Abstract
Aim
The aim of this study was to systematically review the literature to answer the questions: (i) “Is periodontal treatment effective to improve clinical and immunological conditions in obese subjects?”; (ii) “Do obese subjects present different clinical and immunological response after periodontal therapy when compared to non-obese subjects?”
Methods
Searches were performed in six databases up to August 2014. Interventional studies were included if the following data were described: (1) Obesity/overweight assessment; (2) definition of periodontal disease; (3) periodontal therapy; (4) inflammatory marker in serum/plasma, and/or clinical parameters of periodontal disease. Assessment of quality was performed with the Downs and Black scale. Meta-analyses were conducted with the available data.
Results
Of 489 articles, 5 were included, and only 3 proceeded to meta-analysis of clinical outcomes. Included studies presented fair methodological quality. Statistical analysis demonstrated that periodontal therapy in obese subjects was effective to improve clinical outcomes. No clinical differences between post-therapy results of obese and non-obese were observed. Effects of periodontal therapy on inflammatory markers remain unclear.
Conclusions
Periodontal treatment seems to be effective to improve healing in obese individuals. No differences on periodontal healing between obese and non-obese subjects were observed; however, only limited and fragile base of evidence was available for analysis.
Clinical relevance
Periodontal treatment is effective to improve clinical and immunological periodontal parameters in adults. Also, obesity seems to not modify the periodontal healing after treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A plausible biologic relationship between obesity and periodontal disease may exist. Studies in adults and in children have investigated this possible relationship, and majority of them found a positive association between both conditions [1–3]. In addition, systematic reviews have exhaustedly explored this connection, supporting the evidences from clinical and epidemiological studies [4, 5]. However, the causal mechanisms behind this positive association remain unclear, once the cross-sectional design adopted by most of the studies does not allow a temporal relationship between presumed exposure and outcome [6].
Some mechanisms try to explain this association, considering different approaches such the socioeconomic, the behavioral, the life-course but always emphasizing the biologic one [7]. In essence, the white adipose tissue (WAT) is responsible for secretion of many inflammatory cytokines, such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), and specific adipocytokines such as leptin and adiponectin [8]. Additionally, some adipocytes could suffer hypoxia due to WAT hypertrophy. The dying adipocytes have the potential to secret pro-inflammatory cytokines and to increase the chemoattraction of monocytes to the WAT, exacerbating the cytokine secretion. Serum increase of pro-inflammatory mediators is responsible for producing a low-grade systemic inflammation and insulin resistance, influencing the host immune response [9]. Thus, the ability to identify and combat possible infectious agents is totally unbalanced, becoming the obese subjects more susceptible to systemic infections [10]. It has been shown that toll-like receptors (TLR) in periodontal tissues of obese subjects are desensitized by pro-inflammatory cytokines, which become less effective to periodontopathogen recognition, resulting in development and progression of periodontal diseases [5].
Periodontal therapy conceptually aims to eliminate both living bacteria in the microbial biofilm and calcified biofilm microorganisms. From a practical point of view, this therapy intends to reduce the number of biofilm microorganisms and disturb the ecology the microbial biofilm. As a consequence, the host immune response can better cope with the remaining microorganisms, decreasing the inflammatory load present in the affected sites [11]. The response of periodontal therapy in obese subjects has been discretely studied. As similarly observed in type II diabetes, periodontal therapy success may present some specificities due to the potential modifier effect caused by obesity on periodontal tissues [12]. Previously conducted reports presented a low number of patients enrolled and a lack of consensus between the clinical results; hence, the effects of periodontal therapy on clinical and immunological parameters of obese subjects are not totally clear. Two systematic reviews have been published on the topic, but their results should be carefully considered. The first included observational and interventional studies without performing a meta-analysis, which limits the authors’ results [13]. The latter included participants with systemic disease affecting the periodontal response, such as diabetes. Even though the authors had mentioned they intended to control their results, it was not possible [14]. Thus, the question of whether obesity impacts on periodontal healing remains to be answered.
Since obesity may interfere on periodontal disease development and progression and given the knowledge obtained from diabetes studies, the effect of periodontal therapy on obese subjects is unsure. Also, it is expected that obese individuals might present different clinical and immunological outcomes after periodontal therapy when compared to eutrophic subjects. Based on that, the aim of the present study was to systematically review the literature in order to explore the clinical and systemic immunological impact of periodontal therapy on obese subjects and also to compare obese and non-obese individuals regarding the clinical parameters and immunological markers level after periodontal treatment.
