Abstract
Objectives
The objective of this study is to determine the potential for microcracks in the radicular dentin of first maxillary premolars using three different mechanized endodontic instrumentation systems.
Methods
Eighty extracted maxillary first premolars with two root canals and no externally visible microcracks were selected. Root canal instrumentation was performed with either the ProTaper file system, the WaveOne primary file, or the self-adjusting file (SAF). Teeth with intact roots served as controls. The roots were cut into segments and examined with an intensive, small-diameter light source that was applied diagonally to the entire periphery of the root slice under ×20 magnification; the presence of microcracks and fractures was recorded. Pearson’s chi-square method was used for statistical analysis, and significance was set at p < 0.05.
Results
Microcracks were present in 30 and 20 % of roots treated with the ProTaper and WaveOne systems, respectively, while no microcracks were present in the roots treated with the SAF (p = 0.008 and p = 0.035, respectively). Intact teeth presented with cracks in 5 % of the roots. The intensive, small-diameter light source revealed microcracks that could not be detected when using the microscope’s light alone.
Conclusions
Within the limitations of this study, it could be concluded that mechanized root canal instrumentation with the ProTaper and WaveOne systems in maxillary first premolars causes microcracks in the radicular dentin, while the use of the SAF file causes no such microcracks.
Clinical relevance
Rotary and reciprocating files with large tapers may cause microcracks in the radicular dentin of maxillary first premolars. Less aggressive methods should be considered for these teeth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nickel titanium (NiTi) rotary file systems are currently the leading technology in root canal instrumentation [1, 2]. Nevertheless, a study by Shemesh et al. in 2009 [3] first revealed that the efficiency of rotary instrumentation might also have a biomechanical cost by creating microcracks in the radicular dentin.
Since this initial report [3], more than 15 studies have been published that support the findings of the initial report [4–18]. Such microcracks can eventually propagate and lead to the formation of full-thickness fractures in the radicular dentin, which are also known as vertical root fractures (VRFs) [5, 7, 9]. Two recent studies have questioned the cause-effect relationship between instrumentation of the root canals with rotary/reciprocating files and the presence of microcracks in the radicular dentin [19, 20].
Many terms have been used in the above-mentioned studies, including “craze lines” [3, 7, 21], “microcracks” [7], and the comprehensive term “dentinal defects” [3, 4, 6, 7, 22], which includes all of the above.
The field of fracture mechanics defines a “catastrophic fracture” as a fracture that breaks the tested material or object into fully separate parts. Such a fracture often starts with microscopic cracks (microcracks) in the material that can propagate under additional stress and eventually result in a fracture [23]. In the present report, we have used the proper mechanical term microcracks to describe all partial discontinuities in the dentin, which are usually observed with transmitted light under a microscope [7, 11, 14], and the terms “fracture” and “full-thickness fracture” to describe a full-thickness discontinuity in the radicular dentin wall.
VRFs can appear in many types of teeth; however, the maxillary premolars are among those most susceptible to VRFs [24–26]. This susceptibility has been attributed to the high convexity of the shape of these roots [21, 24, 27–29]. Such high convexity is the factor that is common to all roots that are highly susceptible to VRFs [26, 30]. The phenomenon of microcracks has been most commonly studied in the mandibular premolars [3–6, 31]. Maxillary first premolars are different because they often have relatively thin roots that could potentially make them more susceptible to microcrack formation, particularly when mechanized instrumentation is applied.
The present research was designed to study the phenomenon of microcracks that could potentially be caused by mechanized endodontic instrumentation in maxillary first premolars.
