Abstract
Five non-symbiotic hemoglobins (nsHb) have been identified in rice (Oryza sativa). Previous studies have shown that stress conditions can induce their overexpression, but the role of those globins is still unclear. To better understand the functions of nsHb, the reactivity of rice Hb1 toward hydrogen peroxide (H2O2) has been studied in vitro. Our results show that recombinant rice Hb1 dimerizes through dityrosine cross-links in the presence of H2O2. By site-directed mutagenesis, we suggest that tyrosine 112 located in the FG loop is involved in this dimerization. Interestingly, this residue is not conserved in the sequence of the five rice non-symbiotic hemoglobins. Stopped-flow spectrophotometric experiments have been performed to measure the catalytic constants of rice Hb and its variants using the oxidation of guaiacol. We have shown that Tyrosine112 is a residue that enhances the peroxidase activity of rice Hb1, since its replacement by an alananine leads to a decrease of guaiacol oxidation. In contrast, tyrosine 151, a conserved residue which is buried inside the heme pocket, reduces the protein reactivity. Indeed, the variant Tyr151Ala exhibits a higher peroxidase activity than the wild type. Interestingly, this residue affects the heme coordination and the replacement of the tyrosine by an alanine leads to the loss of the distal ligand. Therefore, even if the amino acid at position 151 does not participate to the formation of the dimer, this residue modulates the peroxidase activity and plays a role in the hexacoordinated state of the heme.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globins are ubiquitous proteins found in all kingdoms of life [1, 2]. The globin family members share several common features. First, they adopt a globin-type fold with 6–8 α-helices (named A–H). Second, the active site of globins carries a heme prosthetic group (Fe-protoporphyrin IX) which is embedded inside the protein. The heme iron can be oxidized or reduced and is able to reversibly bind different small molecules, such as O2, NO, CO, and CN−, depending on its redox state.
Hemoglobin (Hb) from red blood cell was the first protein identified in the globin family. Together with myoglobin from vertebrate muscles, they represent a model system for globins. Hb from erythrocytes and Mb are hexacoordinated globins with the heme iron interacting with the four nitrogen atoms of the porphyrin, with the side chain of proximal histidine ligand localized on the F helix and a water molecule as the distal ligand.
In 2000, Burmester discovered a third vertebrate globin called neuroglobin (Ngb), because they found that this monomeric protein was predominantly expressed in the central and peripheral nervous system in vertebrates [3]. Then, Ngb has been also observed in the retina [4], in the spinal cord [5], in the auditory system [6], and also in some tumor cells [7,8,9]. The role of Ngb is still unclear, but it seems that its function is cell-dependent.
In 2001, a fourth vertebrate globin named cytoglobin (Cgb) has been identified [10]. Despite intensive research over the past decade, the cellular function of Cgb is still not clearly established. Unlike erythrocyte Hb and myocyte Mb, Ngb and Cgb are hexacoordinated proteins with the sixth coordination provided by the side chain of a distal histidine situated in the E helix. The reactivity of the globin toward ligands is controlled by the nature of the distal ligand (endogenous distal histidine or water molecule). The removal of the distal histidine residue by side-directed mutagenesis leads to the production of heme protein where the distal histidine has been replaced by a water molecule. This variant exhibits an increased affinity for ligands [11,12,13].
In plants, three hemoglobin types have been identified so far: truncated, symbiotic, and non-symbiotic Hbs (for review, see [14, 15]) often referred to phytoglobins [16]. Truncated Hbs are usually shorter than the other plant hemoglobins and exhibit an abbreviated 2-over-twofold. Their physiologic functions are not clearly identified (for review, see [17]). The symbiotic Hbs which are expressed in plant nodules are involved in the oxygen supply regulation for nitrogen-fixing bacteria [18, 19]. The non-symbiotic hemoglobins (nsHb) have been found in different plants: maize [20], spinach [21], rice [22], Lotus japonicus [23], Arabidopsis thaliana [24], and barley [25]. nsHb family is divided into two phylogenetic groups termed class 1 and class 2, depending on their oxygen affinity. Class 1 nsHbs exhibit high affinity for O2 due to a low O2-dissociation rate constant [22]. In contrast, class 2 nsHbs have a lower O2 affinity likely due to the existence of only a small fraction of hexacoordinated heme [24, 26]. The nsHb functions are still unclear, but those globins could play different roles in plant metabolism, including O2-transport, O2-sensing, NO-scavenging, and also redox-signaling. It has been reported that those proteins could also participate in cellular protection mechanism during oxidative stress [27]. Knock-out studies in Arabidopsis thaliana have shown that at least one functional nsHb gene is essential for plant survival [14, 28].
Rice non-symbiotic hemoglobins (rHb) have been first described in 1997 [22]. Among them, hemoglobins from a Japanese rice, called Oryza sativa, are the most studied members. Up to now, five different rHbs in Oryza sativa rice have been discovered and named rHb1–rHb5 [29, 30]. rHb1, which belongs to class 1, is the only rHbs whose X-ray structure has been resolved [27]. In the absence of an external ligand, this 18-kDa globin is a hexacoordinated globin, like Cgb and Ngb, with His F8 and His E7 as the fifth (proximal) and the sixth (distal) iron ligand, respectively [26].
rHbs have been studied in vivo [30,31,32,33] and in vitro [22, 34, 35], but the functions of the different rHbs have not been clearly established. Their expressions in active tissues (seeds, leaves, stems, and roots) suggest that those globins play an important role in plant metabolism (for review see [36]). Moreover, it has been demonstrated that genes’ expression of nsHb from rice and other plants is modified with different stress stimuli as osmotic stress and high salinity [24, 37,38,39] and under low O2 conditions [31]. The treatments with nitrate, nitrite, and nitric oxide have also shown to induce a change in the pattern of nsHb genes’ expression [40,41,42,43]. This result suggests that the function of the rHb is related to the response of plants to stress. The stress conditions are known to induce an increase of the cellular concentration of reactive oxygen species (ROS) which are converted to H2O2 [44]. H2O2 can be used by the plant as a signaling molecule in the transduction of stress signals. Indeed, this molecule could modify the patterns of plants’ genes’ expression to regulate cell-cycle processes [45,46,47]. However, at high concentration, H2O2 becomes a molecule critical for the viability of the cells, so it is necessary that plants develop systems to control the concentration of H2O2 [48]. It has been demonstrated that an Arabidopsis thaliana line overexpressing hemoglobin 1 (AtGLB1) maintains a low endogenous H2O2 level after hypoxic stress [49]. In vertebrates, it has been shown that hemoglobin from erythrocyte cells can make the H2O2 level decrease by acting as an anti-oxidative peroxidase [50].
In vitro, the first article dealing with peroxidase activity of a globin has been published in the early 1950s [51,52,53]. In this paper, the decomposition of H2O2 by myoglobin has been observed. From this discovery, the peroxidase activity of several myoglobins from different taxa has been evaluated. It has been shown that the reaction of some globins with hydrogen peroxide (H2O2) leads to the formation of a transient ferryl heme (FeIV = O) called compound II [54]. When Ngb and Cgb have been identified in the early 2000s, their ability to react with H2O2 has been tested. Interestingly, those two vertebrate hexacoordinated globins react with this ligand differently. Cgb has been reported to have considerable peroxidase activity consuming both hydrogen peroxide and lipid peroxides [10, 55], whereas native Ngb does not react with H2O2 at all [56]. Moreover, protein oligomerization has been observed for several hemoproteins in the presence of H2O2. For example, human myoglobin (hMb) forms a dimer in the presence of H2O2 due to the formation of a disulfide bond between two cysteines located on the G helix at position 110 [57]. Since this discovery, the reactivity of hMb with H2O2 has been intensively studied. Addition of H2O2 to hMb leads to a thiyl radical formation at residue Cys110 that is facilitated by an electron transfer between Tyr103 and Cys110. Indeed, the variant Tyr103Ala presents a decrease in the peroxidase activity [58]. Although the Cys110Ala mutation in human Mb prevents thiyl radical formation, the apparent rate constant for the reaction with H2O2 is not modified by the mutation [57]. Up to now, no other myoglobin has been involved in a protein dimerization through a disulfide bridge, but other globins have been shown to dimerize through dityrosine cross-links in the presence of H2O2 [59,60,61,62,63,64].