Methods
Review questions
Is periodontal treatment effective to improve clinical and immunological conditions in obese subjects?
Are clinical and immunological responses after periodontal therapy of obese subjects different when compared to non-obese subjects?
Inclusion and exclusion criteria
Potential articles to be included in this review were independently examined by two reviewers (GGN and FRML) according to the following inclusion criteria:
-
1.
Interventional studies assessing the effects of periodontal therapy on obese individuals and/or comparing the effects of periodontal therapy on obese and non-obese subjects;
-
2.
Adults enrolled in the study (≥18-year-old);
-
3.
Obesity/overweight assessment (e.g., body mass index, waist/hip ratio, waist circumference);
-
4.
Definition of periodontal disease;
-
5.
Presence of periodontal therapy (non-surgical therapy: supra, sub gingival scaling and root planing; surgical therapy: open flap debridement with or without the use of regenerative materials);
-
6.
Evaluation of at least one inflammatory marker in serum; and/or evaluation of clinical parameters of periodontal disease before and after periodontal therapy.
Additionally, the following exclusion criteria were observed:
-
1.
Use of systemic medication (anti-inflammatory, antibiotics) 6 months previous to periodontal therapy;
-
2.
Presence of systemic conditions that could affect the progression of periodontitis and/or gain/loss of weight;
-
3.
Cell culture or animal studies;
-
4.
Observational studies;
-
5.
Letters to the editor, reviews, and conference abstracts.
In cases of disagreement, articles were discussed between the reviewers until a consensus was reached.
Strategy search
Literature was searched in a structured way to identify papers that analyzed the effect of periodontal therapy on clinical and/or immunological parameters in obese/overweight and eutrophic subjects. Electronic database searches in PubMed via Medline, Scopus, Embase, Web of Knowledge, Latin-American and Caribbean Center on Health Sciences Information (LILACS) and Scientific Electronic Library Online (SciELO) were performed up to and including August 2014. Initially, the search was conducted in PubMed via Medline using the following strategy: “Periodontal Diseases”[Mesh] OR “Periodontitis”[Mesh] OR “Gingivitis”[Mesh]) AND “Obesity”[Mesh] OR “Obesity, Abdominal”[Mesh] OR “Body Fat Distribution”[Mesh] OR “Abdominal Fat”[Mesh] OR “Intra-Abdominal Fat”[Mesh] AND “Therapeutics”[Mesh] OR “Root Planing”[Mesh] OR “Dental Scaling”[Mesh] OR “Periodontal Debridement”[Mesh] OR “Periodontal Treatment” OR “Periodontal Therapy.” No date and language restrictions were applied. Duplicate studies were identified and discarded using the software EndNote X7 (Thomson Reuters, Carlsbad, CA, USA, 2013). Titles, abstracts, and key words of potential articles were screened independently considering the inclusion and exclusion criteria by two reviewers. Lists were compared, and in case of disagreement, a consensus was reached by discussion. Assessment of the full articles retrieved after an initial screening was performed independently by the same two reviewers. In addition to the electronic search, a hand search was performed in the reference list of all included studies by the same reviewers. Predefined data-collection worksheets were employed for the assessment of each selected publication.
Data extraction and quality assessment
Articles with potential to be included in the study were submitted to a detailed analysis comprehending information regarding author’s name and year of publication, inflammatory markers evaluated, study design with sample size, periodontal disease definition, obesity definition, mean age of enrolled subjects, follow-up period, type of periodontal therapy, and main results. In addition, in order to perform a meta-analysis, results regarding mean values and standard deviation of inflammatory markers and/or clinical parameters were registered. Authors were contacted in order to clarify any queries on the study methodology or result.
With the intention to assess quality of selected studies, the scale proposed by Downs and Black [15] was used. As with previous studies [16], this checklist was modified slightly: the scoring for question 27 on power was simplified to zero or one-point, according to presence or absence of sufficient power in the study to detect a clinically significant effect. Therefore, articles reporting power of less than 0.80 with alpha at 0.05 obtained a zero score. The maximum rate for this modified scale was 28 with all specific items scored as either yes (=1) or no/unable to determine (=0), with the exception of item 5, “Are the distributions of principals confounders in each group of subjects to be compared clearly described?” in which responses were rated as yes (=2), partially (=1), and no (=0). The ranges of scores were grouped into four different categories as follows: excellent (26 to 28), good (20 to 25), fair (15 to 19), and poor (14 and less) [16]. Articles were evaluated by the same two reviewers independently, and in case of disagreement, a consensus was obtained after discussion.
