Abstract
Backgrounds
Semaphorin 3A (Sema3A) was demonstrated to exert an osteoprotective effect by both inhibiting osteoclastic bone resorption and promoting osteoblastic bone formation. The effect of Sema3A on fracture healing of osteoporotic rats was investigated in this study.
Methods
Twelve weeks after bilateral ovariectomy, all animals underwent unilateral transverse osteotomy on the proximal tibiae, and were then randomly divided into two groups. Rats received vehicle (control) or weekly local injection of Sema3A (500 μg/kg) into the injury site (group Sema3A) after fracture surgery until sacrifice at 4 and 8 weeks. Specimens were harvested and examined by radiography, iDXA, histology, micro-CT, and three-point bending test.
Results
Compared to control, Sema3A treatment significantly increased bone mineral density, percent bone volume and biomechanical strength of the callus at 4 and 8 weeks post-fracture. At 8 weeks after fracture, the bone volume of callus showed no difference between groups, while the average cross-sectional area of callus in the control group was 43.8 % higher than that of Sema3A group. Histological images showed increased callus formation at 4 weeks post-fracture and better callus ossification in the Sema3A group, while callus remodeling in the control group seemed to be delayed and not well bridged.
Conclusions
Results in this study indicated that Sema3A treatment increased callus volume and density at 4 weeks post-fracture, and induced promoted callus ossification and remodeling at 8 weeks post-fracture compared to control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postmenopausal osteoporosis is a skeletal disorder characterized by low bone mass and deterioration of bone microarchitecture due to increased bone turnover induced by estrogen deficiency [1]. Previous studies show that osteoporotic bodies with fracture are usually subject to depressed callus quality and prolonged healing time than normal, even nonunion [2, 3]. Thus, there has been interest in treatments that can suppress excessive bone resorption or enhance new bone formation during bone regeneration, such as estrogen, selective estrogen receptor modulators (SERM), calcitonin, bisphosphonates (BPs), parathyroid hormone (PTH), strontium ranelate, sclerostin antibodies, and dickkopf-1 antibodies [4–7].
Bone tissue is densely innervated, and there is increasing evidence that neuronal regulation is critical for bone metabolism, and that semaphorin 3A (Sema3A) is up-regulated in response to nerve injury [8, 9]. In patients with traumatic brain injury and extremity fractures, accelerated speed of fracture healing and overgrowth of callus and heterotopic ossification are often encountered in clinical practice [10, 11]. A recent study found that Sema3A exerted an osteoprotective effect by both suppressing osteoclastic bone resorption and increasing osteoblastic bone formation. After intravenous administration of Sema3A, normal mice showed decreased osteoclastic and increased osteoblastic formation and function, mice with drill hole induced bone defects revealed enhanced bone regeneration, and ovariectomized mice showed a decrease in bone loss [12]. However, knowledge about the effect of Sema3A on callus formation and fracture healing in osteoporotic animals was limited.
Thus, this primary study aims to investigate the effects of local injected Sema3A on fracture healing in ovariectomized (OVX) rats. Four and eight weeks after fracture surgery, callus formation and fracture healing were evaluated by radiology, histology, micro-computed tomography (micro-CT) and biomechanical assessment.
Materials and methods
Animals
Seventy-four 3-month-old female Sprague–Dawley rats weighing 245 ± 28 g were included in this study. Every four animals were kept in one cage with climate-controlled conditions (25 °C; 55 % humidity; 12 h of light alternating with 12 h of darkness). Free access to tap water and standard laboratory diet containing 1.56 % calcium, 0.8 % phosphorus and 800 IU/kg vitamin D were permitted. Principles of laboratory animal care (NIH publication No. 85-23, revised 1985) were followed, and the study protocol was approved by the Ethics in Animal Research Committee of Sichuan University.
Surgical procedure
After bilateral ovariectomy (69 rats) or sham operation (5 rats) according to a previous report, 12 weeks were allowed to pass for the establishment of standard osteoporotic animal models [13]. The body weight of animals reached 400 ± 43 g for OVX rats and 345 ± 25 g for sham operated rats. Five randomly selected OVX rats and all the sham operated ones were sacrificed for bone mineral density (BMD) and micro-CT evaluation of the proximal tibiae.