Peroxidase activity is one of the potential functions proposed for globins. Considering that some nsHb seems to protect plants from an H2O2 excess at the cellular level and that some globins are able to degrade H2O2, we have decided to analyze the potential peroxidase activity of nsHb1 from rice and the roles of tyrosines. We focused our study also on the dimerization of rHb in the presence of H2O2. The purpose of this study is to understand if some structural parameters, potentially the tyrosine residues studied in this paper, could control this activity.
Material and methods
Chemicals
Horse heart myoglobin (hhMb) (ref M1882) was obtained from Sigma-Aldrich. The commercial protein was treated with 0.5 mM K3FeCN6 (potassium hexacyanoferrate III) overnight at 4 °C to oxidize heme iron and then dialyzed against phosphate buffer (65 mM, pH 7.0). The purity was confirmed using absorption ratio at aromatic region (280 nm) and at the Soret peak (409 nm). Restriction enzymes were from Takara or New England Biolabs. Pfu DNA polymerase and T4 DNA ligase were purchased, respectively, from Stratagen and Thermo Scientific. Oligonucleotides were synthetized by Eurogentec. Chemical products were purchased from Sigma-Aldrich.
Isolation of rice genomic DNA from rice seeds
5.0 g of Oryza sativa seeds was crushed until a fine powder was obtained. The powder was added into 40 mL of extraction buffer (100 mM Tris HCl pH 8.0, 50 mM EDTA (ethylenediaminetetraacetic acid disodium salt) pH 8.0, 500 mM NaCl, and 4 mM dithiothreitol (DTT), and then, 5.6 mL of 20% sodium dodecyl sulfate (SDS) was added. The obtained solution was mixed gently for 30 min at 65 °C and then centrifuged for 10 min at 10,000g. The supernatant was filtered through a 0.22 µm filter to remove aggregates. Then, 16.7 mL of 5 M ammonium acetate was added and the solution was left for 30 min on ice and then centrifuged for 30 min at 3000g. 30 mL of the supernatant was collected and DNA was precipitated by addition of 24 mL isopropanol. The genomic DNA was collected by centrifugation for 30 min at 3000g and then resuspended in 500 µL of ultrapure water.
Cloning of rHb1 gene
rHb1 gene was first amplified by PCR using genomic DNA as template. Oligonucleotide primers specific to the 5′ and 3′ ends of rHb1 gene (5′GCAGCGGCGCCGCTCTCGTGGAGGGAA3′ and 5′CGATAAGCTTTCACTCAGCAGGCTTC3′), respectively, were used. Two restriction sites (Nar I and Hind III) were incorporated at the 5′-end of each primer to facilitate insertion into the expression plasmid (the restriction sites are underlined). After PCR reaction, the size of the amplified product was analyzed by agarose gel electrophoresis. The DNA fragment was longer than the expected size of rHb1 gene due to introns. The amplified DNA was cloned into polylinker site of the expression vector pPROEX HTA. To eliminate introns, three successive PCR have been performed using primers that overlap two successive coding genes. At each step, the amplified plasmid pPROEX TA rHb1 was sequenced. In another construction pPreSci rHb1, the cleavage site sequence specific of tobacco etch virus (TEV) protease has been modified to be recognized by PreScission.
The Y112A rHb1, Y151A rHb1, and double mutant Y112A Y151A rHb1 gene were constructed using the QuickChange Site Directed Mutagenesis Kit (Stratagene) and the mutations were verified by DNA sequencing.
Expression and purification of rice Hb1
WT rHb1 and mutants were expressed by the host strain Escherichia coli BL21 (λDE3). Transformed bacteria were grown overnight at 37 °C in a 10 mL LB (Luria–Bertani) medium supplemented with 100 µg/mL of ampicillin. Then, the overnight culture was transferred into 500 mL TB (terrific broth) medium-containing 5-aminolevulinic acid (0.5 mM) and ampicillin (100 µg/mL). The culture was shaken at 37 °C at 200 rpm until an optical density (OD600nm) of 1.2 was reached. The expression of the protein was induced by the addition of 0.4 mM IPTG (Isopropyl β-d-1-thiogalactopyranoside), and the incubation was performed at 25 °C for 16 h at 200 rpm. The reddish cells were collected by centrifugation at 3000g for 15 min and stored at − 80 °C. To extract the proteins, the bacterial pellet was resuspended in 50 mL Tris HCl (50 mM, pH 8), DNAse I (0.02 mg mL−1), and phenylmethylsulphonyl fluoride (1 mM), and lysed by sonication. The crude lysate was centrifuged for 30 min at 40,000g at 4 °C and the supernatant was filtered through 0.22 µm filter to remove aggregates. The His-tag protein was isolated by incubation for 1 h with 20 mL Ni–NTA resin buffered with Tris HCl (50 mM, pH 8) and imidazole (5 mM). Then, the solution was poured into a column and the non-specific proteins were eluted with a buffer containing Tris HCl (50 mM, pH 8.0) and imidazole (5 mM) until DO280nm of the washing buffer was below 0.1. The His-tag protein was eluted with buffer containing Tris HCl (50 mM, pH 8.0) and imidazole (300 mM). The imidazole was removed by dialyzing against phosphate buffer (65 mM, pH 7.0). The purest and the most concentrated fractions were pulled together. The His-tag fragment was cleaved with PreScission or with TEV protease depending on the plasmid used. For each protease, the ratio of enzyme/substrate has been optimized. Then, the Ni–NTA resin was used to remove his-tagged protease (in the case of TEV protease), the non-cleavaged Hb1, and His-tag fragments. To discard non-specific proteins, the solution was loaded onto a Sephacryl S200 exclusion size column. Fractions containing proteins were collected and the purity was ≥ 95% as assessed by SDS-PAGE gels and Soret/280 absorbance ratios. The solutions containing recombinant proteins and hhMb were treated with 0.5 mM K3FeCN6 (potassium hexacyanoferrate III) overnight at 4 °C to oxidize heme iron and then dialyzed against phosphate buffer (65 mM, pH 7.0) before being stored at − 80 °C. The absence of apoprotein is checked according to [40].
Proteins analysis
For routine experiments, UV–visible spectra were recorded on a DU 800 Beckman Coulter spectrophotometer at ambient temperature. Protein concentrations were determined spectrophotometrically using Soret band extinction coefficients measured in this study.