Statistical analysis
Each clinical sign of periodontal disease was considered an independent outcome as follows: percentage of sites with bleeding on probing (BoP), probing depth (PD), and clinical attachment level (CAL). Data regarding systemic inflammatory markers were not available to be included in the pooled analysis. In order to answer our review questions, different analyses were performed: (1) to observe the effect of periodontal therapy only among obese subjects; (2) to compare the effect of periodontal treatment between obese and non-obese. Meta-analyses were performed at the longest follow-up. When heterogeneity was not statistically significant (P > 0.05), the fixed-effects model was used; otherwise, the random-effects model was employed [17]. The software RevMan 5.2 (The Nordic Cochrane Center, Copenhagen, Denmark, 2011) was used to perform the meta-analyses.
Results
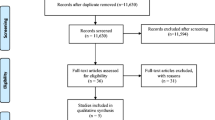
Results from the literature search comprised 489 articles. One hundred forty-four articles were duplicated and removed, remaining 345 articles for consideration. After that, title, abstracts, and key words were evaluated and 335 articles excluded. Finally, 10 articles were fully read, and five included in the final review (Fig. 1). Excluded articles and main reason for exclusion are presented in Table 1.
Main results of included articles are present in Table 2. Of all included studies, two of them evaluated serum levels of C-reactive protein [18, 19], two determined the levels of IL-6 and TNF-α [19, 20], two evaluated levels of leptin [19, 21], and only one assessed levels of IL-1 and INF-Ɣ [20] and levels of adiponectin [21].
All studies presented clinical data of periodontal parameters at baseline and after treatment in obese and in non-obese subjects. Two of them found no difference on periodontal response between obese and normal-weight individuals [18, 20], whereas the remaining studies observed a worse periodontal healing in obese [19, 21, 22]. Three studies also evaluated the levels of circulating pro-inflammatory cytokines with obese subjects presenting significantly higher levels than their counterparts at baseline [18–20]. Additionally, all studies detected clinical and biochemical differences after periodontal therapy in comparison with baseline values (Fig. 2 and Fig. 3).
The assessment of quality of included studies revealed that all of them were classified as fair quality with values ranging from 15 to 19.
Three studies presented available data to be included in the meta-analysis considering the effects of periodontal treatment on some clinical parameters of periodontal disease [19–21]; one study presented data only concerning BoP [20]. The latter authors were contacted in order to obtain data of remaining outcomes; nevertheless, the original dataset was not available. Other authors were also contacted to collect missing data, but no response was obtained. Even with the reduced number of studies enrolled, the effect of periodontal therapy could be observed in obese subjects, comparing all clinical parameters at baseline and follow-up (BoP P < 0.0001, I2 0 %; PD P < 0.0001, I2 0 %; CAL P < 0.0001, I2 0 %). In all analyses, as no heterogeneity was observed, the fixed model was employed. Considering the effects of periodontal therapy on clinical signs of periodontal disease of obese and non-obese subjects after periodontal treatment, no difference could be observed for all evaluated parameters (BoP P = 0.19, I 2 0 %; PD P = 0.93, I 2 83 %; CAL P = 0.92, I2 87 %). As heterogeneity was present only for PD and CAL, the random-effects model was used for those analyses.
Discussion
The results of this systematic review and meta-analysis demonstrated that periodontal therapy could improve clinical signs of periodontal disease in obese subjects. Additionally, this systemic condition seems to exert no influence on periodontal healing, when comparing after-treatment results of this group with non-obese subjects. It is evident that our results should be carefully considered, once few studies presented available data to be included in the systematic review. Additionally, all articles were classified as fair quality, reinforcing our concerns with the obtained results.
