Abstract
Objectives
The aim of this study was to investigate the plaque inhibitory effect of a new 0.03 % chlorhexidine digluconate (CHX) and 0.05 % cetylpyridinium chloride (CPC) mouthrinse formulation and to explore patients’ experience and side effects after its use.
Materials and methods
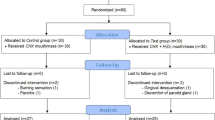
This short-term, randomized, double blind, parallel, clinical trial enrolled 150 periodontally healthy patients. These volunteers were randomly allocated to one of following mouthrinse groups (n = 50/group): 0.12 % CHX + 0.05 % CPC (Perio-Aid® Treatment alcohol-free), 0.03 % CHX + 0.05 % CPC new test formulation or to the placebo group. Clinical parameters (plaque, gingival, and stain indexes) and microbiological samples were taken at baseline, before supragingival cleaning, and after 4 days of undisturbed plaque growth, rinsing twice/day with one of the mouthrinses.
Results
Plaque reduction was similar for the 0.12 % CHX (−0.52 ± 0.55) and 0.03 % CHX (−0.47 ± 0.49) groups. Both showed significant reductions in plaque accumulation compared to the placebo (p < 0.001). The new formulation had less of a negative impact on taste perception when compared to the 0.12 % CHX solution. The new CHX mouthrinse was also able to control bacterial loads and reduce some periodontopathogens.
Conclusions
This study indicated that the new 0.03 % CHX + 0.05 % CPC formulation exerted clinical efficacy similar to that achieved by an already-marketed 0.12 % CHX + 0.05 % CPC mouthrinse, but with slightly fewer side effects.
Clinical relevance
Lower CHX mouthrinse formulations could be effective in the inhibition of plaque regrowth with reduced unpleasant subjective side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal disease prevention is based on controlling supragingival biofilm [1] in order to reduce the levels of potentially pathogenic species in the oral cavity [2]. Daily plaque control and regular maintenance visits are also essential for maintaining long-term clinical stability after periodontal therapy [3, 4].
Because for a portion of the population, plaque control is difficult or insufficient to maintain gingival health, chemical agents have been used to improve oral hygiene and to reduce the incidence of gingivitis. Chlorhexidine digluconate (CHX) is considered the gold standard oral antiseptic due to its clinical superiority and its antimicrobial effects [5]. CHX is a positively charged biguanide, which can adsorb to negatively charged surfaces, such as mucous membranes in the oral cavity, the salivary pellicle on teeth, and biofilm components including bacteria, extracellular polysaccharides, and glycoproteins [6–8]. CHX’s anti-plaque effect is achieved through an immediate bactericidal effect at high concentrations, followed by a prolonged bacteriostatic effect at low concentrations [9, 10]. CHX exhibits high substantivity, of up to 12 h on the tooth surface [11].
A recent systematic review confirmed that the use of CHX significantly reduced levels of plaque and gingivitis, regardless of its concentration (between 0.2 and 0.06 %), but depending on the total dosage [12]. The optimum dose of CHX is generally considered to be approximately 20 mg twice daily [13]. Nevertheless, there is a positive correlation between the concentration of CHX and the incidence of side effects [14], which increase considerably at concentrations exceeding 0.1 % [15, 16]. Tooth staining and taste alterations are the most important of CHX’s side effects. Around 30 % of subjects who rinse with CHX report non-serious side effects [17], which may cause more non-compliance among patients. With the aim of reducing CHX’s side effects, the focus has shifted to low concentration CHX formulae [18, 19] because similar plaque inhibition can be achieved with larger volumes of lower concentration solutions [20, 21].
To prevent decreased efficacy of low concentration CHX formulations, re-formulations were required, involving the addition of another antimicrobial agent, such as cetylpyridinium chloride (CPC). CPC is a cationic surface-active agent belonging to the quaternary ammonium group [22], with moderate anti-plaque and bactericidal activity against Gram-positive bacteria, fungi, and yeasts [23]. This molecule seems to enhance CHX’s antimicrobial activity through a synergistic effect [24].