Analysis of peroxidase reaction products by gel electrophoresis
58 µg of WT rice Hb1 (10 µL of stock solution at 320 µM) was incubated with 10 µL of H2O2 to obtain a final H2O2 concentrations of 0, 32, 40, 80, 160, 800, 1600, 3200, and 6400 µM in phosphate buffer (65 mM, pH 7.0) at 20 °C for 5 min. The reaction was stopped by adding 7 µL of catalase at the concentration of 0.01 mg mL−1 for 10 min. The reaction mixture was then treated with DTT at the final concentration of 10 mM for 10 min. The samples were heated at 95 °C for 5 min and electrophoresis was carried out on a 12% SDS-PAGE gel. Proteins were visualized by staining gel with Coomassie Blue. Precision Plus Protein Dual Xtra Standards from Biorad was used as ladder. The yield of the dimer was estimated using Gene Tools, an optical densitometry software from Syngene. To quantify precisely the amount of dimers produced during the reaction with H2O2, a range of known amounts of rHb1 were deposited on a gel from 0.5 (30 pmol) to 100 µg (6 nmoles). After electrophoresis, protein bands stained with Coomassie Blue were analyzed by Gene Tools. The area of each peak was measured to make a calibration curve. The curve showing the peak as a function of amount of protein describes a linear relationships until 20 µg of rHb1 (i.e., 1.2 nmoles). This reference scale was used to estimate the quantity of dimer formed during the reaction. The weight of dimer was then converted into molar quantity and the percentage of dimer produced was calculated by dividing this value by the amount of monomer (3.2 nmoles) used to initiate the reaction. For experiences in presence of sodium ascorbate, 58 µg of WT rice Hb1 in a phosphate buffer (65 mM, pH 7.0) was treated with H2O2 for 10 min (line 2), or treated with sodium ascorbate for 10 min, and then, H2O2 was added for 10 min (line3), or treated with H2O2 for 10 min, and then, sodium ascorbate was added for 10 min (line 4). The ratio protein:H2O2:sodium ascorbate was equal to 1:1:1. Line 1 corresponds to the protein without any treatment.
Determination of Soret band extinction coefficient of WT Hb1 and its variants
Extinction coefficients were determined by pyridine hemi-hemochromogen method [65,66,67]. Spectroscopic measures were conducted in triplicate for each experiment, and independent experiments were performed using at least three batches of proteins. Extinction coefficients for deoxyglobins were calculated by measuring the absorbance of the Soret band after addition of a small amount of freshly prepared solution of sodium dithionite.
Peroxidase activity assay
Peroxidase activities of WT rHb1 and mutants were determined with an Applied Photophysics SX20 stopped-flow equipped with a 150W Xenon arc lamp and a temperature-controlled bath. The catalytic constants were evaluated using guaiacol (0–80 mM) as substrate and H2O2 (100 mM) as oxidant. The precise concentration of H2O2 was measured using the extinction coefficient ε240nm = 43.6 M−1 cm−1 [68]. The kinetic measurements were carried out at 20 °C in a phosphate buffer (65 mM, pH 7.0). The final concentration of rHb1 varied from 1 to 5 µM depending on the variant. Due to a lower peroxidase activity, the hhMb concentration was adjusted from 5 to 20 µM. The reaction was initiated by the addition of the solution containing H2O2/guaiacol. The kinetics of appearance of tetraguaiacol, the tetrameric form of guaiacol, were followed at 470 nm using an extinction coefficient ε470nm = 26.6 mM−1 cm−1 [69]. For each concentration of guaiacol, four acquisitions with 8000 time points were performed on a time scale of 10 s. The kinetic traces were analyzed using Origin 8 Pro software. The initial rates were calculated based on the initial linear changes of the absorbance at 470 nm. The plots of initial rates versus guaiacol concentration were fitted with the Michaelis–Menten equation: Vi/[E]) = kcat [S]/(([S] + KM) [70]. The reported kcat and KM are the average of at least five independent experiments using different batches of protein.
Results
H2O2 is a reactive oxygen species which is involved in various physiological pathways in plants. Indeed, it has been reported that H2O2 has regulatory effects on growth, development, and quality of fruit (for review see [71]), but in stress conditions, an excess of H2O2 can induce irreversible damages to cells [48]. Previous studies showed that an overexpression of rHb1 is observed under stress conditions [22, 31], and as H2O2 promotes oligomerization of several members of the globin family, we have investigated, as a first step, the influence of H2O2 on recombinant rHb1 oligomerization. In a second time, we have characterized rHb1 peroxidase activity in vitro.
Oligomerization of WT rice Hb1 in presence of H 2 O 2
As a first approach to evaluate the reactivity of nsHb1 toward H2O2, we have analyzed the impact of H2O2 on WT rHb1 oligomerization form. The protein was incubated with increasing concentrations of this oxidant at pH 7. Different ratios of H2O2:protein were tested from 0.1:1 for the lowest one to highest one 40:1. The reaction products were analyzed by SDS-PAGE (Fig. 1).
SDS-PAGE analysis of H2O2-reacted WT rHb 1 (at final concentration 160 µM) at pH 7 (upper), pH 6 (middle), and pH 5 (below). Increasing H2O2 concentrations have been used 0 µM, 16 µM (1:10), 40 µM (1:4), 80 µM (1:2), 160 µM (1:1), 800 µM (5:1), 1600 µM (10:1), 3200:µM (20:1), and 6400 µM (40:1). Molecular weights for ladder (1) are listed in kDa. The letters M and D identify the position of the monomeric and the presumed dimeric form of the protein, respectively
Addition of H2O2 induces the appearance of a new band around 37 kDa which corresponds to the dimeric form of the protein. The amount of dimers has been determined using Gene Tools, an optical densitometry software from Syngene. The dimer amount increases as the H2O2:protein increases until a plateau observed at a ratio equal to 1:1. It is interesting to notice that only about 24% of protein dimerized in presence of H2O2. The small percentage of dimer observed during the reaction cannot be attributed to saturated intensity of bands. Indeed, the quantity calibration has shown that the intensity of the peaks is linear with protein amount up to 20 µg of protein. At higher concentration of H2O2, bands appear around 50–75 kDa. From their molecular masses, we can speculate that those bands correspond to a trimer and a tetramer. Indeed, it has been show that centrin with a single tyrosine can form multimers in presence of oxidizing radicals [72, 73].
To confirm that rHb1 catalyzes a peroxidase reaction, the cross-linking experiments have been repeated in presence and in absence of a reductant (sodium ascorbate). Sodium ascorbate has been shown to react both with ferryl and ferric myoglobin [74]. As we can see in Fig. S1, the addition of H2O2 to rHb1 leads to the formation of a dimer (around 37 kDa). If the rHb1 is pretreated with sodium ascorbate before addition of H2O2, no dimer appears, whereas if sodium ascorbate is added after treatment of H2O2, a dimer is formed. Therefore, the presence of sodium ascorbate before addition of H2O2 reduces efficiently the dimer formation.
Our result is in contrast with a previous study where no dimer formation was detected on SDS-PAGE [35]. The difference between these two experiments can be explained by the amount of protein deposited into the wells (7.7 µg vs 58 µg in the present study). Moreover, in that paper, the pH of the reaction was not mentioned. To check the influence of pH on dimerization, the WT rHb1 was incubated with different concentrations of H2O2 at pH 5, pH 6, or pH 7. The analysis of the electrophoresis gel clearly shows that the diminution of the pH leads to a sensible reduction of the dimer formation (Fig. 1). At pH 6, the amount of dimer decreases of about a factor 2 below the ratio 1:1 compared to pH 7. Beyond ratio 1:1, the percentage of dimer is similar for the two pH (24% for pH 7 versus 20% for pH 6). The diminution of the pH from 7 to 5 has a greater impact on the rHb1dimerization. Indeed, at ratio 1:1, the percentage of monomer which dimerizes is only 6% and rises to 10% when the ratio reaches the value 40:1. Low pH can induce the dissociation of heme from the protein core. To test if the decrease of dimer quantity observed at pH 5 is not induced by the acidic condition, the UV–visible spectrum of rHb1 has been recorded at pH 7, pH 6, and pH 5 (data not shown). For each pH, the ratio of the absorbance at 409 nm and 390 nm is used to assess the heme release from the protein. No significant difference for this value (around 1.9) is observed as a function of pH. Therefore, we can conclude that the dissociation of the heme is not responsible for the reduction of the dimer amount observed at pH 5.