Materials and methods
Eighty-five intact maxillary first premolars with two separate root canals, straight roots, and closed, mature apices were selected from a random collection of recently extracted teeth. All teeth were pre-examined with an operating microscope (Kaps 1100, Karl Kaps, Asslar/Wetzlar, Germany) under ×12 magnification using an intensive, small-diameter (1.6 mm) light source (HDP-2, Radiant Lighted Instrument System, Q-Optics, Duncanville, TX, USA) that was applied around the root while rotating the root manually. This procedure was performed to exclude any externally detectable defects or cracks in the roots. Such cracks and defects were found in 5 (5.8 %) of the initially collected teeth that appeared intact. Teeth with these defects/microcracks were excluded from the present study, leaving 80 teeth with no defects/microcracks that were randomly divided into 4 groups of 20 teeth each. Both canals of each premolar were instrumented in the present study.
Access cavities were prepared, and initial canal length was determined using #10 K files (Dentsply Maillefer, Ballaigues, Switzerland), which were inserted into the root canal until the tip of the file was observed at the apical foramen. Only teeth with apical patency were included. Working length was established at 1 mm short of the initial canal length. To avoid dehydration, all teeth and root segments (see below) were stored in water and kept wet throughout the experimental procedures. A single experienced operator (DE) performed all procedures.
The crowns of all teeth were removed using a diamond-coated burr with a water-air cooling spray, and the remaining roots were approximately 16 mm in length. Silicon impression material (vinyl polysiloxane impression material, 3M ESPE, Seefeld, Germany) was used to coat the cement surfaces of the roots to simulate a periodontal ligament. After the silicone coating had set, all teeth were embedded in acrylic blocks and randomly divided into four groups.
Root canal preparation
All canals were prepared with a #20 NiTi hand file to the working length to establish a glide path, except for the intact control group, which was left unprepared.
Group 1: untreated controls (n = 20)
The canals in this group were unprepared and served as controls.
Group 2: ProTaper files (n = 20)
The canals were prepared with the ProTaper system following the manufacturer’s instructions. The ProTaper files (Dentsply Maillefer) were applied using a WaveOne electric motor (WaveOne endo motor, Dentsply Maillefer), which was operated at 300 rpm according to the manufacturer’s instructions. A ProTaper SX file was used to enlarge the coronal third of the canal. A ProTaper S1 file was advanced into the canal to working length, followed by S2, F1, F2, and F3 files applied sequentially to working length. Irrigation with 3 % sodium hypochlorite was applied between each instrument using a syringe and a 30-gauge needle. The needle was inserted as far as it could go at any stage of instrumentation and was withdrawn 2 mm before irrigation was applied. A total of 12 mL of sodium hypochlorite was used in each canal. Glide (Dentsply Maillefer) lubricant was applied to each instrument before insertion into the canal.
Group 3: WaveOne primary file (n = 20)
The canals were prepared with the WaveOne file following the manufacturer’s instructions. The canals were prepared with the WaveOne primary file (Dentsply Maillefer) using the WaveOne electric motor operated with a 1:6 reducing handpiece. The motor was set for the angles of reciprocation and the speed of the WaveOne instruments according to the manufacturer’s instructions. The files were used with pecking movements with minimal apical pressure. After every two pecking motions, the instrument was withdrawn, the flutes were cleaned with gauze, and the canal was irrigated. The procedure was repeated until the working length was reached. Irrigation with 3 % sodium hypochlorite was applied between each insertion of the instrument using a syringe and a 30-gauge needle. The needle was inserted as far as it could go at any stage of instrumentation and was withdrawn 2 mm before irrigation was applied. A total of 12 mL of sodium hypochlorite was used in each canal. Glide lubricant was applied to the file before each insertion into the canal.
Group 4: self-adjusting file (n = 20)
The canals were prepared with the SAF system following the manufacturer’s instructions. A glide path for the SAF instrument was first prepared using a ProFile 20/04 file (Dentsply Maillfer), which was used to working length. This was done as an integral part of the SAF procedure following the manufacturer’s instructions. A 1.5-mm SAF file (ReDent-Nova, Raanana, Israel) was then dipped in the Glide lubricant and tested manually in the canal to verify that it could reach working length. The file was then attached to the RDT handpiece head, which was operated at 5000 rpm and resulted in 5000 in-and-out vibrations per minute with an amplitude of 0.4 mm (RDT3, ReDent-Nova). The file was used with a pecking motion to working length for 4 min in each canal. Continuous simultaneous irrigation with 3 % sodium hypochlorite was applied using a peristaltic pump (VATEA, ReDent-Nova) at a flow rate of 3 mL/min. A total of 12 mL of irrigant was used in each canal.