It is important to note that distinction between antiseptic mouthrinses is due to the formulation rather than the concentration of the ingredients [25], the presence of a certain agent [26], or the bioavailability of CHX [27]. These differences may be due to variances in formulation (the absence of alcohol or the addition of CPC or sodium fluoride) or to other unknown factors related to the formulation as a whole. For example, 0.12 % CHX + sodium fluoride has been proven to yield inferior clinical results compared to 0.12 % CHX with or without alcohol [28, 29]. A 0.12 % CHX has proven to be more active when combined with alcohol than when formulated in the absence of alcohol, while 0.12 % CHX + 0.05 % CPC have shown antimicrobial effects superior to 0.12 % CHX + alcohol [24]. CHX efficacy, therefore, can be compromised by the chemical interaction between active ingredients and excipients present in the formulation since chlorhexidine is a highly cationic molecule and thus reacts with any anionic molecule, resulting in its inactivation. Is not uncommon to find two CHX mouthwashes containing the same concentration of CHX and differing significantly in their relative efficacy, bioavailability, biofilm penetrability, and substantivity [24, 30]. Modifications in the chlorhexidine formulation may affect its proven efficiency and therefore should be appropriately tested [24].
The aim of this study was to evaluate the inhibitory effect on bacterial plaque accumulation and the occurrence of side effects of a new mouthrinse formulation, containing 0.03 % CHX + 0.05 % CPC.
The current study tested the hypothesis that this new solution:
-
1.
Yields similar clinical results regarding the inhibition of de novo plaque growth compared to those achieved with an already marketed 0.12 % CHX mouthrinse.
-
2.
Causes fewer side effects than the marketed 0.12 % CHX formula.
-
3.
Has no negative microbiological effects, but controls total bacterial loads.
Materials and methods
Study population
One hundred fifty (150) subjects were consecutively screened and enrolled by the same investigator (CM) at the University Dental Clinic of Universitat Internacional de Catalunya (UIC), Sant Cugat, Barcelona, between October 2012 and July 2013; these subjects were medical, nursing, and physiotherapy students and students in their first or second year of dentistry at the UIC. The clinical protocol was approved by the Ethics Committee of the UIC (study number PER-ECL-2011-06-NF) and complied with the principles of the Declaration of Helsinki and current regulations applying to the execution of research studies in human beings. Confidentiality was upheld for all participants, thereby meeting the requirements of Spanish Law 15/1999 on data protection.
Subjects were selected on the basis of being 18–30 years old, being in good overall health without medical history or having taken medications that could interfere with the study conduct, having a minimum of six teeth per quadrant, and absence of probing depths ≥4 mm. Exclusion criteria included having allergy to CHX or CPC, continuous use of CHX or of any other oral antiseptic in the months prior to the study, any adverse medical background or long-term medications that could affect gingival conditions, having taken antibiotics in the previous 3 months, having moderate to severe gingivitis (bleeding on probing ≥40 %) [31], pregnancy or breastfeeding, smokers of more than five cigarettes per day, wearing orthodontic appliances, fixed or removable prostheses, having systemic diseases that increase the risk for gingival diseases (diabetes mellitus, immunosuppression), or severe dental crowding.
Study design
A 4-day, randomized, double-blind, parallel, placebo-controlled clinical trial was designed. Three mouthrinse formulations were evaluated, and a total of 50 subjects were assigned to each of the following groups:
-
Negative control placebo mouthrinse (NC): a saline solution (0.9 % w/w) [23, 24].
-
Positive control mouthrinse (PC): 0.12 % CHX + 0.05 % CPC (Perio-Aid® Treatment, alcohol free; Dentaid, Spain).
-
New formulation mouthrinse (Test): 0.03 % CHX + 0.05 % CPC.
The new formulation was developed by the Dentaid Research Center (Dentaid, Spain).
Screening visit
The volunteers were informed about the objectives and details of the study, and if interested, a written informed consent was obtained. A detailed oral health evaluation was carried out and socio-demographic data were collected.
Baseline visit (T-0)
At baseline, Löe and Silness gingival index (GI) [32] and Brecx tooth stain index (SI) [33] were recorded at four sites per tooth. For the microbial analysis, pooled subgingival samples from the buccal sulci around teeth 16, 21, 36, and 41 were taken, with one sterile paper point (absorbent paper point size 30; Dentsply, Maillefer, Ballaigues, Switzerland) per tooth, 20 s in place. Before sampling, cotton rolls were placed in the vestibule and the tooth surface was air-dried. Paper points were transferred into a sterile screw-capped vial, containing 1 ml of reduced transport medium [34]. Samples were sent to the microbiology laboratory at Dentaid Research Center at a controlled temperature of 4 °C for processing. The microbial analyses were blinded.
Subsequently, plaque index was assessed by applying an erythrosine solution (Plac-Control®; Dentaid, Spain) with the Modified Quigley Hein plaque index (MPI) [35], scored at four sites per tooth. All subjects then received professional ultrasonic debridement and all teeth were polished to remove remaining plaque and staining.