Identification of the amino acids involved in protein oligomerization
It has been shown that cysteines or tyrosines could be involved in globin dimerization upon treatment with H2O2 [75]. The analysis of WT rHb1 sequence reveals the presence of one cysteine at position 82 on E helix and two tyrosines at position 112 and 151 on FG loop and H helix, respectively. To identify which residue is involved in the oligomerization, the protein solution was treated with 10 mM DTT before being loaded onto the gel (Fig. 1). This treatment did not prevent the dimer formation, indicating that the dimerization is not caused by a disulfide bridge between two monomers. To confirm that a tyrosine is probably responsible for the covalent cross-linkage, both tyrosines at position 112 and 151 have been mutated into alanine to investigate their role in the dimerization.
The WT protein and the three variants (Y112A, Y151A, and Y112AY151A) were incubated with H2O2 (protein/H2O2 ratio of 1:5) at pH 7.0 for 10 min. After addition of 10 mM DTT and denaturation at 95 °C for 5 min, the reaction products were analyzed on an SDS-PAGE gel (Fig. 2).
SDS-PAGE analysis of rHb 1 WT (160 µM) and its variants (160 µM) with or without H2O2 (800 µM) to obtain a ratio H2O2:protein of 5: 1. The experiments have been carried out at pH 7.0. (1) Ladder in kDa. (2) WT, (3) WT + H2O2, (4) Y112A, (5) Y112A + H2O2, (6) Y151A, (7) Y151A + H2O2, (8) Y112A/Y151A, and (9) Y112AY151A + H2O2. Letters M and D identify the position of the monomeric and the dimeric form, respectively
The addition of H2O2 to WT and Y151A rHb1 leads to the formation of a dimer and a tetramer. In contrast, no dimer or higher oligomer was detected for the variants Y112A and Y112AY151A. We can thus conclude that Tyr112 is probably the amino acid involved in the dimerization of the protein in the presence of H2O2. Indeed, this result is in good agreement with the three-dimensional structure of the protein [27]. The X-ray structure of rice Hb1 (PDB 1D8U) shows that both tyrosines are localized in the vicinity of heme iron (Fig. 3).
While Tyr112 is localized in the FG loop and its side chain points toward the solvent, Tyr151 is localized in the helix H and its side chain is oriented toward the heme pocket.
However, we cannot exclude that the mutation Y112A inhibits the peroxidase activity of the protein which leads to prevent the formation of dimer. To further investigate, the properties of rHb1variants have been analyzed.
Characterization of rHb1 variants: UV–visible spectra
The nature and the position of amino acids in the heme pocket are critical for the globins conformation. Therefore, modifying the amino acid side chains close to the heme can change the heme coordination and by the way its ligand affinity. To further investigate the impact of tyrosine residues located in the heme-binding pocket on the electronic properties of the prosthetic group, the UV–visible spectra of the different variants were recorded at pH 7.0 (Fig. 4).
UV–visible spectra of rHb WT (line), rHb Y112A (dash), rHb Y151A (dash dot), rHb Y112AY151A (short dot), and hhMb (short dash) at pH 7.0 in the met-form (A) and deoxy-form (B). Samples were treated with [Fe(CN)6]3− (A) and sodium dithionite (B) to fully oxidized or reduced heme iron, respectively. Inserts at upper area of the graphics focus on the Q bands between 480 and 700 nm
The spectrum of WT rHb is typical of a hexacoordinated low-spin heme with a Soret band situated at 408.5 nm and β and α bands located at 533 and 560 nm, respectively. The mutation of residue Tyr112 does not change significantly the absorption spectrum of the protein. In contrast, the replacement of Tyr151 by Ala induces a hypochromic shift of the Soret band, a decrease in the absorbance of the Q bands and the appearance of a new band around 630 nm. The absorption spectra of Tyr151Ala and Tyr112Ala Tyr151Ala variants are very similar to the spectrum of hhMb and are characteristic of a heme iron with proximal histidine and water axial ligands [76]. We can conclude that the mutation of residue 151 modifies the heme cavity and prevents heme iron coordination with the distal histidine at position 73. The absorption spectra of WT and Tyr112Ala variants recorded after addition of a small amount of sodium dithionite are consistent with the bis-histidine coordination of Fe2+ heme iron as evident by the characteristic β and α bands at 527 and 556 nm (Fig. 4B). The absence of noticeable β and α bands in Tyr151Ala mutants confirms the absence of His73 in the position of the distal ligand. These results suggest that the replacement of Tyr112 by Ala does not impact the electronic structure of the heme iron, whereas substitution of Tyr151 by Ala leads to a rearrangement of the heme pocket and makes the distal histidine interactions with the heme iron weaker.
Determination of extinction coefficient of rHb1 and its mutants.
Extinction coefficient (ε) is a critical parameter for quantifying the concentrations of globins in solution. In a previous publication [77], we showed that only one residue mutation in the vicinity of the heme could modify the molar extinction coefficient of the Soret band without affecting its λmax. The extinction coefficients of WT rHb1 and tyrosine variants were determined by pyridine hemi-hemochromogen method [65]. As a control, the extinction coefficient for WT hhMb was measured under the same conditions and the values are summarized in Table 1.
The value obtained for hhMb is in good agreement with the literature [78]. In the case of WT rHb1, the values of molar extinction coefficient are 143,000 ± 8,000 M−1.cm−1 and 200,000 ± 10,000 M−1.cm−1, respectively, for the met and deoxy-form. These values are slightly higher than those determined in a previous study [27].
We can notice that the Tyr 112Ala mutation induces only a slight decrease of the extinction coefficient value compared to the WT protein for met- and deoxy-form. In contrast, the replacement of Tyr by Ala in position 151 leads to an increase of the extinction coefficient for met-form and a decrease for the deoxy-form. This result underlines the impact of the mutation on the conformation of the heme pocket and the electronic structure of the prosthetic group. We have used the values of molar extinction coefficient determined here to quantify the peroxidase activity of rHb1 after addition of H2O2. Indeed, to determinate the kinetic parameters, the concentration of the enzyme should be known precisely.
Peroxidase activity of WT rHb1 and its variants
We investigated the influence of tyrosine in the vicinity of the heme on peroxidase activity using H2O2 as oxidant and guaiacol as substrate. The guaiacol is a typical substrate used for estimating peroxidase activity of heme proteins like sperm whale myoglobin (swMb) [79,80,81,82,83,84,85], horse heart myoglobin (hhMb) [86], Cgb [60], cytochrome C [87], cytochrome b5 [88], and horseradish peroxidase (HRP) [89, 90]. The oxidation of guaiacol leads to tetraguaiacol formation which can be followed spectrophotometrically at λ = 470 nm.
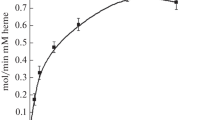
As a control, we have analyzed the peroxidase activity of hhMb which has been well studied in the past. Plots of the initial velocities divided by enzyme concentration (vi/[E]) as a function of guaiacol concentration [S] are presented in Fig. 5.
The kinetic data for WT rHb1 and its variants show a behavior characteristic of a substrate inhibition, as evidenced from the decrease of the initial velocity with increasing concentrations of the substrate, a common deviation from Michaelis–Menten kinetics [91]. In contrast, reaction with hhMb exhibits no inhibition until the substrate concentration reaches 80 mM. Inhibition substrate has already been reported for swMb mutants with non-classical distal ligand [92].
At lower substrate concentrations, the kinetic parameters kcat and KM follow the classical Michaelis–Menten equation Vi/[E]) = kcat [S]/(([S] + KM), so kcat and KM data can be obtained by fitting the plot of Vi/[E] as a function of [S], as shown in Fig. 5.B.
The fitting parameters for hhMb, rHb1 and its variants are summarized in Table 2.