Sectioning and microscopic examination
All roots were sectioned perpendicular to their long axes at 3, 6, and 9 mm from the apex using a diamond-coated saw (Isomet 1000 precision saw, Bueller, Lake Bluff, IL, USA) under a continuous water stream.
Each specimen was then checked for the presence of dentinal defects/microcracks. The specimens were checked independently by two observers who were blinded to the group to which a given sample belonged and who were initially checked for inter-observer reproducibility. The examination was performed using an operating microscope (Kaps) at ×20 magnification. The sliced surface was first checked using the light source of the microscope. The light source was then turned off, and an intensive light source with a small (1.6 mm) diameter was applied diagonally (at approximately 45° to the sectioned plane) to the entire periphery of the root slice; both root canals of all premolars were examined for dentinal microcracks or complete, full-thickness fractures. “No defect” was defined as root dentin that presented with no visible microcracks or fractures. “Defect” was defined by microcracks or complete fractures in the root dentin. Each tooth with both its roots/canals comprised the counting unit. A tooth with a microcrack or fracture in one or more of its segments was defined as a tooth with defects.
Lines connecting the two canals in a single root often represented an isthmus-like structure and were not considered defects. In addition, any visible line at the border between two types of dentin (primary and secondary or tertiary) was not counted as a defect.
Digital images of sections with defects were captured at ×31 magnification using a digital camera (Sony Alpha NEX-7, Sony, Tokyo, Japan) attached to the microscope. A total of 60 segments were examined in each group for a total of 240 root segments. The results were expressed as the number and percentage of teeth with defects or cracks out of the total number of teeth in each group.
Statistical analysis
Pearson’s chi-square test was used to compare the groups, and the significance level was set at p < 0.05.
Results
Detectable defects in the total initial sample
A total of 85 recently extracted and visually intact maxillary first premolars were initially screened for the presence of microcracks or fractures that could be visualized externally using magnification and an intensive, small-diameter light source. In five of these teeth (5.8 %), such defects were found. These five teeth were excluded from the present study, leaving 80 teeth with no detectable microcracks or fractures.
Incidence of microcracks after instrumentation
In the ProTaper-treated group, 6 of 20 (30 %) teeth presented with microcracks or fractures in the radicular dentin (Fig. 1). In the WaveOne-treated teeth, 4 of 20 (20 %) presented with microcracks, while in the non-treated control group, one tooth (5 %) presented with a microcrack. In the group instrumented with the SAF system, no microcracks were found (Fig. 1). The difference between the untreated controls and the ProTaper-treated group was significant (Table 1). The differences between both the ProTaper-treated and the WaveOne-treated groups compared to the SAF-treated group were significant (p = 0.008 and p = 0.035, respectively) (Table 1). The other differences, including the difference between the ProTaper- and the WaveOne-treated groups, were not significant.
Microcracks and fractures caused by instrumentation of the root canals of first maxillary premolars. Percentage of roots presenting with microcracks or fractures. Control untreated control, PT ProTaper, WO WaveOne, SAF self-adjusting file. Similar letters indicate no significant difference while different letters indicate a significant difference
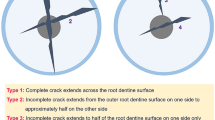
Type and location of the defects
In the ProTaper-treated group, two roots (10 %) presented with full-thickness fractures, while the remainder of the 10 defects found in all groups in this study were defined as microcracks (Table 2). These microcracks started at either the inner surface (3/10) or the outer surface (7/10) of the root (Table 2). Most of the microcracks and fractures (7/12) were found at 6 mm, 4 of the 12 were found at 3 mm, and 1 was found at 9 mm from the apex.