All clinical parameters were recorded and plaque samples collected by the same examiner (CM) under the same conditions.
For the intra-examiner correlation, ten participants were examined twice with a 1-h interval. The Kappa concordance correlation coefficient was 0.725. This clinician was unaware of the mouthrinse assigned to participants.
Mouthrinse allocation was randomized by an independent clinician (AP) who offered three envelopes to each participant, who then randomly chose one of these three envelopes, containing a mouthwash group. Each subject received a bottle (all bottles had the same outer appearance) containing 150 ml of mouthrinse from the corresponding group (coded A, B, or C). All subjects were masked to their group allocation. Participants were asked to rinse every 12 h, with 15 ml for 30 s, and after spitting, to refrain from rinsing or eating for at least 60 min. Participants were asked to discontinue all forms of oral hygiene for the next 4 days.
Day 4 visit (T-1)
The subjects were asked about the occurrence of any adverse effects and the bottles were collected. A new subgingival microbiological sample was collected and all clinical parameters were recorded as at baseline. All subjects completed a questionnaire with a visual analog scale (VAS) from 0 to 5, designed to assess perceptions regarding the mouthrinse used and all teeth were polished.
Microbiological assessment
DNA extraction method
Genomic DNA was isolated from the subgingival samples using an ATP Genomic DNA Mini Kit (ATP Biotech Inc., Taipei City, Taiwan) following the manufacturer’s instructions. DNA was quantified using Nanodrop® ND-1000 technology (Nanodrop Technologies, Wilmington, DE, USA).
Conventional PCR conditions
Treponema denticola (Td) and Tannerella forsythia (Tf) 16S rDNA genes were amplified using specific primers [36, 37]. PCR mixes were performed in 25 μl with 100 ng of DNA, 2.5 mM of MgCl2, 1 μM of each primer, and 0.5 U Taq DNA polymerase (Takara Bio, Otsu, Japan). The PCR amplification program included initial DNA denaturation at 95 °C for 5 min and a total of 35 PCR cycles, each cycle consisting of 1 min of denaturation at 95 °C, 1 min of annealing at 55 °C, and 1 min of extension at 72 °C. The negative control was sterile Milli-Q water. As a positive control, the genomic DNA of type strains was used. PCR reactions were done in triplicate. Amplification results were evaluated by electrophoresis using 4 % agarose gel with ethidium bromide (0.5 g/ml) in 1× TAE buffer.
QPCR conditions
Quantitative PCR was performed using a LightCycler® 480 II (Roche Diagnostics, Penzberg, Germany). The specific primers used (Invitrogen Life Technologies, Carlsbad, CA, USA) and TaqMan probes (Applied Biosystems, UK and Roche Diagnostics) were previously described [38–42]; their concentrations ranged between 0.5 and 1 μM for primers and 0.2 μM for probes. The qPCR reaction was conducted in a 20-μl volume, containing LightCycler® 480 II Probes Master (Roche Diagnostics), primers, probe, 5 μl of genomic DNA, and PCR-grade sterile water. The qPCR program was done using an initial cycle of 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing for 30 s, and extension at 72 °C for 1 s. The annealing temperatures in all cases were between 55 and 65 °C. Positive and negative controls and test samples were done in triplicate. Standard curves were developed for the qPCR of each bacterial species using known concentrations of genomic DNA as a template. Tenfold serial dilutions in PCR-grade sterile water were made to prepare standard DNA ranging from 102 to 109 cells. Standard curves were constructed by plotting crossing point (Cp) values versus log cells and were used to quantify the number of cells of each species, as required, based on their respective Cps. When analyzing the samples, each run of qPCR was conducted with a standard DNA curve. Data analysis and Cp values were calculated by LightCycler® 480 Software 1.5 (Roche Diagnostics) using the second derivative maxim method.
Data analysis
Based on a previous study, the expected difference between the experimental and placebo groups is 0.25 for plaque index with a standard deviation of 0.34 [23]. Our study included 150 subjects (50 per arm). This sample size ensures an alpha of 0.05 and a power of 90 %. A drop-out rate of 10 % was anticipated. For qualitative clinical variables, frequency and percentage distribution were determined. For quantitative variables, central tendency measures (mean), measures of position (quartiles), and dispersion (standard deviation) were used. Normal distribution of the variables was analyzed using Kolmogorov-Smirnov test. The main study variables are MPI, GI, and SI. For these, the differences between baseline and day 4 were calculated and compared between the three groups using Kruskal-Wallis test. Finally, differences were compared to each other via the Duncan test. A p value of <0.05 was accepted as a significant difference. Data management and analysis were performed using a statistical software program (version 18; SPSS Inc., Chicago, IL, USA). The microbiological data are presented descriptively only.