Subsequently, all the remaining animals (64 rats) underwent unilateral transverse osteotomy on the right tibiae according to previous studies [14]. General anesthesia was obtained by abdominal injections of ketamine (100 mg/kg; 3B Scientific Corporation, USA) and xylazine (10 mg/kg; Atomax Chemicals Co., Ltd., China). Briefly, a transverse osteotomy was made at the proximal one-third of the right tibia, then fracture fragments were contacted and a stainless steel wire (1.0 mm in diameter, Shanghai Medical Devices Co., Ltd; China) was inserted through the tibial nod surface across the fracture ends via the medullary cavity. X-ray analysis was performed using RADspeed (RadSpeed M CH with FPD; SHIMADZU Corporation) to assess the location and quality of fractures immediately after surgery (Fig. 1a). All the animals received an intramuscular antibiotic and analgesic injection for 3 postoperative days. Unrestricted activity was allowed after wake-up from anesthesia.
a Radiograph of the fractured tibia taken immediately after surgery: the arrow shows the fracture line. b Scheme of the three-point bending test of the fractured tibia; specimen was placed in a repeated position with its medial surface facing downward on two lower support bars 15-mm apart, and the arrow shows the pressure through the central part of the callus
Pharmaceutical treatment
During fracture operation, all the animals were randomly divided into two groups (32 rats per group) for vehicle or Sema3A treatment. Animals from the Sema3A group accepted injection of Sema3A dissolved in sterile PBS (1 μg/μl), and rats from the vehicle group accepted injection of sterile PBS. The pharmaceutical treatment plan was blinded for the surgeon on the operation day. Sema3A or vehicle was locally injected exactly to the fracture gap during surgery and subcutaneously at the fracture site afterwards. Dosage of local application of Sema3A in rats was very limited. In this primary study, the dose of recombinant human Sema3A/Fc Chimera (R&D Systems) was 500 μg/kg weekly. During the treatment period, body weight of animals was measured weekly, and the drug dosage was adjusted accordingly.
Specimen harvest
At the end of the observation time (4 and 8 weeks after fracture), animals were euthanized by cardiac puncture under general anesthesia and fractured tibiae were collected carefully. After complete excision of soft tissues, half the specimens from each group were stored at −80 °C until biomechanical testing, and the other half were kept in 70 % alcohol for radiological, micro-CT and undecalcified section analysis.
Radiography
Radiographs of the fractured tibiae were taken using RADspeed at settings of 40 kilovolts and 1.2 milliamps immediately after harvesting. Fracture healing was evaluated in a blinded manner according to the classification of Goldberg et al. [15] mainly as follows: nonunion, possible union, or complete union.
Callus BMD analysis
BMD analysis of the callus was performed using Lunar iDXA (GE Healthcare Lunar, USA), with the hand regional high resolution and small-animal scan mode. Briefly, the fractured tibiae were placed in deionized water with the same position for X-ray scan. The region of interest (ROI) was chosen as a longitudinal rectangle covering the whole callus area. BMD values (mg/cm2) were obtained for statistical analysis.
Micro-CT examination
For micro-CT examination, specimens were prepared as 10-mm-long blocks with callus and kept in 70 % ethanol. All blocks were scanned on a μCT system (μCT 80 scanner Scanco Medical, Bassersdorf, Switzerland) with an isotropic voxel size of 10 μm and reconstructed for a detailed qualitative and quantitative evaluation. The constrained 3D Gaussian filter (σ = 1.2, support = 1) was used for partial noise suppression in the image. The volume of interest (VOI) was defined as a cylinder covering the whole callus area; the newly formed bone tissue was defined on the basis of the threshold level. In brief, the high and low radio-opacity mineralized tissues were differentially segmented by a two-level global thresholding procedure, with low attenuation = 250 and high attenuation = 500 in per mille of maximal image gray value [16, 17]. After segmentation, bone volume (BV), percent bone volume (BV/TV), and average cross-sectional area (CsAr) were quantified within the VOI using software attached to the μCT 80 scanner running on OpenVMS operating system.