The effect of type of illumination on the detection of microcracks
The type of illumination used for checking the root segments had a substantial effect on the detection of microcracks (Fig. 2). When examined with the intensive, small-diameter light source applied diagonally, microcracks could be observed in root slices but could not be detected using the light source of the microscope alone. The difference was that the diagonal application of the intensive light allowed the depth of the slice to be checked, while the light source of the microscope only allowed observation, which was limited to the cut surface. Wetness on the surface of the root segment also had a negative effect on the detection of microcracks, and a short blast of air was therefore used to remove wetness.
Intensive small-diameter light source vs. microscope illumination. Diagonal illumination with an intensive, small-diameter light source allows for the examination of what occurs in the depth of the root slice, while the light source of the microscope has a tendency to show only the surface of the slice. a Root slice illuminated with the microscope light source: no microcracks are visible. b The same root slice as in a, illuminated diagonally with an intensive small-diameter light source: a microcrack is clearly visible. c A suspected line, as seen under microscope illumination. d The same root slice as in c: diagonal illumination with a small-diameter intensive light source revealed the real nature of the suspected line: a microcrack
Discussion
Microcracks are often found in intact extracted teeth. In the present study, 5.8 % of the initial sample of apparently intact extracted maxillary premolars presented with externally detectable microcracks. Similar findings have been reported in other studies. Such pre-existing microcracks could originate from either normal or excessive wear and tear in vivo [32, 33], could be related to the extraction procedure [34, 35], or both. Arias et al. [19] found that 50 % or more of the untreated control roots in their study had microcracks. This might be explained by the nature of their sample, which consisted of teeth from cadavers of a selective elderly population with a mean age of 82.8 (±14.6) years. Therefore, there should be no question that some microcracks do exist in roots before any endodontic treatment is applied.
More than 19 studies [3–20] have been published that address the issue of whether certain mechanized endodontic instrumentation systems might result in a higher incidence of microcracks.
Such studies can be divided into those that present a control group with no microcracks or almost no microcracks [3–7, 9–17] and those that either failed to present such a group [18] or reported a high incidence of microcracks in the untreated control group [8, 19, 20].
The difference in results between the two groups of studies might have arisen from the pre-selection of teeth for the studies. In some studies, such as Arias et al. [19], no such pre-selection was possible because the model attempted to reproduce the in vivo situation by instrumenting the teeth while they were still in the jaw of a cadaver. Other studies have also indicated a high incidence of microcracks in the non-treated control group [8, 20]. Pre-existing microcracks could be a confounding variable that limits the interpretation and the statistical power of the sample, particularly when relatively small sample sizes are used. The detectable cracks that were found in the teeth that were rejected from the present study could not be seen unless the intensive, small-diameter light source was used. Simple microscopic observation using the light source of the microscope might have failed to detect many of these cracks during the selection process.
The three mechanized file systems used in the present study differ significantly, and each was used according to its manufacturer instructions, which naturally differ substantially. Both the WaveOne and ProTaper protocols recommend glide path preparation with hand files. On the other hand, the current SAF protocol recommends the use of a size 20/04 rotary file for glide path preparation.
The WaveOne primary file was selected for this study because the next file in this system, the Wave One Large, is apical size 40 with an apical taper of 0.08 and was considered too large for maxillary first premolars. The selection of F3 (apical size 30 with an apical taper of 0.09) as the final file of the ProTaper sequence was based on previous studies that indicate that the smaller size ProTaper (F2, with apical size 20) is too small for the canals of these teeth, the apical size of which is often larger than #25 [36, 37]. F3 was also selected to facilitate comparisons to previous studies [7, 8, 10] that also used the ProTaper F3 as the final file. It seems that the differences in size and taper as well as mode of action may have contributed to the differences in the incidence of microcracks reported here.