Results
Study population
One hundred fifty patients (109 women [72.7 %] with a mean age of 21.6 years [SD = 3.4]) were enrolled in this study. At baseline, the clinical parameters were MPI 1.15 (SD = 0.42) and GI 2.03 (SD = 0.8); tooth staining was not observed. Baseline demographic and clinical characteristics for each group are shown in Table 1. One hundred fifty-two patients were initially enrolled, but two patients dropped out voluntarily..
Clinical outcome variables
At baseline, no differences were found between the three mouthrinse solutions, for any of the variables (p > 0.40).
Significant differences were observed overall between groups for all of the post-intervention follow-up variables.
Modified Quigley Hein plaque index (MPI, Turesky et al. 1970)
For post-MPI, differences were found when comparing the test and PC groups with the placebo group (p < 0.001) in each case. The reduction in MPI during the 4 days of rinsing (Fig. 1a) was very similar for test (−0.47 ± 0.49) and PC (−0.52 ± 0.55) and statistically higher than for the NC group which showed an increase (+0.58 ± 0.53) (p < 0.05). (Table 2).
a Change in modified Quigley Hein plaque index (MPI) between baseline and day 4 (whiskers plot). b Change in Löe and Silness gingival index (GI) between baseline and day 4 (whiskers plot). c Change in Brecx tooth stain index (SI) between baseline and day 4 (whiskers plot). The median and 95 % confidence interval (n = 50 per mouthrinse) are shown for the following mouthrinses respectively: NC = placebo, test = 0.03 % CHX + 0.05 % CPC, and PC = 0.12 % CHX + 0.05 % CPC
Löe and Silness gingival index (GI, Löe and Silness 1963)
Post-GI was found to differ between test and PC groups versus the placebo group (p < 0.001). The change in GI between baseline and day 4 (Fig. 1b) was also similar for the two CHX solutions, with reductions of −0.97 ± 0.65 for the test and −1.14 ± 0.63 for the PC. In the NC group, the decrease in GI was significantly smaller (−0.23 + 0.71) (p < 0.001) (Table 2).
Brecx tooth stain index (SI, Brecx et al. 1993)
Staining Index differed when comparing the test group with the placebo group (p = 0.001), and no differences were found between the PC group and the placebo group (p = 0.165). The SI slightly increased similarly for the CHX mouthrinses (Fig. 1c) (test = +0.05 ± 0.13 and PC = +0.02 ± 0.06). For the NC group, staining was negligible (Table 2).
Microbiological assessment
Total microbial load
At baseline, the total microbial load (estimated via a universal 16S rRNA evaluation) was comparable in the three groups (Table 3). After use of the NC and, to a lower extent, of the test rinse, these counts increased (from 6.1 to 6.8 log and from 5.9 to 6.3, respectively). For the 0.12 % CHX rinse, the changes remained negligible over time (from 6.0 to 5.9). Therefore, the bacterial load was maintained in the three study groups from baseline to the end of the study; this is especially true for Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Streptococcus mutans, and Escherichia coli. After treatment with placebo, the bacterial loads increased for most of the bacterial species. No variation was observed in the load of most of the species studied after applying the test mouthrinse treatment, except for the total bacterial pool and Streptococcus gordonii. While in the positive control group, the only species that seems to increase is S. gordonii.
Detection frequency of specific bacterial species
The frequency of bacterial detection was similar at baseline. The detection frequency of specific species also changed after 4 days of rinsing. The negative control showed a slight increase in detection frequency and counts of most pathogens. For the test rinse, the incidence of T. forsythia and the prevalence of Prevotella intermedia and S. mutans decreased after treatment. The PC rinse showed reductions both in detection frequency and in counts for most species, especially for T. denticola, T. forsythia, P. intermedia, S. gordonii, and S. mutans. For Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum, the changes were very small.
Proportions of total microbiota
No inter-group differences were observed at baseline. The three mouthrinses controlled bacterial loads, and no increase was observed for any of the species, except for S. gordonii, which increased in the placebo group.
Patient-based variables
Compliance
The evaluation of the remaining liquid in the bottles showed that the majority of the participants complied optimally with the treatment, 93.5 % of them returning <50 ml of mouthrinse after 4 days.
