based on a comparison between baseline and follow-up results in the same patients.
To conclude, since immunosuppression after organ transplantation may trigger latent oral infections and may thus predispose the patient to severe systemic complications due to hematogenic spread of these infections, oral infections must be diagnosed and treated properly before transplantation. For the same reason, all transplantation patients should then be referred for oral disease prevention as well as for a regular dental maintenance program.
References
Sanchez-Escuredo A, Alsina A, Diekmann F et al (2015) Economic analysis of the treatment of end-stage renal disease treatment: living-donor kidney transplantation versus hemodialysis. Transplantation Proc 47(1):30–33
Jensen CE, Sorensen P, Petersen KD (2014) In Denmark kidney transplantation is more cost-effective than dialysis. Dan Med J 61(3):A4796
Ruospo M, Palmer SC, Craig JC et al (2014) Prevalence and severity of oral disease in adults with chronic kidney disease: a systematic review of observational studies. Nephrol Dial Transplant 29(2):364–375
Eckardt KU, Coresh J, Devuyst O et al (2013) Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 382:158–169
Fisher MA, Taylor GW, Shelton BJ et al (2008) Periodontal disease and other nontraditional risk factors for CKD. Am J Kidney Dis 51(1):45–52
Kaswan S, Patil S, Maheshwari S, Wadhawan R (2015) Prevalence of oral lesions in kidney transplant patients: a single center experience. Saudi J Kidney Dis Transpl 26(4):678–683
Rezvani G, Davarmanesh M, Azar MR et al (2014) Oral manifestations of allograft recipients before and after renal transplantation. Saudi J Kidney Dis Transpl 25(2):278–284
Seymour RA, Thomason JM, Nolan A (1997) Oral lesions in organ transplant patients. J Oral Pathol Med 26(7):297–304
Vesterinen M, Ruokonen H, Leivo T et al (2007) Oral health and dental treatment of patients with renal disease. Quintessence Int 38(3):211–219
Vesterinen M, Ruokonen H, Furuholm J, Honkanen E, Meurman JH (2011) Oral health in predialysis patients with emphasis on diabetic nephropathy. Clin Oral Investig 15(1):99–104
Nylund K, Meurman JH, Heikkinen AM, Honkanen E, Vesterinen M, Ruokonen H (2015) Oral health in predialysis patients with emphasis on periodontal disease. Quintessence Int 46(10):899–907
Mattila KJ, Nieminen MS, Valtonen VV et al (1989) Association between dental health and acute myocardial infarction. Br Med J 25(298):779–781
Lindy O, Suomalainen K, Mäkelä M, Lindy S (2008) Statin use is associated with fewer periodontal lesions: a retrospective study. BMC Oral Health 8(16)
Page RC, Eke PI (2007) Case definition for use in population-based surveillance of periodontitis. J Periodontol 78(Suppl.7):1387–1399
Bots CP, Brand HS, Poorterman JH et al (2007) Oral and salivary changes in patients with end stage renal disease (ESRD): a two year follow-up study. Br Dent J 202:E7
Thorman R, Neovius M, Hylander B (2009) Clinical findings in oral health during progression of chronic kidney disease to end-stage renal disease in a Swedish population. Scand J of Urol 43:154–159
Närhi TO, Meurman JH, Ainamo A (1999) Xerostomia and hyposalivation causes, consequences and treatment in elderly. Drugs Aging 15(2):103–116
Ortiz F, Aronen P, Koskinen PK et al (2014) Health-related quality of life after kidney transplantation: who benefits the most? Transpl Int 27:1143–1151
Laupacis A, Keown P, Pus N et al (1996) A study of the quality of life and cost-utility of renal transplantation. Kidney Int 50:235–242
Haapio M, Helve J, Kyllonen L, Gronhagen-Riska C, Finne P (2013) Modality of chronic renal replacement therapy and survival—a complete cohort from Finland, 2000-2009. Nephrol Dial Transplant 28:3072–3081
Pippias M, Stel VS, Abad Diez JM et al (2015) Renal replacement therapy in Europe: a summary of the 2012 ERA-EDTA Registry Annual Report. Clin Kidney J 8(3):248–261
Greenberg MS, Cohen G (1977) Oral infection in immunosuppressed renal transplant patients. Oral Surg Oral Med Oral pathol 43(6):879–885
Lucas VS, Roberts GJ (2005) Oro-dental health in children with chronic renal failure and after renal transplantation: a clinical review. Pediatr Nephrol 20(10):1388–1394
Zwiech R, Bruzda-Zwiech A (2013) Does oral health contribute to post-transplant complications in kidney allograft recipients? Acta Odontol Scand 71(3–4):756–763
Helenius-Hietala J, Åberg F, Meurman JH, Isoniemi H (2013) Increased infection risk postliver transplant without pretransplant dental treatment. Oral Dis 19:271–278
Acknowledgments
This study was funded by hospital research funding under EVO grant TYH20012128 (EVO comes from the Finnish words “erityisvaltionosuusrahoitus,” which in English means “discretionary state funding”) and by the Finnish Dental Society Apollonia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by hospital research funding under EVO grant TYH20012128 (EVO comes from the Finnish words “erityisvaltionosuusrahoitus,” which in English means “discretionary state funding”) and by the Finnish Dental Society Apollonia.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
The ethical committee of the Helsinki and Uusimaa Hospital District had approved the study, which was conducted according to the principles of the Declaration of Helsinki (Dnro 305/13/03/02/2012).
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Nylund, K.M., Meurman, J.H., Heikkinen, A.M. et al. Oral health in patients with renal disease: a longitudinal study from predialysis to kidney transplantation. Clin Oral Invest 22, 339–347 (2018). https://doi.org/10.1007/s00784-017-2118-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2118-y