Periodontal therapy seems to be an effective method to improve clinical signs of periodontal disease among obese individuals. Even though the effects of periodontal therapy on serum levels of inflammatory markers could not be measured in this review, it is expected a decrease of pro-inflammatory cytokines at least in gingival crevicular fluid. This local alteration could be responsible for improving the immune response towards inflammation resolution in the site-level, promoting periodontal healing. Similar findings were observed among type II diabetes individuals, even though both conditions have distinct etiological pathways [12]. Type II diabetes, besides altering the host immune response by increasing the levels of inflammatory markers, also interferes with both the delivery of nutrition and the migration of defensive immune cells to the gingival tissues. This, in turn, reduces the oxygen diffusion and elimination of metabolic waste, exacerbating the disease [23].
Surprisingly, our findings comparing the post-treatment results of obese and non-obese subjects suggested that obesity did not modify the effects of periodontal treatment. However, it is worth pointing out that only one study followed up the enrolled subjects up to 6 months [21], against 2 to 3 months in the others. This study revealed that obese subjects presented lower improvement of clinical parameters, suggesting that obesity could modify periodontal healing in the long-term. Thus, given that periodontal healing can occur until 24 months after therapy [24] and that it is influenced by local and systemic factors, it is not possible to assume that periodontal therapy would have similar outcomes in obese and in non-obese in the long-term. Additionally, as periodontal disease development and resolution are strongly influenced by oral habits [25], and since it seems that obese patients are more likely to present neglected general and oral health hygiene habits, it is not possible to presume the long-term effects of the therapy in this specific group [26–28].
Considering the effects of periodontal therapy on pro-inflammatory cytokines, no statistical analysis could be performed in our study. Many pro-inflammatory cytokines were investigated, such as IL-1β, IL-6, TNF-α, as well as specific adipokines, like leptin and adiponectin. Despite this variety of mediators studied, results presented no consensus, once in some reports therapy was responsible for decreasing serum levels of the studied protein, whereas in others not. Even with an improvement of periodontal status among enrolled obese patients post-therapy when compared to baseline, many of them retained several inflamed residual periodontal pockets, which might be responsible for maintaining serum levels of pro-inflammatory cytokines. It was also observed a lack of goal to be achieved by plaque or BoP levels in order to classify patients as clinically healthy at the end of therapy. Given the nature of periodontitis, which presents itself in cycles of progression and latency, a combination of those variables with more reliable measures of periodontal conditions, such as CAL and PD, is strongly recommended [29, 30]. Hence, our results suggest that the effect of periodontal treatment on systemic mediators of obese subjects should be further investigated.
Even with many reports in the medical field suggesting that obesity may influence the immune-inflammatory response [31], this is not evident in the periodontal tissues. Some investigators have studied the effects of adipokines in this process, looking at the role played by the interaction between leptin, visfatin, and adiponectin [32, 33]. According to Deschner and colleagues [34], the increase in the first two cytokines, combined with the decrease in the last one, could be considered a “pathomechanistic link between obesity and compromised periodontal healing.”
In the present review, several limitations should be taken into consideration. Firstly, the results obtained in the meta-analysis are fragile, once few studies presented data to be analyzed. Even with other studies presenting potential data to be pooled, authors did not respond to any request. Secondly, the small number of studies precluded the assessment of heterogeneity by using meta-regression and subgroup analyses, as well as the assessment of publication bias by using funnel plot and the Egger’s test. Thirdly, it was not possible to combine the results of immunological markers possibly affected by periodontal therapy. Thus, it was not possible to measure quantitatively the effects of periodontal treatment on these mediators. Fourthly, the low number of subjects enrolled in the included studies and the short follow-up could misrepresent the actual impact of obesity on the healing of periodontal tissues. As most studies did not present a sample size calculation, it was not possible to determine if the absence of difference in periodontal healing after therapy between obese and non-obese subjects was real or if it was due to a lack of statistical power to identify it. Fifthly, it is worth pointing out that the design of included studies could also have misrepresented our findings. Even though it has been suggested that obese subjects tend to present neglected hygiene habits [26–28] and low socioeconomic status [35, 36], no study concerned about pairing subjects enrolled in obese and non-obese groups. This might have compromised the comparability between groups. Finally, all studies presented a fair quality, signaling that included studies also presented important limitations in their designs and findings.