Satisfaction questionnaire
Patient experience with the different mouthrinses is summarized in Fig. 2. Overall, the new CHX solution scored clearly better than the PC, especially for general opinion, which was more positive for 0.03 % CHX solution. The 0.03 % CHX had less of a negative impact on taste perception, dry mouth, and tooth staining when compared to the 0.12 % CHX solution. For sensitivity, burning, and numbness, the two CHX solutions scored similarly.
Subjective ratings (visual analog scale ranging from 0 to 5 on anonymous questionnaire) concerning general opinion, permanence of flavor, food perception, sensitivity, burning or dry mouth, numbness of tongue, and tooth staining. The following values were indicated: 0 = unacceptable, taste remains short time in mouth, no change in food perception, no sensitivity, no burning, no dry mouth, no numbness, no staining; and 5 = optimal opinion, taste remains long time in mouth, dramatic reduction in taste perception of food, high sensitivity, intense burning, intense dry mouth, intense numbness, extreme staining over the 4-day follow-up period, for the following mouthrinses: NC = placebo; test = 0.03 % CHX + 0.05 % CPC; PC = 0.12 % CHX + 0.05 % CPC
Adverse events
In the 0.03 % CHX group, two patients reported itching of the oral mucosa for 1 or 2 days, and one patient mentioned noting a bitter taste in the oral cavity. For the 0.12 % CHX rinse, three patients reported an itching sensation of the oral mucosa.
Discussion
A short-term, de novo plaque growth model in the absence of oral hygiene measures was chosen for this study as a characteristic model for assessing the anti-plaque effect of new mouthrinse formulations [43]. All mouthrinses were used in accordance with the instructions provided by the manufacturer.
In this clinical trial, the beneficial effects of a new 0.03 % CHX formulation compared to placebo has been observed in terms of plaque reduction and gingival inflammation. Plaque control was similar for the 0.03 % CHX and the 0.12 % CHX rinses, although the total amount of CHX used per day was 36 mg for PC, four times higher than for the test group (9 mg).
This is the first time that such a low concentration of CHX (0.03 %) has been tested. Until now, several studies have proven the anti-plaque efficacy of low concentration CHX mouthwash formulations, such as 0.06 % [27, 44] and 0.05 % [18, 45–47], which were also better perceived by patients [18, 46]. No clinical differences were observed after rinsing with 15 ml of 0.1 % CHX or 15 ml of 0.06 % CHX [27], or after rinsing with 0.05 % CHX + 0.05 % CPC or 0.2 % CHX + alcohol [46]. A 0.05 % CHX + 0.05 % CPC rinse has proven to yield a satisfactory anti-plaque effect in patients undergoing periodontal maintenance [45, 46], even in patients who did not comply with maintenance visits, by providing an additional reduction in plaque level and bleeding on probing, in a 6-month study [47].
Reduced CHX side effects have been reported with lower CHX concentrations [18, 19]. In fact, 0.05 % CHX + 0.05 % CPC showed fewer side effects than long-term use with 0.2 % CHX + alcohol [46]. A 0.05 % CHX/herbal extract combination has also resulted in less tooth staining versus 0.1 % CHX [18]. The greatest staining effect of CHX digluconate can be observed in the first molars after the third day of use [48], and therefore, this 4-day study can be considered sufficient to assess a CHX mouthrinse’s tooth staining effect. Unexpectedly, in our study, no significant differences were detected with regard to the appearance of tooth staining when comparing the CHX groups; therefore, our hypothesis that the new formulation would exert fewer adverse effects was not met in terms of staining. This is in accordance with one study where no significant differences were found between the tooth staining of mouthrinses containing 0.1 % CHX and 0.05 % CHX [18] up to 4 weeks or with another study involving a mouthrinse containing 0.05 % CHX + 0.05 % CPC and an identical mouthrinse without the active ingredients, in which the results revealed no significant staining by the active ingredients in up to 2 weeks of use [45]. Perhaps a longer-term study could confirm the hypothesis that the new 0.03 % CHX formulation would result in less staining than the 0.12 % CHX mouthrinse. In vitro studies have shown 0.1 % CHX to stain more than 0.2 % CHX [49], and similar staining has been observed when comparing 0.05 % CHX and 0.1 % CHX [19]. Although the reason for these results is unclear, as in our case, possible explanations could include mechanisms of competitive binding to the pellicle, saturation of receptors, or changes in valency of the dicationic molecule.
Advanced formulation techniques allow for increased bioavailability of active ingredients, which, in turn, permits the use of lower concentrations of these ingredients while maintaining high efficacy. Although the different elements contained in formulas may adversely affect the bioavailability of active substances, hence different mouthrinses with different CHX concentrations have similar behavior in terms of staining. Further research is needed to clarify this issue.