The catalytic efficiency kcat/KM obtained for WT hhMB is very similar to the one determined in a previous study (kcat/KM = 11 s−1.M−1) [86], but the absolute kcat and KM values are tenfold smaller (kcat = 2.8 vs 0.36 s−1 and KM = 230 vs 32 mM). The observed difference can be explained by the concentration of H2O2 used in both studies. In our experiment, the concentration was tenfold higher (100 mM vs 9.7 mM). To determine the influence of H2O2 concentration on kinetic constants, we have performed peroxidase reaction with H2O2 concentration at 10 mM. The plot Vi/[E] as a function [S] is shown in Fig. S2 and the fitting of the curve by Michaelis–Menten equation gives the following kinetic parameters 0.45 ± 0.1 s−1 and 64 ± 20 mM, respectively, for kcat and KM. The values are closer that those obtained previously [86]. Therefore, we can conclude that the differences observed were due to the H2O2 concentration.
Using the same H2O2 concentration fixed at 100 mM, our results show that the peroxidase activity of WT rHb1 determined at pH 7 exhibits a kcat/KM 200 higher than WT hhMb. Several experiments have been performed in the past to determine the peroxidase activity parameters of globins using guaiacol as substrate with a concentration of H2O2 fixed at 100 mM at pH 7.0. The sperm whale Mb (swMb) exhibited kcat = 0.4 s−1, Km = 3.53 mM and kcat/KM = 110 M−1 s−1 [80]. It is interesting to notice that another study on swMb with a concentration of H2O2 fixed at 4.8 mM leads to comparable kcat/KM = 115 s−1.M−1 but with values of kcat and KM which are 12- fold lower [81, 83]. Therefore, the peroxidase activity of rHb1 reported here is higher than the one measured for swMb and hhMb under the same experimental conditions (pH 7, [H2O2] fixed at 100 mM). From our study and the literature, we can conclude that the value kcat/KM can be ranged in this order rHb1 >> swMb >> hhMb.
The impact on peroxidase activity of the tyrosine mutations close to the heme environment has been analyzed. Whereas the mutation Tyr151Ala does not prevent the dimer formation in presence of H2O2, this variant has catalytic efficiency (kcat/KM) similar to WT protein. But, the value kcat is increased by a factor 3.4 and the substrate affinity is lowered (KM = 2.1 mM for WT and 3.7 mM for Tyr151A). Similar behavior is observed for the double mutant. The introduction of mutation Tyr151Ala on variant Tyr112Ala leads to an enhancement of the kcat value by a factor of 3.8 and an increase of KM value by a factor of 1.6 compared to the single mutant Tyr112Ala. Hence, the mutation Tyr151Ala reduces the affinity of the protein for the guaiacol substrate. This observation is important, because the UV–visible spectrum has shown that the mutation Tyr151Ala prevents heme iron from being coordinated by distal histidine. Therefore, in the case of rHb1, the change of distal ligand from endogenous histidine to probably a water molecule improves the turnover number of the peroxidase reaction.
In contrast, the mutation Tyr112Ala leads to a reduction of KM (1.5 mM vs 2.1 mM, respectively, for rHb1Tyr110A and WT protein). Therefore, this mutant has a better affinity for the substrate, but the turnover number kcat is reduced by a factor of 3 (1.3 s–1 vs 4 s–1, respectively, for rHb1Tyr110 and WT protein). Therefore, the amino-acid substitution at position 112 leads to a reduction in catalytic efficiency kcat/KM (900 vs 1900s−1.M−1).
Substrate inhibition
The inhibition kinetic parameters have been calculated using the graphical method applied to substrate inhibition [93]. The inhibition constants were determined from the plot (Vi /(Vmax – Vi)) as a function of 1/[S] using Vi values measured at higher concentrations of guaiacol (Fig. S3) and the slope of the linear fit corresponds to KSi.
For WT rHb1, the KSi value has been calculated to 18.6 mM and a similar KSi value was observed for the Y112A mutant (Table 2). The substitution of Tyr151 by Ala leads to the increase in the substrate inhibition constant, KSi = 110 ± 60 mM, and increased Ksi value with respect to the WT was also determined for the double mutant KSi = 55 ± 8 mM. These data suggest that the substitution of Tyr 151 by Ala decreases substrate affinity for the inhibitory site. This result is in good agreement with the changes in the active site of the protein induced by Tyr151Ala mutation.
Discussion and perspectives
In this article, peroxidase activities of rHb1 were measured following guaiacol oxidation. We show that rHb1 activity is one of the highest one among the globins tested up to now. This fact is very interesting, because some authors have postulated that the hexacoordination of the heme could be responsible for the absence of globin reactivity toward H2O2 [63]. Indeed, WT Ngb exhibits no peroxidase activity, and by contrast, Ngb His64Val variant produces a peroxidase-like ferryl FeIV = O intermediate [63]. On the other hand, a recent study has shown that the mutation of distal histidine at position 81 in human hexacoordinated Cgb into a valine or a tyrosine leads to a significant decrease of the peroxidase activity [60], suggesting that the coordination state of the heme iron is not the only element which controls the reactivity with H2O2. Therefore, it is interesting to determine parameters that influence peroxidase activity for globins. Some authors have suggested that the distance between heme iron and the nitrogen ring (Nε) of distal histidine in myoglobin could modify the peroxidase activity of globins [85, 94]. Unfortunately, no crystallographic structure of rHb1 with exogenous ligand exists and we cannot verify this hypothesis. Nevertheless, for hexacoordinated proteins, we have calculated this distance from the three-dimensional structures of rHb1 (PDB 1D8U), Ngb (PBD 4MPM), and Cgb (PBD 2DC3). No significant difference was found between these proteins, but we can notice that in the double mutant Cgb C38S C83S (PBD IUMO) which prevents the formation of the intramolecular disulfide bridge, the distance between the heme iron and the Nε of the distal histidine is considerably increased. This double mutation induces a significant decrease of peroxidase activity compared to native protein [95]. Other experiments should be performed to determine which elements control peroxidase activity of hexacoordinated proteins with two histidines.
Using variants, we demonstrate that in the presence of H2O2, rHb1 dimerizes through a dityrosine bond between the Tyr112 of a protein and the Tyr 112 of another one. This finding is in good agreement with the three-dimension structure of rHb1, since the side chain of Tyr112, localized in the FG loop points toward the solvent. In interesting ways, the yield of oligomerization is pH-dependent. Indeed, we have demonstrated that the dimerization is facilitated at pH 7 and decreases as the pH drops. The dimerization through dityrosine cross-links in presence of H2O2 has been well documented for numerous proteins [96] and for globins: swMb [59], human cytoglobin (hCgb) [60], and Mycobacterium tuberculosis truncated hemoglobin O (trHbO) [61]. The reaction between WT human Ngb and H2O2 does not lead to a dimer but Ngb H64V, a variant of metNgb lacking the distal coordination bond dimerizes through Tyr88–Tyr88 link [63]. The reaction of H2O2 with sperm whale Mb (swMb) leads to a dimer formation through a covalent bond between Tyr103 located on G helix of one protein and Tyr151 (at the C-terminal part) of another one [59, 62, 64], while horse heart Mb (hhMb) and red kangaroo Mb which have a phenylalanine at position 151 do not exhibit dimers in the presence of H2O2 [59, 97]. Tyr55 on E helix and Tyr115 on H helix are involved in the dimerization of trHbO in presence of H2O2 [61]. Human Cgb also dimerizes via dityrosine cross-linking in the presence of H2O2, but, up to now, the tyrosine(s) involved in the dimerization has (have) not been identified [60]. Interestingly, our study highlights for the first time a tyrosine located in the FG loop as the residue involved in the dimerization of a globin. Moreover, even if tyrosine 112 is involved in the dimerization in presence of H2O2, the replacement of that residue with an alanine does not change the electronic environment of the heme. No significant modification of the UV–visible spectrum was indeed observed for the Tyr112A variant. Moreover, the extinction coefficients of the Soret band are quite the same for the WT rHb1 and rHb Tyr112A. The distance between the hydroxyl of tyrosine 112 and the iron heme is 9.70 Å and its side chain points toward the solvent. These two elements could explain why the mutation Tyr112A has a low impact on electronic environment of the iron. However, this mutation leads to a significant decrease of peroxidase activity compared to the WT protein. Indeed, we have shown using guaiacol as substrate that at pH 7, the mutation Tyr112Ala induces a decrease in overall catalytic efficiency by a factor of 2.4 compared to WT protein. Tyr112 is a residue which is not conserved among the rice Hbs family. Tyrosine112 is also present in rHb2 but is not conserved in other rHbs (Asn for rHb3 and rHb4, Ala for rHb5) (Fig. 6).