In summary, and considering the limits of this systematic review, only restricted information comparing the effects of periodontal therapy on obese and non-obese is available in the current literature. Overall, our results suggest that periodontal therapy is effective to improve clinical outcomes of periodontal disease in obese subjects. Additionally, obesity seems not exert a modifier effect on periodontal healing, once obese and non-obese subjects presented similar improvement of clinical conditions post-therapy. Nevertheless, interventional prospective case-controls with long-term follow-up, larger number of subjects enrolled, and more solid methodological aspects are required for further investigations.
References
Han DH, Lim SY, Sun BC, Paek DM, Kim HD (2010) Visceral fat area-defined obesity and periodontitis among Koreans. J Clin Periodontol 37:172–179. doi:10.1111/j.1600-051X.2009.01515.x
Furuta M, Shimazaki Y, Takeshita T, Shibata Y, Akifusa S, Eshima N, Kiyohara Y, Ninomiya T, Hirakawa Y, Mukai N, Nagata M, Yamashita Y (2013) Gender differences in the association between metabolic syndrome and periodontal disease: the Hisayama study. J Clin Periodontol 40:743–752. doi:10.1111/jcpe.12119
Nascimento GG, Seerig LM, Vargas-Ferreira F, Correa FO, Leite FR, Demarco FF (2013) Are obesity and overweight associated with gingivitis occurrence in Brazilian schoolchildren? J Clin Periodontol 40:1072–1078. doi:10.1111/jcpe.12163
Chaffee BW, Weston SJ (2010) Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol 81:1708–1724. doi:10.1902/jop.2010.100321
Suvan J, D'Aiuto F, Moles DR, Petrie A, Donos N (2011) Association between overweight/obesity and periodontitis in adults. A systematic review. Obes Rev 12:e381–e404. doi:10.1111/j.1467-789X.2010.00808.x
Khader YS, Bawadi HA, Haroun TF, Alomari M, Tayyem RF (2009) The association between periodontal disease and obesity among adults in Jordan. J Clin Periodontol 36:18–24. doi:10.1111/j.1600-051X.2008.01345.x
Nascimento GG, Leite FR, Correa MB, Horta BL, Peres MA, Demarco FF (2014) Relationship between periodontal disease and obesity: the role of life-course events. Braz Dent J 25:87–89
Kopelman PG (2000) Obesity as a medical problem. Nature 404:635–643. doi:10.1038/35007508
Cancello R, Clement K (2006) Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. Bjog 113:1141–1147. doi:10.1111/j.1471-0528.2006.01004.x
Bullo M, Garcia-Lorda P, Megias I, Salas-Salvado J (2003) Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res 11:525–531. doi:10.1038/oby.2003.74
Adriaens PA, Adriaens LM (2004) Effects of nonsurgical periodontal therapy on hard and soft tissues. Periodontol 2000 36:121–145. doi:10.1111/j.1600-0757.2004.03676.x
Correa FO, Goncalves D, Figueredo CM, Bastos AS, Gustafsson A, Orrico SR (2010) Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol 37:53–58. doi:10.1111/j.1600-051X.2009.01498.x
Keller A, Rohde JF, Raymond K, Heitmann BL (2015) The association between periodontal disease and overweight and obesity: a systematic review. J Periodontol 86(6):1–15. doi:10.1902/jop.2015.140589
Papageorgiou SN, Reichert C, Jager A, Deschner J (2015) Effect of overweight/obesity on response to periodontal treatment: systematic review and a meta-analysis. J Clin Periodontol. doi:10.1111/jcpe.12365
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52:377–384
Tan L, Wang MJ, Modini M, Joyce S, Mykletun A, Christensen H, Harvey SB (2014) Preventing the development of depression at work: a systematic review and meta-analysis of universal interventions in the workplace. BMC Med 12:74. doi:10.1186/1741-7015-12-74
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Al-Zahrani MS, Alghamdi HS (2012) Effect of periodontal treatment on serum C-reactive protein level in obese and normal-weight women affected with chronic periodontitis. Saudi Med J 33:309–314
Altay U, Gurgan CA, Agbaht K (2013) Changes in inflammatory and metabolic parameters after periodontal treatment in patients with and without obesity. J Periodontol 84:13–23. doi:10.1902/jop.2012.110646