In reference to taste, the test mouthrinse was the most widely accepted by the participants. Meanwhile, the literature reflects greater taste acceptance by patients of 0.05 % CHX [46] and 0.12 % CHX in comparison to 0.2 % CHX [15, 29], and there were no significant differences between the opinion regarding the flavor of a mouthrinse containing 0.05 % CHX + 0.05 % CPC compared to that of an identical mouthrinse without the active ingredients in a long-term study [47].
In this study, all CHX solutions, even the 0.03 % CHX mouthrinse, controlled the total bacterial load. Other studies have concurred, where low CHX concentrations, such as 0.05 %, have shown a reduction of total bacterial load [45–47]. In fact, one study showed no significant differences between 0.05 and 0.2 % CHX in terms of bacterial load at 6 months [46]. Some studies have also confirmed the antimicrobial efficacy of 0.05 % CPC formulations [45, 50], although the high heterogeneity in the results highlights the importance of an adequate formulation and CPC bioavailability [23]. In the present study, qPCR was used as a complementary method to microbiological culture [51, 52]. Our hypothesis that the new formulation controls the total bacterial load has been confirmed.
The reduction in gingival index is similar when comparing the 0.03 % CHX solution with the 0.12 % CHX mouthrinses. However, long-term studies are needed to confirm these observations because it is believed that CHX concentrations below 0.1 % may not be effective for controlling gingival inflammation [44].
In conclusion, although the new 0.03 % CHX rinse provides a CHX amount that is four times lower than the standard formulation, a similar plaque control efficacy has been observed, which ultimately enhances the value of the entirely new formulated product as a whole and confirms the main hypothesis in that similar plaque control can be observed with both formulations: 0.03 and 0.12 % CHX. The control of bacterial load was similar for the CHX solutions. The new 0.03 % CHX formulation had a better patient appreciation for taste and a lower degree of alteration in taste perception.
Although a 4-day study may have limitations in terms of long-term results, mainly for its anti-gingivitis efficacy, it is an adequate period for studying anti-plaque efficacy and early occurrence of undesirable adverse effects [53]. Nevertheless, longer studies are needed to evaluate the use of this new 0.03%CHX + 0.05 % CPC formulation as an adjunct to the hygienic phase and its long-term effect.
References
Van der Weijden F, Slot DE (2011) Oral hygiene in the prevention of periodontal diseases: the evidence. Periodontal 2000 55:104--123
Socransky SS, Haffajee AD (2002) Dental biofilms: difficult therapeutic targets. Periodontol 2000 28:12–55
Axelsson P, Lindhe J (1981) The significance of maintenance care in the treatment of periodontal disease. J Clin Periodontol 8:281–294
Lindhe J, Westfelt E, Nyman S, Socransky SS, Haffajee AD (1984) Long-term effect of surgical/non-surgical treatment of periodontal disease. J Clin Periodontol 11:448–458
Jones CG (1997) Chlorhexidine: is it still the gold standard? Periodontol 2000 15:55–62
Gjermo P, Bonesvoll P, Rölla G (1974) Relationship between plaque-inhibiting effect and retention of chlorhexidine in the human oral cavity. Arch Oral Biol 19:1031–1034
Bonesvoll P, Lökken P, Rölla G, Pous P (1974) Retention of chlorhexidine in the human oral cavity after mouth-rinses. Arch Oral Biol 19:1025–1029
Kozlovsky A, Artzi Z, Moses O, Kamin-Belsky N, Greenstein RB (2006) Interaction of chlorhexidine with smooth and rough types of titanium surfaces. J Periodontol 77:1194–1200
Hugo WB, Longworth AR (1964) Some aspects of the mode of action of chlorhexidine. J Pharm Pharmacol 16:655–662
Jenkins S, Addy M, Wade W (1988) The mechanisms of action of chlorhexidine. A study of plaque growth on enamel inserts in vivo. J Clin Periodontol 15:415–424
Schiott C, Löe H, Jensen SB, Killian M, Davies RM, Glanvind K (1970) The effect of chlorhexidine mouthrinses on the human oral flora. J Periodontal Res 5:84–89
Van Strydonck DAC, Slot DE, van der Velden U, van der Weijden F (2012) Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol 39:1042–1055