Therefore, we can conclude that tyrosine 112 is one of the key residues which controls peroxidase activity efficiency and which is responsible for dimerization of rHb in the presence of H2O2. Tyr 112 confers unique properties to rHb1 and probably to rHb2.
Our study shows that rHb1 react with H2O2 in vitro, however, in vivo, this heme protein does not probably play the role of peroxidases. Indeed, the peroxidases are widely distributed in plant kingdom [98]. In rice, these class III peroxidases exist as multigene family [99] and compared to this class of proteins, rHb1 exhibits a low peroxidase activity using guaiacol as substrate, as we have shown in this study. Therefore, probably, rHb1 does not act as a peroxidase in vivo. However, it is possible that under stress conditions, the reaction of rHb1 with H2O2 induces the formation of a tyrosine radical on the protein, and we can speculate on the future of reactive intermediate in vivo. Either, one molecule of rHb1 reacts with another one to form a homodimer, but the concentration of this hemoprotein is low (~ 50–100 nM) [36] and the likelihood of a homodimer being formed is low. However, we cannot exclude that a heterodimer is produced. This cross-linking could modulate the structural stability and/or the function of the globin. Indeed, it has been shown that the oligomeric cytochrome c can lead to tertiary structural changes with enhanced peroxidase activity [87]. To test this hypothesis for rHb1, it would be interesting to analyze the peroxidase activity of rice hemoglobin dimer. Moreover, as rice Hb1 is apparently in the cytoplasm [100] and has no signal peptide [22], an interaction with a partner could lead to a relocalization of this protein in the cells. This study raises another interrogation concerning the type of residue involved in the dimerization. In fact, either a cysteine or a tyrosine could react to form a disulfide bridge and a dityrosine link, respectively. The disulfide-bond formation is reversible under reduced conditions, whereas the dityrosine cross-link is more stable. Therefore, the nature of the linkage could be also important to cell function.
In this study, we were also interested in amino-acid Tyr 151 which is the second Tyr residue in rHb1 sequence. As Tyr112, Tyr 151 is located in the vicinity of heme iron, but this amino acid is buried inside the heme pocket. From the X-ray structure of the WT rHb1, the distance between the hydroxyl of the tyrosine and the heme iron is only 8 Å. The comparison of amino-acid sequence of non-symbiotic rice Hb shows that this residue is well conserved (Fig. 6) [22]. We have demonstrated some interesting features concerning Tyr151. First of all, even if this residue is not involved in the dimerization of rHb1 in presence of H2O2, Tyr151 on H helix is found to be important for heme environment. Indeed, we have shown that the Soret band extinction coefficient of the variant Tyr151Ala is clearly higher than the one for WT rHb1 and this mutation leads to a significant change in UV–visible absorption spectra compared to WT one. The optical spectrum of Tyr151Ala rHb1 is very similar to those of Mbs. Indeed, it exhibits bands characteristic of a heme coordinated with the Nε of the proximal histidine and with one molecule of water as sixth ligand. Therefore, tyrosine 151 is essential to position the distal histidine close enough to the heme iron to promote a hexacoordinated state. The heme properties are impacted by the mutation Tyr151Ala and the modification of electronic environment of the heme is correlated with an increase in the catalytic rate constant kcat and in the substrate inhibition constant, KSi. Indeed, the catalytic rate constant of Tyr151Ala rHb1 is clearly enhanced by 3.4-fold relative to native rHb1 and the catalytic efficiency (kcat/KM) is increased by a factor of 1.5. This result was not surprising, in fact, it has been reported that the loss of the distal ligand activates peroxidase activity [63, 101]. The presence of a tyrosine in the vicinity of the heme has been shown to be important for peroxidase activity. Indeed, the mutation Phe43 (C7)Tyr in swMb increases the kcat/KM by 28-fold using guaiacol as substrate [82], and the mutation Tyr103Phe localized in G helix in human Mb leads to the diminution of the kapp value, the apparent rate of ion ferryl formation by a factor of 1.4 [58].
Our findings raise also the question about the influence of the heme coordination on the peroxidase activity. Indeed, surprisingly, whereas two hexacoordinated globins: Cgb and rHb1 react with hydrogen peroxide [60, 102], Ngb, another hexacoordinated globin, exhibits no peroxidase activity, so other parameters come into play to modulate the reactivity of globins.
Data availability
The research data associated with this article are available from the corresponding author [VD] on request.
Abbreviations
- Ala (A):
-
Alanine
- Cgb:
-
Cytoglobin
- DTT:
-
Dithiothreitol
- ε:
-
Extinction coefficient
- EDTA:
-
Ethylenediaminetetraacetic acid disodium salt
- His (H):
-
Histidine
- hMb:
-
Human myoglobin
- hhMb:
-
Horse heart myoglobin
- Ngb:
-
Neuroglobine
- nsHb:
-
Non-symbiotic hemoglobin
- Phe (P):
-
Phenylalanine
- rHb:
-
Rice hemoglobin
- ROS:
-
Reactive oxygen species
- SDS:
-
Sodium dodecyl sulfate
- swMb:
-
Sperm whale myoglobin
- Tyr (Y):
-
Tyrosine
- WT:
-
Wild Type
References
Vinogradov SN et al (2005) Three globin lineages belonging to two structural classes in genomes from the three kingdoms of life. Proc Natl Acad Sci U S A 102(32):11385–11389
Vinogradov SN et al (2006) A phylogenomic profile of globins. BMC Evol Biol 6:31–31
Burmester T et al (2000) A vertebrate globin expressed in the brain. Nature 407:520–523
Schmidt M et al (2003) How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem 278(3):1932–1935
D’Aprile A et al (2014) Hematopoietic stem/progenitor cells express myoglobin and neuroglobin: adaptation to hypoxia or prevention from oxidative stress? Stem Cells 32(5):1267–1277
Reuss S et al (2016) Neuroglobin expression in the mammalian auditory system. Mol Neurobiol 53(3):1461–1477
Zhang J et al (2013) Neuroglobin, a novel intracellular hexa-coordinated globin, functions as a tumor suppressor in hepatocellular carcinoma via Raf/MAPK/Erk. Mol Pharmacol 83(5):1109–1119
Fiocchetti M et al (2020) Extracellular neuroglobin as a stress-induced factor activating pre-adaptation mechanisms against oxidative stress and chemotherapy-induced cell death in breast cancer. Cancers (Basel) 12(9):2451
Solar Fernandez V, Marino M, Fiocchetti M (2021) Neuroglobin in retinal neurodegeneration: a potential target in therapeutic approaches. Cells 10(11):3200
Kawada N et al (2001) Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J Biol Chem 276(27):25318–25323
Tiso M et al (2011) Human neuroglobin functions as a redox-regulated nitrite reductase. J Biol Chem 286(20):18277–18289
Van Doorslaer S et al (2003) Nitric oxide binding properties of neuroglobin: a characterization by EPR and flash photolysis. J Biol Chem 278(7):4919–4925
Dewilde S et al (2001) Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem 276(42):38949–38955