An interesting finding of the present study was that even though a rotary mechanized file (ProFile 20/04) was used for glide path preparation in the SAF group, no microcracks were found in this group. This finding might be explained by two studies by Kim et al. [22, 38]. In both, a finite element analysis model was used to analyze the stress generated at the surface layer of the radicular dentin during instrumentation of the root canal. When large rotary files (Profile 30/06 and ProTaper F3) were tested, von Mises stresses as high as 311 and 386 MPa were found in the outer layer of dentin for the ProFile 30/06 and ProTaper F3, respectively. Such values are three times greater than the tensile strength of the dentin, which is 106 MPa [39]. Such stress can result in a continuity failure of the dentin (microcracks) at the area of stress concentration. By contrast, when smaller files (ProFile 20/06 and ProTaper F1) were used in the same model, in the second study by Kim et al. [38], the stress recorded was much smaller, at 86.7 and 98.1 MPa for ProFile 20/06 and ProTaper F1, respectively. These last values were smaller than those recorded with the larger files in these same series, and they were lower than the reported tensile strength of dentin [39].
These last findings might also potentially explain the differences found in the present study: the ProFile 20/04 that was used for glide path formation in the SAF group caused no microcracks, while the full sequence of ProTaper files and the Primary WaveOne file caused microcracks in 30 and 20 % of the teeth, respectively. It is likely that it was not the rotary or reciprocating action of the file per se that was the determining factor, but rather, the size and taper of the files combined with the rotary/reciprocating motion were the potential causative factors.
The finding that the SAF file did not cause microcracks is in agreement with previous studies, which found either no cracks [7] or very small numbers of cracks [31] in roots treated with this mechanized instrument. The second study by Kim et al. [38] also explained the above results: The SAF, which was also tested in that study, caused almost no stress in the radicular dentin when operated in their root model [38]. It is likely that the absence of a central metal core in the SAF file and its extreme compressibility [40–42] might explain the difference between this file and the other rotary or reciprocating file systems.
It is important to note that the intensive, small-diameter light source applied diagonally around the perimeter of the root segments when examining the root slices could greatly affect the results (Fig. 2).
The finding that one (5 %) of the teeth in the untreated control group had a microcrack might have resulted from the limitations of the selection process. It is likely that even when the teeth are carefully examined with the intensive light source, as in the present study, some internal microcracks are not detected and are found only after the root segments are examined. Such sporadic findings might represent “background noise” in the selection process and might also explain the single tooth that was found to have a microcrack in an SAF-treated group previously reported by Hin et al. [31]. Such potential “background noise” might require that larger groups of teeth be used in future studies to reduce its potential effect on the results. As to a potential effect of such “noise” in the present study, even if one tooth with a defect is to be deducted from both the WaveOne and ProTaper groups, the significant difference between these two groups and the SAF group still holds.
The results of the present study and their interpretation are in agreement with most of the previously published reports [3–18]. The present results were in disagreement with two recent studies. This is most likely due to the very high incidence of microcracks in the control groups [19, 20], which was a confounding factor that was common to both studies.
Conclusions
Within the limitations of the present study, it could be concluded that mechanized root canal instrumentation with a ProTaper up to F3 or a WaveOne primary file in maxillary first premolars caused microcracks in the radicular dentin, while the use of the SAF file caused no such microcracks. An intensive, small-diameter light source should be used in similar studies because it could detect microcracks that could not otherwise be seen.