Cumming BR, Löe H (1973) Optimal dosage and method of delivering chlorhexidine solutions for the inhibition of dental plaque. J Periodontal Res 8:57–62
Flötra L, Gjermo P, Rölla G, Waerhaug J (1971) Side effects of chlorhexidine mouth washes. Scand J Dent Res 79:119–125
van Strydonck DAC, Timmerman MF, van der Velden U, van der Weijden GA (2005) Plaque inhibition of two commercially available chlorhexidine mouthrinses. J Clin Periodontol 32:305–309
Pizzo G, Guiglia R, Imburgia M, Pizzo I, D’Angelo M, Giuliana G (2006) The effects of antimicrobial sprays and mouthrinses on supragingival plaque regrowth: a comparative study. J Periodontol 77:248–256
McCoy LC, Wehler CJ, Rich SE, Garcia RI, Miller DR, Jones JA (2008) Adverse events associated with chlorhexidine use: results from the Department of Veterans Affairs Dental Diabetes Study. J Am Dent Assoc 139:178–283
Duss C, Lang NP, Cosyn J, Persson GR (2010) A randomized, controlled clinical trial on the clinical, microbiological, and staining effects of a novel 0.05 % chlorhexidine/herbal extract and a 0.1 % chlorhexidine mouthrinse adjunct to periodontal surgery. J Clin Periodontol 37:988–997
Hofer D, Meier A, Sener B, Guggenheim B, Attin T, Schmidlin PR (2011) Biofilm reduction and staining potential of a 0.05 % chlorhexidine rinse containing essential oils. Int J Dent Hyg 9:60–67
Bonesvoll P, Germo P (1978) A comparison between chlorhexidine and some quaternary ammonium compounds with regard to retention, salivary concentration and plaque inhibiting effect in the human mouth after mouthrinses. Arch Oral Biol 23:289–294
Segreto VA, Collins EM, Beiswanger BB (1986) A comparison of mouthrinses containing two concentrations of chlorhexidine. J Periodontol Res 21:23–32
Mandel ID (1988) Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol 15:488–498
Costa X, Serrano J, Laguna E, Herrera D, Serrano J, Alonso B, Sanz M (2013) Efficacy of a new mouth rinse formulation based on 0.07 % cetylpyridinium chloride in the control of plaque and gingivitis: a 6-month randomized clinical trial. J Clin Periodontol 40:1007–1015
Herrera D, Roldán S, Santacruz I, Santos S, Masdevall M, Sanz M (2003) Differences in antimicrobial activity of four commercial 0.12 % chlorhexidine mouthrinse formulations: an in vitro contact test and salivary bacterial counts study. J Clin Periodontol 30:307–314
Addy M, Jenkins S, Newcombe R (1991) The effect of some chlorhexidine-containing mouthrinses on salivary bacterial counts. J Clin Periodontol 18:90–93
Harper PR, Milsom S, Wade W, Addy M, Moran J, Newcombe RG (1995) An approach to efficacy screening of mouthrinses: studies on a group of French products (II). Inhibition of salivary bacteria and plaque in vivo. J Clin Periodontol 22:723–727
Claydon N, Smith S, Stiller S, Newcombe RG, Addy MA (2002) Comparison of the plaque-inhibitory properties of stannous fluoride and low-concentration chlorhexidine mouthrinses. J Clin Periodontol 29:1072–1077
Mendieta C, Vallcorba N, Binney A, Addy M (1994) Comparison of 2 chlorhexidine mouthwashes on plaque regrowth in vivo and dietary staining in vitro. J Clin Periodontol 21:296–300
Quirynen M, Avontroodt P, Peeters W, Pawels M, Coucke W, Van Steenberghe D (2001) Effect of different chlorhexidine formulations in mouthrinses on de novo plaque formation. J Clin Periodontol 28:1127–1136
Barnett ML (2003) The role of therapeutic antimicrobial mouthrinses in clinical practice: control of supragingival plaque and gingivitis. J Am Dent Assoc 134:669–704
Van der Weijden GA, Timmerman MF, Niiboer A, Reijerse E, Van der Velden U (1994) Comparison of different approaches to assess bleeding on probing as indicators of gingivitis. J Clin Periodontol 21:589–594
Löe H, Silness J (1963) Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 21:533–551
Brecx M, Macdonald LL, Legary K, Cheang M, Forgay MG (1993) Long-term effects of meridol and chlorhexidine mouthrinses on plaque, gingivitis, staining and bacterial vitality. J Dent Res 72:1194–1197
Syed SA, Loesche WJ (1972) Survival of human dental plaque flora in various transport media. Appl Microbiol 24:638–644
Turesky S, Gilmore ND, Glickman I (1970) Reduced plaque formation by the chloromethyl analogue of vitamin C. J Periodontol 41:41–43
Ashimoto A, Chen C, Bakker I, Slots J (1996) Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol 11:266–273
Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257–266
Loozen G, Boon N, Pauwels M, Quirynen M, Teughels W (2011) Live/dead real-time polymerase chain reaction to assess new therapies against dental plaque-related pathologies. Mol Oral Microbiol 26:253–261
Àlvarez G, González M, Isabal S, Blanc V, León R (2013) Method to quantify live and dead cells in multi-species oral biofilm by real-time PCR with propidium monoazide. AMB Express 3:1
Boutaga K, van Winkelhoff AJ, Vandenbroucke-Grauls CM, Savelkoul PH (2003) Comparison of real-time PCR and culture for detection of Porphyromonas gingivalis in subgingival plaque samples. J Clin Microbiol 41:4950–4954
Seow WK, Lam JHC, Tsang AKL, Holcombe T, Bird PS (2009) Oral Streptococcus species in pre-term and full-term children—a longitudinal study. Int J Paediatr Dent 19:406–411
Taskin B, Gozen AG, Duran M (2011) Selective quantification of viable Escherichia coli in biosolids by quantitative PCR with propidium monoazide modification. Appl Environ Microbiol 77:4329–4335
Addy M, Willis L, Moran J (1983) Effect of toothpaste rinses compared with chlorhexidine on plaque formation during 4-day period. J Clin Periodontol 10:89–99
Hoffmann T, Bruhn G, Richter S, Netuschil L, Brecx M (2001) Clinical controlled study on plaque and gingivitis reduction under long-term use of low-dose chlorhexidine solutions in a population exhibiting good oral hygiene. Clin Oral Investig 5:89–95
Santos S, Herrera D, Lopez E, O’Connor A, González I, Sanz M (2004) A randomized clinical trial on the short-term clinical and microbiological effects of the adjunctive use of a 0.05 % chlorhexidine mouthrinse for patients in supportive periodontal care. J Clin Periodontol 31:45–51
Quirynen M, Soers C, Desnyder M, Dekeyser C, Pauwels M, van Steenberghe D (2005) A 0.05 % cetyl pyridinium chloride/0.05 % chlorhexidine mouth rinse during maintenance phase after initial periodontal therapy. J Clin Periodontol 32:390–400
Escribano M, Herrera D, Morante S, Teughels W, Quirynen M, Sanz M (2010) Efficacy of low-concentration chlorhexidine mouth rinse in non-compliant periodontitis patients attending a supportive periodontal care programme: a randomized clinical trial. J Clin Periodontol 37:266–275
Bagis B, Baltacioglu E, Özcan M, Ustaomer S (2011) Evaluation of chlorhexidine gluconate mouthrinse-induced staining using a digital colorimeter: an in vitro study. Quintessence Int 42:213–223
Addy M, Mahdavi SA, Loyn T (1995) Dietary staining in vitro by mouth-rinses as a comparative measure of antiseptic activity and predictor of staining in vivo. J Dent 23:95–99
Boyd T, Vazquez J, Williams M (2010) Reduction of VSC and salivary bacteria by a multibenefit mouthrinse. J Breath Res 2:017013
Abiko Y, Sato T, Mayanagi G, Takahashi N (2010) Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J Periodontal Res 45:389–395
Maurin M (2012) Real-time PCR as a diagnostic tool for bacterial diseases. Expert Rev Mol Diagn 12:731–754
Gunsolley JC (2006) A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J Am Dent Assoc. 137:1649–1657
Acknowledgments
The authors wish to thank Vanessa Blanc, Rubén León, and Ann Bangle for their help during the study design and their suggestions during manuscript preparation.
Disclosure form
This study was funded by the Universitat Internacional de Catalunya and partially supported by a grant from Dentaid (Cerdanyola del Valles, Barcelona, Spain). Dentaid provided us with the RTF vials and conducted microbiological analysis in the Dentaid Research Center. The authors report no conflicts of interest related to this study. There is no financial relationship between any author and the commercial firm.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov Identifier: NCT02194023
Electronic supplementary material
ESM 1
(DOC 83.5 kb)
Rights and permissions
About this article
Cite this article
Mor-Reinoso, C., Pascual, A., Nart, J. et al. Inhibition of de novo plaque growth by a new 0.03 % chlorhexidine mouth rinse formulation applying a non-brushing model: a randomized, double blind clinical trial. Clin Oral Invest 20, 1459–1467 (2016). https://doi.org/10.1007/s00784-015-1625-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1625-y