Dordas C (2009) Nonsymbiotic hemoglobins and stress tolerance in plants. Plant Sci 176(4):433–440
Hoy JA, Hargrove MS (2008) The structure and function of plant hemoglobins. Plant Physiol Biochem 46(3):371–379
Hill R, Hargrove M, Arredondo-Peter R (2016) Phytoglobin: a novel nomenclature for plant globins accepted by the globin community at the 2014 XVIII conference on oxygen-binding and sensing proteins. F1000Res 5:212
Garrocho-Villegas V, Gopalasubramaniam SK, Arredondo-Peter R (2007) Plant hemoglobins: what we know six decades after their discovery. Gene 398(1–2):78–85
Appleby CA (1992) The origin and functions of haemoglobin in plants. Sci Progress 76(3/4):301–302 (pp. 365–398)
Ott T et al (2005) Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr Biol 15(6):531–535
Aréchaga-Ocampo E et al (2001) Cloning and expression analysis of hemoglobin genes from maize (Zea mays ssp. mays) and teosinte (Zea mays ssp. parviglumis). Biochim Biophys Acta (BBA) Gene Struct Expr 1522(1):1–8
Bai X et al (2016) Overexpression of spinach non-symbiotic hemoglobin in Arabidopsis resulted in decreased NO content and lowered nitrate and other abiotic stresses tolerance. Sci Rep 6(1):26400
Arredondo-Peter R et al (1997) Rice hemoglobins. Gene cloning, analysis, and O2-binding kinetics of a recombinant protein synthesized in Escherichia coli. Plant Physiol 115(3):1259–1266
Calvo-Begueria L et al (2017) Characterization of the heme pocket structure and ligand binding kinetics of non-symbiotic hemoglobins from the model legume Lotus japonicus. Front Plant Sci 8:407
Trevaskis B et al (1997) Two hemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghemoglobins. Proc Natl Acad Sci USA 94(22):12230–12234
Duff SM, Wittenberg JB, Hill RD (1997) Expression, purification, and properties of recombinant barley (Hordeum sp.) haemoglobin. Optical spectra and reactions with gaseous ligands. J Biol Chem 272(27):16746–16752
Smagghe BJ et al (2009) Review: correlations between oxygen affinity and sequence classifications of plant hemoglobins. Biopolymers 91(12):1083–1096
Goodman M, Hargrove M (2001) Quaternary structure of rice nonsymbiotic hemoglobin. J Biol Chem 276:6834–6839
Hebelstrup KH et al (2006) Hemoglobin is essential for normal growth of Arabidopsis organs. Physiol Plant (København 1988) 127(1):157–166
Garrocho-Villegas V et al (2008) Expression and in silico structural analysis of a rice (Oryza sativa) hemoglobin 5. Plant Physiol Biochem 46(10):855–859
Lira-Ruan V, Ruiz-Kubli M, Arredondo-Peter R (2011) Expression of non-symbiotic hemoglobin 1 and 2 genes in rice (Oryza sativa) embryonic organs. Commun Integr Biol 4(4):457–458
Lira-Ruan V et al (2001) Synthesis of hemoglobins in rice (Oryza sativa var. Jackson) plants growing in normal and stress conditions. Plant Sci 161(2):279–287
Ross EJ et al (2004) Activation of the Oryza sativa non-symbiotic haemoglobin-2 promoter by the cytokinin-regulated transcription factor, ARR1. J Exp Bot 55(403):1721–1731
Wang X, Hargrove MS (2013) Nitric oxide in plants: the roles of ascorbate and hemoglobin. PLoS ONE 8(12):e82611
Butcher D et al (2017) Role of ionic strength and pH in modulating thermodynamic profiles associated with CO escape from rice nonsymbiotic hemoglobin 1. J Phys Chem B 121(2):351–364
Violante-Mota F et al (2010) Analysis of peroxidase activity of rice (Oryza sativa) recombinant hemoglobin 1: implications for in vivo function of hexacoordinate non-symbiotic hemoglobins in plants. Phytochemistry 71(1):21–26
Arredondo-Peter R, Moran J, Sarath G (2014) Rice (Oryza) hemoglobins. F1000Research 3:253
Taylor ER et al (1994) A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol Biol 24(6):853–862
Zhao L et al (2008) A nonsymbiotic hemoglobin gene from maize, ZmHb, is involved in response to submergence, high-salt and osmotic stresses. Plant Cell, Tissue Organ Cult 95(2):227–237
Almada R et al (2013) Class 1 non-symbiotic and class 3 truncated hemoglobin-like genes are differentially expressed in stone fruit rootstocks (Prunus L.) with different degrees of tolerance to root hypoxia. Tree Genet Genom 9:1051–1063
Wang R et al (2000) Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell 12(8):1491–1509
Sakamoto A et al (2004) Three distinct Arabidopsis hemoglobins exhibit peroxidase-like activity and differentially mediate nitrite-dependent protein nitration. FEBS Lett 572(1–3):27–32
Ohwaki Y et al (2005) Induction of class-1 non-symbiotic hemoglobin genes by nitrate, nitrite and nitric oxide in cultured rice cells. Plant Cell Physiol 46(2):324–331
Sasakura F et al (2006) A class 1 hemoglobin gene from Alnus firma functions in symbiotic and nonsymbiotic tissues to detoxify nitric oxide. Mol Plant Microbe Interact 19(4):441–450
Hung S-H, Yu C-W, Lin C (2005) Hydrogen peroxide functions as a stress signal in plants. Bot Bull Acad Sin 46:1–10
Slesak I et al (2007) The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim Pol 54(1):39–50
Petrov VD, Van Breusegem F (2012) Hydrogen peroxide-a central hub for information flow in plant cells. AoB PLANTS 2012:pls014
Saxena I, Srikanth S, Chen Z (2016) Cross talk between H2O2 and interacting signal molecules under plant stress response. Front Plant Scince 7:00570
Gechev TS, Hille J (2005) Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol 168(1):17–20
Yang L-X et al (2005) AtGLB1 enhances the tolerance of arabidopsis to hydrogen peroxide stress. Plant Cell Physiol 46(8):1309–1316
Widmer CC et al (2009) Hemoglobin can attenuate hydrogen peroxide-induced oxidative stress by acting as an antioxidative peroxidase. Antioxid Redox Signal 12:185–198
Keilin D, Hartree EF (1950) Reaction of methaemoglobin with hydrogen peroxide. Nature 166(4221):513–514
George P, Irvine DH (1951) Reaction of metmyoglobin with hydrogen peroxide. Nature 168(4265):164–165
George P, Irvine DH (1952) The reaction between metmyoglobin and hydrogen peroxide. Biochem J 52(3):511–517
Harel S, Kanner J (1988) The generation of ferryl or hydroxyl radicals during interaction of haemproteins with hydrogen peroxide. Free Radic Res Commun 5(1):21–33
Lv Y et al (2008) Cytoglobin: a novel potential gene medicine for fibrosis and cancer therapy. Curr Gene Ther 8(4):287–294
Trandafir F et al (2007) Neuroglobin and cytoglobin as potential enzyme or substrate. Gene 398(1–2):103–113
Witting PK, Douglas DJ, Mauk AG (2000) Reaction of human myoglobin and H2O2. Involvement of a thiyl radical produced at cysteine 110. J Biol Chem 275(27):20391–20398
Witting PK, Mauk AG, Lay PA (2002) Role of tyrosine-103 in myoglobin peroxidase activity: kinetic and steady-state studies on the reaction of wild-type and variant recombinant human myoglobins with H(2)O(2). Biochemistry 41(38):11495–11503
Tew D, Ortiz-de-Montellano PR (1988) The myoglobin protein radical. Coupling of Tyr-103 to Tyr-151 in the H2O2-mediated cross-linking of sperm whale myoglobin. J Biol Chem 263(33):17880–17886
Beckerson P, Svistunenko D, Reeder B (2015) Effect of the distal histidine on the peroxidatic activity of monomeric cytoglobin. F1000Res 4:87