References
Ersev H, Yılmaz B, Çiftçioğlu E, Özkarslı ŞF (2010) A comparison of the shaping effects of 5 nickel-titanium rotary instruments in simulated S-shaped canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:e86–e93
Narayan GS, Venkatesan SM, Karumaran CS, Indira R, Ramachandran SMR (2012) A comparative evaluation on the cleaning and shaping ability of three nickel titanium rotary instruments using computerized tomography—an ex vivo study. Contemp Clin Dent 3:S151–S155
Shemesh H, Bier CA, Wu MK, Tanomaru-Filho M, Wesselink PR (2009) The effects of canal preparation and filling on the incidence of dentinal defects. Int Endod J 42:208–213
Bier CAS, Shemesh H, Tanomaru-Filho M, Wesselink PR, Wu MK (2009) The ability of different nickel-titanium rotary instruments to induce dentinal damage during canal preparation. J Endod 35:236–238
Shemesh H, Roeleveld AC, Wesselink PR, Wu M-K (2011) Damage to root dentin during retreatment procedures. J Endod 37:63–66
Adorno CG, Yoshioka T, Suda H (2011) Crack initiation on the apical root surface caused by three different nickel-titanium rotary files at different working lengths. J Endod 37:522–525
Yoldas O, Yilmaz S, Atakan G, Kuden C, Kasan Z (2012) Dentinal microcrack formation during root canal preparation by different NiTi rotary instruments and the self-adjusting file. J Endod 38:232–235
Barreto MS, Moraes RA, da Rosa RA, Moreira CHC, So MVR, Bier CAS (2012) Vertical root fractures and dentin defects: effects of root canal preparation, filling and mechanical cycling. J Endod 38:1135–1139
Bürklein S, Tsotsis P, Schäfer E (2013) Incidence of dentinal defects after root canal preparation: reciprocating versus rotary instrumentation. J Endod 39:501–504
Liu R, Xiang Hou B, Wesselink PR, Wu M-K, Shemesh H (2013) The incidence of root microcracks caused by 3 different single-file systems versus the ProTaper system. J Endod 39:1054–1056
Liu R, Kaiwar A, Shemesh H, Wesselink PR, Hou B, Wu MK (2013b) Incidence of apical root cracks and apical dentinal detachments after canal preparation with hand and rotary files at different instrumentation lengths. J Endod 39:129–132
Ashwinkumar V, Krithikadatta J, Surendran S, Velmurugan N (2014) Effect of reciprocating file motion on microcrack formation in root canals: an SEM study. Int Endod J 47:622–627
Kumaran P, Sivapriya E, Indhramohan J, Gopikrishna V, Savadamoorthi KS, Pradeepkumar AR (2013) Dentinal defects before and after rotary root canal instrumentation with three different obturation techniques and two obturating materials. J Conser Dent 16:522–526
Kansal R, Rajput A, Talwar S, Roongta R, Verma M (2014) Assessment of dentinal damage during canal preparation using reciprocating and rotary files. J Endod 40:1443–1446
Çapar ID, Arslan H, Akcay M, Uysal B (2014) Effects of ProTaper Universal, ProTaperNext, and HyFlex instruments on crack formation in dentin. J Endod 40:1482–1484
Karataş E, Gündüz HA, Kırıcı DÖ, Arslan H, Topçu MÇ, Kübra Yeşildal Yeter KY (2015) Dentinal crack formation during root canal preparation by Twisted File Adaptive, ProTaper Next, ProTaper Universal and WaveOne instruments. J Endod 41:261–264
Abou El Nasr HM, Abd El Kader KG (2014) Dentinal damage and fracture resistance of oval roots prepared with single-file systems using different kinematics. J Endod 40:849–851
Pop I, Manoharan A, Zanini F, Tromba G, Patel S, Foschi F (2014) Synchrotron light-based μCT to analyse the presence of dentinal microcracks post-rotary and reciprocating NiTi instrumentation. Clinl Oral Invest. doi:10.1007/s00784-014-1206-5
Arias A, Lee YHBS, Peters CI, Gluskin AH, Peters OA (2014) Comparison of 2 canal preparation techniques in the induction of microcracks: a pilot study with cadaver mandibles. J Endod 40:982–985
De-Deus G, Silva EJ, Marins J, Souza E, Neves Ade A, Gonçalves Belladonna Alves H, Lopes RT, Versiani MA (2014) Lack of causal relationship between dentinal microcracks and root canal preparation with reciprocation systems. J Endod 40:1447–1450