Histological analysis
For histological sections, specimens were dehydrated in graded ethanol (80–100 %), and embedded in methylmethacrylate (Technovit 7200 VCL; Exact Apparatbau, Nordenstedt, Germanny) without decalcification. Coronal sections 5-μm thick were prepared (Leica, Germany) and stained in 1 % toluidine blue. Images were acquired under light microscopy (Eclipse 80i, Nikon, Japan) with a digital camera. The percent of bone union, i.e., rate of the length of bone union to the total length of the osteotomy, was calculated with Leica DMI 6000 B micro-systems (Germany). Bone-to-bone binding was defined as bone union, while fibrous binding was defined as non-union.
Biomechanical testing
A three-point bending test on the fractured calluses was performed by a commercial material testing system (Instron 4302; Instron, Norwood, MA, USA). Briefly, all specimens were placed in a repeated position with the medial surface facing downward on two lower support bars 1.5 cm apart, and the testing area was defined as the central part of the callus (Fig. 1b). The specimen was then compressed at the velocity of 1 mm/min until breakage. From the load–deflection curve recorded by a connected computer, the ultimate load at failure (N; maximum force that the tibia could bear), stiffness (N/mm; slope of the load–deflection curve from the linear part), and total energy absorption (mJ; energy absorbed by tibia during compression progress) were calculated.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Statistical analyses were performed using the statistics package SPSS 13.0 (SPSS, Chicago, IL, USA). Comparisons of means were carried out using Student’s t-test, whereas comparisons of proportions were performed by Fisher’s exact test. The significance level of 0.05 was applied for all analyses.
Results
Establishment of osteoporotic model
Twelve weeks after sham or OVX operation, micro-CT cross-slices showed the changes of bone mass and microarchitecture of the proximal tibiae before and after sham or OVX operation. In quantitative analysis, OVX operation decreased the BV/TV by 40.6 %, the mean trabecular number by 44.1 %, the mean trabecular thickness by 42.3 %, but increased the mean trabecular separation by 55.4 % when compared to sham operation (Fig. 2).
Micro-CT cross-slices of the proximal tibiae before (baseline) or 12 weeks after sham or OVX operation on rats, as well as the results of quantitative analysis. Data are expressed as mean ± SD, n = 5 specimens per group. *p < 0.05 OVX vs sham group (Student’s t-test). BV/TV percent bone volume, Tb. N the mean trabecular number, Tb. Th the mean trabecular thickness, Tb. Sp the mean trabecular separation
Radiography
Four weeks after fracture, distinct fracture lines were observed on the X-ray films taken from rats in the control group, but those of the Sema3A group had reached possible union. Eight weeks after fracture, the fracture gap reached bony union in both groups. However, specimens from the Sema3A group showed higher radiodensity of the callus compared to the control (Fig. 3).
Callus BMD analysis
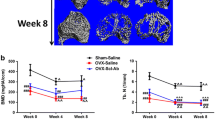
Results of callus BMD analysis showed that both groups showed significantly increased BMD values at 8 weeks than that of 4 weeks after fracture (p < 0.05, data not shown). For group comparison, no significant difference was observed at 4 weeks after fracture. However, callus BMD value of the Sema3A group was 12.4 % higher than the control at 8 weeks after fracture (p < 0.05, data not shown).
Micro-CT examination
Representative 2D images from coronal cutaways and transverse slices through the center of the callus are presented in Fig. 4, and the results of quantitative analysis within VOI were expressed as BV, BV/TV, and CsAr in Table 1. At 4 weeks post-fracture, Sema3A increased the value of BV by 55.7 %, BV/TV by 32.8 %, and CsAr by 51.6 % compared to the control (p < 0.05). At 8 weeks post-fracture, Sema3A increased the value of BV/TV by 44.4 % compared to control (p < 0.05); the value of BV showed no difference between groups; while the value of CsAr in the control group was 43.8 % higher than that of Sema3A group (p < 0.05).