Zuza EP, Barroso EM, Carrareto AL, Pires JR, Carlos IZ, Theodoro LH, Toledo BE (2011) The role of obesity as a modifying factor in patients undergoing non-surgical periodontal therapy. J Periodontol 82:676–682. doi:10.1902/jop.2010.100545
Dias Goncalves TE, Feres M, Zimmermann GS, Faveri M, Figueiredo LC, Braga PG, Duarte PM (2014) Effects of scaling and root planing on clinical response and serum levels of adipocytokines in obese patients with chronic periodontitis. J Periodontol 86(1):53–61. doi:10.1902/jop.2014.140266
Suvan J, Petrie A, Moles DR, Nibali L, Patel K, Darbar U, Donos N, Tonetti M, D'Aiuto F (2014) Body mass index as a predictive factor of periodontal therapy outcomes. J Dent Res 93:49–54. doi:10.1177/0022034513511084
Kumar M, Mishra L, Mohanty R, Nayak R (2014) Diabetes and gum disease: the diabolic duo. Diabetes Metab Syndr 8:255–258. doi:10.1016/j.dsx.2014.09.022
Graziani F, Laurell L, Tonetti M, Gottlow J, Berglundh T (2005) Periodontal wound healing following GTR therapy of dehiscence-type defects in the monkey: short-, medium- and long-term healing. J Clin Periodontol 32:905–914. doi:10.1111/j.1600-051X.2005.00789.x
van der Weijden F, Slot DE (2011) Oral hygiene in the prevention of periodontal diseases: the evidence. Periodontol 2000 55:104–123. doi:10.1111/j.1600-0757.2009.00337.x
Franchini R, Petri A, Migliario M, Rimondini L (2011) Poor oral hygiene and gingivitis are associated with obesity and overweight status in paediatric subjects. J Clin Periodontol 38:1021–1028. doi:10.1111/j.1600-051X.2011.01770.x
Taggart HM, Mincer AB, Thompson AW (2004) Caring for the orthopaedic patient who is obese. Orthop Nurs 23:204–210
de Castilhos ED, Horta BL, Gigante DP, Demarco FF, Peres KG, Peres MA (2012) Association between obesity and periodontal disease in young adults: a population-based birth cohort. J Clin Periodontol 39:717–724. doi:10.1111/j.1600-051X.2012.01906.x
Savage A, Eaton KA, Moles DR, Needleman I (2009) A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol 36:458–467. doi:10.1111/j.1600-051X.2009.01408.x
Holtfreter B, Albandar JM, Dietrich T, Dye BA, Eaton KA, Eke PI, Papapanou PN, Kocher T, Joint EUUSAPEWG (2015) Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: proposed standards from the joint EU/USA periodontal epidemiology working group. J Clin Periodontol 42:407–412. doi:10.1111/jcpe.12392
Karlsson EA, Beck MA (2010) The burden of obesity on infectious disease. Exp Biol Med (Maywood) 235:1412–1424. doi:10.1258/ebm.2010.010227
Nokhbehsaim M, Keser S, Jager A, Jepsen S, Deschner J (2013) Regulation of regenerative periodontal healing by NAMPT. Mediat Inflamm 2013:202530. doi:10.1155/2013/202530
Nokhbehsaim M, Keser S, Nogueira AV, Cirelli JA, Jepsen S, Jager A, Eick S, Deschner J (2014) Beneficial effects of adiponectin on periodontal ligament cells under normal and regenerative conditions. J Diabetes Res 2014:796565. doi:10.1155/2014/796565
Deschner J, Eick S, Damanaki A, Nokhbehsaim M (2014) The role of adipokines in periodontal infection and healing. Mol Oral Microbiol. doi:10.1111/omi.12070
Thomson WM, Sheiham A, Spencer AJ (2012) Sociobehavioral aspects of periodontal disease. Periodontol 2000 60:54–63. doi:10.1111/j.1600-0757.2011.00405.x
Nascimento GG, Leite FR, Do LG, Peres KG, Correa MB, Demarco FF, Peres MA (2015) Is weight gain associated with the incidence of periodontitis? A systematic review and meta-analysis. J Clin Periodontol 42:495–505. doi:10.1111/jcpe.12417
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Nascimento, G.G., Leite, F.R.M., Correa, M.B. et al. Does periodontal treatment have an effect on clinical and immunological parameters of periodontal disease in obese subjects? A systematic review and meta-analysis. Clin Oral Invest 20, 639–647 (2016). https://doi.org/10.1007/s00784-015-1678-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1678-y