Lertchirakarn V, Palmera JE, Messer HH (2003) Patterns of vertical root fractures: factors affecting stress distribution in the root canal. J Endod 29:523–528
Kim HC, Lee MH, Yum J, Versluis A, Lee CJ, Kim BM (2010) Potential relationship between design of nickel-titanium rotary instruments and vertical root fracture. J Endod 36:1195–1199
Anderson TL (2005) Fracture mechanics: fundamentals and applications, 3rd edn. Taylor & Francis, New York
Tamse A, Zilburg I, Halpern J (1998) Vertical root fractures in adjacent maxillary premolars: an endodontic-prosthetic perplexity. Int Endod J 31:127–132
Tamse A, Fuss Z, Lustig J, Ganor Y, Kaffe I (1999) Radiographic features of vertically fractured, endodontically treated maxillary premolars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88:348–352
Khasnis SA, Kidiyoor KH, Patil AB, Kenganal SB (2014) Vertical root fractures and their management. J Conser Dent 17:103–110
Lertchirakarn V, Palamara JEA, Messer HH (2003) Finite element analysis and strain-gauge studies of vertical root fracture. J Endod 29:529–534
Sathorn C, Palamara JEA, Messer HH, Palamara D (2005) Effect of root canal size and external root surface morphology on fracture susceptibility and pattern: a finite element analysis. J Endod 31:288–292
Chai H, Tamse A (2012) Fracture mechanics analysis of vertical root fracture from condensation of gutta-percha. J Biomechanics 45:1673–1678
Reeh ES, Messer HH, Douglas WH (1989) Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod 15:512–516
Hin ES, Wu M-K, Wesselink PR, Shemesh H (2013) Effects of Self-Adjusting File, Mtwo and ProTaper on the root canal wall. J Endod 39:262–264
Kishen A (2006) Mechanisms and risk factors for fracture predilection in endodontically treated teeth. Endod Top 13:57–83
Tang W, Wu Y, Smales RJ (2010) Identifying and reducing risks for potential fractures in endodontically treated teeth. J Endod 36:609–617
Onnink PA, Davis RD, Wayman BE (1994) An in vitro comparison of incomplete root fractures associated with three obturation techniques. J Endod 20:32–37
Shemesh H, van Soest G, Wu MK, Wesselink PR (2008) Diagnosis of vertical root fractures with optical coherence tomography. J Endod 34:739–742
Kfir A, Rosenberg E, Fuss Z (2006) Comparison in vivo of the first tapered and nontapered instruments that bind at the apical constriction 102; 395–398
Hecker H, Bartha T, Löst C, Weiger R (2010) Determining the apical preparation size in premolars: part III. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110:118–124
Kim HC, Sung SY, Ha J-H, Solomonov M, Lee J-M, Lee C-J, Kim B-M (2013) Stress generation during Self-Adjusting File movement: minimally invasive instrumentation. J Endod 39:1572–1575
Powers JM, Sakaguchi RL (2006) Craig’s restorative dental materials, 12th edn. Elsevier, New York
Metzger Z, Teperovich E, Zary R, Cohen R, Hof R (2010) The self-adjusting file (SAF). Part 1: respecting the root canal anatomy-a new concept ofendodontic files and its implementation. J Endod 36:679–690
Hof R, Perevalov V, Eltanani M, Zary R, Metzger Z (2010) The self-adjusting file (SAF). Part 2: mechanical analysis. J Endod 36:691–696
Metzger Z (2014) The self-adjusting file (SAF) system: an evidence-based update. J Conser Dent 17:401–441
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study from any source.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This study was done on teeth that were selected from a random collection of extracted teeth, which were extracted for various reasons, not related to this study. Thus, for this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Kfir, A., Elkes, D., Pawar, A. et al. Incidence of microcracks in maxillary first premolars after instrumentation with three different mechanized file systems: a comparative ex vivo study. Clin Oral Invest 21, 405–411 (2017). https://doi.org/10.1007/s00784-016-1806-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1806-3