Histological analysis
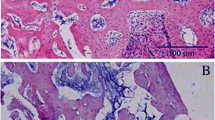
Undecalcified histological images are exhibited in Fig. 5. At 4 weeks post-fracture, increased callus formation and better bone union were observed in the Sema3A group; the bone union rate (%) was 25.7 ± 9.7 in the control and 75.1 ± 20.3 in the Sema3A group (p < 0.05, by Fisher’s exact test). At 8 weeks post-fracture, callus remodeling seemed to be delayed and not well bridged in the control group, while bone union was almost completed in the Sema3A group; the bone union rate (%) was 40.2 ± 11.3 in the control and 87.4 ± 9.5 in the Sema3A group (p < 0.05, by Fisher’s exact test).
Biomechanical testing
Results of biomechanical testing are presented in Table 2. At 4 weeks post-fracture, Sema3A increased the value of ultimate load by 135.2 %, stiffness by 117.4 %, and energy absorption by 134.8 % compared to the control (p < 0.05). At 8 weeks post-fracture, Sema3A increased the value of ultimate load by 76.2 %, stiffness by 70.7 %, and energy absorption by 97.1 % compared to the control (p < 0.05).
Discussion
Over the whole lifetime, bone remodeling is tightly regulated through a complex network of hormones, cytokines and direct cellular interactions by affecting the crosstalk between osteoblasts and osteoclasts. Any disturbance in this regulating network may lead to abnormal bone resorption and formation, such as osteoporosis [1, 18]. Osteoporosis is a systemic bone metabolic disease characterized by low bone mass and microarchitectural deterioration of bone tissue, relating to enhanced bone fragility and a consequent increase in fracture risk. The aging population of humans has continually increased, thus more and more middle-aged and senile patients are affected by post-menopausal or age-related osteoporosis. A previous study reported that over 50 % of women as well as 13 % of men older than 50 might be subject to osteoporosis-related fractures during their lifetime [19]. Patients with osteoporotic fractures have dramatic difficulty in performing daily living activities and suffer from significant pain. Moreover, osteoporotic fractures have been a significant burden on society and healthcare systems [20, 21].
Sema3A, first described as an axon-guiding molecule, is a protein produced by osteoblasts with the ability to inhibit receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclastogenesis and to promote osteoblastic bone formation [12, 22]. Results from radiography, iDXA, histology, micro-CT, and three-point bending test in this study indicated that Sema3A treatment increased callus mass, quality, and biomechanical strength at the early stage of fracture healing (4 weeks post-fracture), and induced promoted callus ossification, remodeling and biomechanical strength at the middle stage of fracture healing (8 weeks post-fracture) compared to the control. Since previous studies showed that osteoporosis impaired callus quality and prolonged fracture healing time compared to normal [2, 3], results in this study suggested that Sema3A held the promise of promoting fracture healing in osteoporotic bodies.
Since osteoporosis is characterized by increased bone resorption, most anti-osteoporotic drugs target osteoclastgenesis by reducing the rate of bone resorption, such as RANKL inhibitor and bisphosphonates [16, 23]. However, these drugs often cause reduction in new bone formation because of the consequent disruption of the linkage between osteoclast and osteoblast activity. Parathyroid hormone (PTH) is the representative anabolic drug which can directly stimulate bone formation, and is currently the only Food and Drug Administration approved anabolic agent for osteoporosis treatment [24]. However, the anabolic effect of PTH is usually related to enhanced bone resorption, and the withdrawal of PTH is reported to lose the therapeutic effects [25]. Strontium ranelate is one of the agents reported to promote osteoporotic fracture healing, with the dual effects of promoting bone formation and inhibiting bone resorption [15, 26, 27].
Information about the effect of Sema3A on fracture healing in osteoporotic animals was limited. Hayashi et al. found that intravenous injection of recombinant Sema3A led to increased osteoblastic parameters and decreased osteoclastic parameters in intact mice; Sema3A enhanced bone regeneration in a mouse model of cortical bone defect; and that Sema3A treatment rescued bone loss in ovariectomized mice [12]. Although it has been suggested that Sema3A regulates bone remodeling directly by regulating the activities of osteoclasts and osteoblasts in vitro [12], in vivo study seems to indicate that decreased expression of Sema3A in bone was not the cause of the bone abnormality. Osteoblast-specific Sema3A-deficient mice were demonstrated to have normal bone mass, although the expression of Sema3A in bone significantly decreased. However, mice lacking Sema3A in neurons showed low bone mass, which seems to indicate that neuron-derived Sema3A is responsible for the decreased bone mass independent of the local role of Sema3A in bone, namely Sema3A regulates bone remodeling by indirectly engaging in sensory nerve innervations [28]. These results are somehow inconsistent with in vitro results that Sema3A directly promotes osteoblastic bone formation and inhibits osteoclastic bone resorption [12].