Ouellet H et al (2007) Reaction of Mycobacterium tuberculosis truncated hemoglobin O with hydrogen peroxide: evidence for peroxidatic activity and formation of protein-based radicals. J Biol Chem 282(10):7491–7503
Lardinois OM, Ortiz de Montellano PR (2003) Intra- and intermolecular transfers of protein radicals in the reactions of sperm whale myoglobin with hydrogen peroxide. J Biol Chem 278(38):36214–36226
Lardinois OM et al (2008) Identification of protein radicals formed in the human neuroglobin-H2O2 reaction using immuno-spin trapping and mass spectrometry. Biochemistry 47(39):10440–10448
Wilks A, Ortiz de Montellano PR (1992) Intramolecular translocation of the protein radical formed in the reaction of recombinant sperm whale myoglobin with H2O2. J Biol Chem 267(13):8827–8833
Paul K et al (1953) The molar light absorption of pyridine ferroprotoporphrin (Pyridine Haemochromogen). Acta Chem Scand 7:1284–1287
Berry EA, Trumpower BL (1987) Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem 161(1):1–15
De Duve C (1948) A spectrophotometric method for the simultaneous determination of myoglobin and hemoglobin in extracts of human muscle. Acta Chem Scand 2(3):264–289
Noble RW, Gibson QH (1970) The reaction of ferrous horseradish peroxidase with hydrogen peroxide. J Biol Chem 245(9):2409–2413
Maehly AC, Chance B (1954) The assay of catalases and peroxidases. Methods Biochem Anal 1:357–424
Leonor-Michaelis MLM, Johnson KA, Goody S (2011) The original michaelis constant: translation of the 1913 Michaelis–Menten paper. Biochemistry 50(39):8264–8269
Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221(3):1197–1214
Brun E et al (2010) Oxidative stress induces mainly human centrin 2 polymerisation. Int J Radiat Biol 86(8):657–668
Gatin A et al (2021) Oxidative radicals (HO(•) or N(3)(•)) induce several di-tyrosine bridge isomers at the protein scale. Free Radic Biol Med 162:461–470
Giulivi C, Cadenas E (1993) The reaction of ascorbic acid with different heme iron redox states of myoglobin Antioxidant and prooxidant aspects. FEBS Lett 332(3):287–290
Gross AJ, Sizer IW (1959) The oxidation of tyramine, tyrosine, and related compounds by peroxidase. J Biol Chem 234(6):1611–1614
Spiro TG, Stong JD, Stein P (1979) Porphyrin core expansion and doming in heme proteins. New evidence from resonance Raman spectra of six-coordinate high-spin iron(III) hemes. J Am Chem Soc 101(10):2648–2655
André E et al (2019) Impact of A90P, F106L and H64V mutations on neuroglobin stability and ligand binding kinetics. J Biol Inorg Chem 24(1):39–52
Antonini EBM (1971) Hemoglobin and myoglobin in their reactions with ligands. Front Biol 21:27–31
Wu L-B et al (2016) Peroxidase activity enhancement of myoglobin by two cooperative distal histidines and a channel to the heme pocket. J Mol Catal B Enzym 134:367–371
Wu L-B et al (2016) An intramolecular disulfide bond designed in myoglobin fine-tunes both protein structure and peroxidase activity. Arch Biochem Biophys 600:47–55
Guo WW et al (2012) Unusual peroxidase activity of a myoglobin mutant with two distal histidines. Chin Chem Lett 23(6):741–744
Liu C et al (2019) Unique Tyr-heme double cross-links in F43Y/T67R myoglobin an artificial enzyme with a peroxidase activity comparable to that of native peroxidases. Chem Commun (Camb) 55(46):6610–6613
Lin Y-W et al (2013) Peroxidase activity of a myoglobin mutant with three distal histidines forming a metal-binding site: implications for the cross-reactivity of cytochrome c oxidase. J Mol Catal B Enzym 91:25–31
Ozaki S et al (2001) Molecular engineering of myoglobin: the improvement of oxidation activity by replacing Phe-43 with tryptophan. Biochemistry 40(4):1044–1052
Matsui T, Liong E, Phillips GN, Watanabe Y (1999) Effects of the location of distal histidine in the reaction of myoglobin with hydrogen peroxide. J Biol Chem 274(5):2838–2844
Hayashi T et al (1999) Peroxidase activity of myoglobin is enhanced by chemical mutation of heme-propionates. J Am Chem Soc 121(34):7747–7750
Wang Z et al (2011) Peroxidase activity enhancement of horse cytochrome c by dimerization. Org Biomol Chem 9(13):4766–4769
Hu S et al (2017) Peroxidase activity of a c-type cytochrome b(5) in the non-native state is comparable to that of native peroxidases. ChemistryOpen 6(3):325–330
Savenkova MI, Newmyer SL, Montellano PR (1996) Rescue of His-42 –> Ala horseradish peroxidase by a Phe-41 –> His mutation. Engineering of a surrogate catalytic histidine. J Biol Chem 271(40):24598–24603
Harris RZ et al (1993) Catalytic properties of horseradish peroxidase reconstituted with the 8-(hydroxymethyl)- and 8-formylheme derivatives. Biochemistry 32(14):3658–3663
LiCata VJ, Allewell NM (1997) Is substrate inhibition a consequence of allostery in aspartate transcarbamylase? Biophys Chem 64(1–3):225–234
Pott M et al (2018) A Noncanonical proximal heme ligand affords an efficient peroxidase in a globin fold. J Am Chem Soc 140(4):1535–1543
Yoshino M, Murakami K (2015) Analysis of the substrate inhibition of complete and partial types. Springerplus 4:292
Wang C et al (2014) Structures of K42N and K42Y sperm whale myoglobins point to an inhibitory role of distal water in peroxidase activity. Acta Crystallogr Sect D 70(11):2833–2839
Jesús-Tejero AAK, Baumgartner MP, Sparacino-Watkins CE, Anthonymutu TS, Vlasova II, Camacho CJ, Gladwin MT, Bayir H, Kagan VE (2016) Peroxidase activation of cytoglobin by anionic phospholipids: mechanisms and consequences. Biochim Biophys Acta 1861(5):391–401
Malencik DA, Anderson SR (2003) Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids 25(3):233–247
Air GM et al (1971) Amino-acid sequences of kangaroo myoglobin and haemoglobin and the date of Marsupial-Eutherian divergence. Nature 229(5284):391–394
Pandey V et al (2017) A comprehensive review on function and application of plant peroxidases. Biochem Anal Biochem 6:100308
Zámocký M et al (2015) Independent evolution of four heme peroxidase superfamilies. Arch Biochem Biophys 574:108–119
Ross EJ et al (2001) Nonsymbiotic hemoglobins in rice are synthesized during germination and in differentiating cell types. Protoplasma 218(3–4):125–133
Tejero J et al (2015) Exploring the mechanisms of the reductase activity of neuroglobin by site-directed mutagenesis of the heme distal pocket. Biochemistry 54(3):722–733
Ferreira JC et al (2015) Intermediate tyrosyl radical and amyloid structure in peroxide-activated cytoglobin. PLoS ONE 10(8):e0136554
Acknowledgements
Eric André was supported by a PhD fellowship from MESRI. The authors would like to thank Dr Emilie Brun for the proofreading of this manuscript. Jean-Philippe Labre is also acknowledged for technical support.
Author information
Authors and Affiliations
Contributions
VD: investigation, validation, and writing—original draft. EA: methodology. SB: conceptualization, writing—review & editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Derrien, V., André, E. & Bernad, S. Peroxidase activity of rice (Oryza sativa) hemoglobin: distinct role of tyrosines 112 and 151. J Biol Inorg Chem 28, 613–626 (2023). https://doi.org/10.1007/s00775-023-02014-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-023-02014-0