Results of this preliminary study indicated that locally applied Sema3A promoted fracture healing and callus remodeling in osteoporotic rats. The sensory nerve projections were not detected in this study, thus it cannot be confirmed whether local Sema3A influences bone remodeling indirectly by regulation of sensory nerve innervations. At 4 weeks post-fracture, increased callus formation in the Sema3A group can be observed in histological sections and confirmed by quantitative micro-CT examination. This result can be easily explained by increased bone formation and inhibited bone resorption due to Sema3A treatment. However, at 8 weeks post-fracture, quantitative micro-CT analysis indicates that the value of BV showed no difference between groups; while the value of CsAr in the control group was 43.8 % higher than that of Sema3A group (p < 0.05). It seems that Sema3A promotes callus remodeling at the middle stage of fracture healing (4–8 weeks after fracture). One plausible explanation may be that Sema3A shortened the period of callus formation, so that the period of callus remodeling started earlier than in thecontrol. Moreover, osteoporosis is usually accompanied by uncoupled bone resorption and formation, thus the different physiological conditions of OVX animals from intact bodies may be another explanation.
There are many other possible functions of Sema3A that may be involved in bone healing when systemic treatment is used, e.g., influences on platelets, hematopoietic stem cells movement, and inflammatory roles. Previous studies indicated that the sensory innervation of bone might play an important role in sensing and responding to low-intensity pulsed ultrasound and explain its effect in promoting fracture healing. Lam et al. [29] found that daily ultrasound treatment significantly increased the rate of union and the volumetric bone mineral density in the fracture callus in the neurally intact rats, but this stimulating effect was absent in the rats with sciatic neurectomy. Nordsletten et al. studied the effect of sciatic nerve resection on tibial fracture healing. Higher bone mineral content but low mechanical strength in three-point cantilever bending was observed in the nerve-resected rats, implying a defective organization of the large callus [30]. These results suggest that neural regulation plays a role in the type of fracture healing, direct or indirect, and in the amount and quality of the callus.
Conclusions
The results of this study demonstrated that Sema3A treatment could increase callus formation, bone mineral density and biomechanical strength at the early stage of fracture healing (4 weeks post-fracture), and promote callus ossification, remodeling and biomechanical strength at the middle stage of fracture healing (8 weeks post-fracture). It was suggested that Sema3A induced improved callus quality and shortened healing time in osteoporotic fracture settings.
References
Consensus Development Conference. Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–50.
Kubo T, Shiga T, Hashimoto J, Yoshioka M, Honjo H, Urabe M, Kitajima I, Semba I, Hirasawa Y. Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J Steroid Biochem Mol Biol. 1999;68(5–6):197–202.
Namkung-Matthai H, Appleyard R, Jansen J, Lin HJ, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28(1):80–6.
Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002;17(12):2237–46.
Li X, Luo X, Yu N, Zeng B. Effects of salmon calcitonin on fracture healing in ovariectomized rats. Saudi Med J. 2007;28(1):60–4.
Agholme F, Isaksson H, Kuhstoss S, Aspenberg P. The effects of Dickkopf-1 antibody on metaphyseal bone and implant fixation under different loading conditions. Bone. 2011;48(5):988–96.
Nozaka K, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Shimada Y. Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone. 2008;42(1):90–7.
De Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, Pasterkamp RJ, Gispen WH, Verhaagen J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175(1):61–75.
Hashimoto M, Ino H, Koda M, Murakami M, Yoshinaga K, Yamazaki M, Moriya H. Regulation of semaphorin 3A expression in neurons of the rat spinal cord and cerebral cortex after transection injury. Acta Neuropathol. 2004;107(3):250–6.
Kushwaha VP, Garland DG. Extremity fractures in the patient with a traumatic brain injury. J Am Acad Orthop Surg. 1998;6(5):298–307.
Gautschi OP, Cadosch D, Frey SP, Skirving AP, Filgueira L, Zellweger R. Serum-mediated osteogenic effect in traumatic brain-injured patients. ANZ J Surg. 2009;79(6):449–55.
Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485(7396):69–74.
Varkey M, Kucharski C, Doschak MR, Winn SR, Brochmann EJ, Murray S, Matyas JR, Zernicke RF, Uludag H. Osteogenic response of bone marrow stromal cells from normal and ovariectomized rats treated with a low dose of basic fibroblast growth factor. Tissue Eng. 2007;13(4):809–17.
Wang JW, Xu SW, Yang DS, Lv RK. Locally applied simvastatin promotes fracture healing in ovariectomized rat. Osteoporos Int. 2007;18(12):1641–50.
Goldberg VM, Powell A, Shaffer JW, Zika J, Bos GD, Heiple KG. Bone grafting: role of histocompatibility in transplantation. J Orthop Res. 1985;3(4):389–404.
Gerstenfeld LC, Sacks DJ, Pelis M, Mason ZD, Graves DT, Barrero M, Ominsky MS, Kostenuik PJ, Morgan EF, Einhorn TA. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res. 2009;24(2):196–208.
Müller R, Rüegsegger P. Micro-tomographic imaging for the nondestructive evaluation of trabecular bone architecture. Stud Health Technol Inform. 1997;40:61–79.
Nakahama K. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 2010;67(23):4001–9.
Chrischilles EA, Butler CD, Davis CS, Wallace RB. A model of lifetime osteoporosis impact. Arch Intern Med. 1991;151(10):2026–32.
Wasnich RD, Ross PD, Heilbrun LK, Vogel JM. Prediction of postmenopausal fracture risk with use of bone mineral measurements. Am J Obstet Gynecol. 1985;153(7):745–51.
Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, Mascioli SR, Scott JC, Seeley DG, Steiger P. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263(5):665–8.
Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75(2):217–27.
McDonald MM, Dulai S, Godfrey C, Amanat N, Sztynda T, Little DG. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone. 2008;43(4):653–62.
Tashjian AH Jr, Gagel RF. Teriparatide [human PTH(1-34)]: 2.5 years of experience on the use and safety of the drug for the treatment of osteoporosis. J Bone Miner Res. 2006;21(3):354–65.
Tanizawa T, Yamamoto N, Takano Y, Mashiba T, Zhang L, Nishida S, Endo N, Takahashi HE, Fujimoto R, Hori M. Effects of human PTH(1-34) and bisphosphonate on the osteopenic rat model. Toxicol Lett. 1998;28(102–103):399–403.
Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42(1):129–38.
Habermann B, Kafchitsas K, Olender G, Augat P, Kurth A. Strontium ranelate enhances callus strength more than PTH 1-34 in an osteoporotic rat model of fracture healing. Calcif Tissue Int. 2010;86(1):82–9.
Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, Ma C, Xu C, Bando W, Fujita K, Shinomiya K, Hirai T, Asou Y, Enomoto M, Okano H, Okawa A, Itoh H. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497(7450):490–3.
Lam WL, Guo X, Leung KS, Kwong KS. The role of the sensory nerve response in ultrasound accelerated fracture repair. J Bone Joint Surg Br. 2012;94(10):1433–8.
Nordsletten L, Madsen JE, Almaas R, Rootwelt T, Halse J, Konttinen YT, Hukkanen M, Santavirta S. The neuronal regulation of fracture healing. Effects of sciatic nerve resection in rat tibia. Acta Orthop Scand. 1994;65(3):299–304.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No. 81300858).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no conflict of interest.
About this article
Cite this article
Li, Y., Yang, L., He, S. et al. The effect of semaphorin 3A on fracture healing in osteoporotic rats. J Orthop Sci 20, 1114–1121 (2015). https://doi.org/10.1007/s00776-015-0771-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-015-0771-z