Abstract
Se is an environmental concern as it can be toxic if present in high concentrations even though it is a dietary requirement for all animals. Se levels are a special concern in the Fountain Creek Watershed located in southeastern Colorado whose geological source is the Se-rich Pierre Shale. Segments of Fountain Creek have Se water levels that exceed the current EPA limit of 5 µg/l. In the studies described here, the effects of river water containing selenium were examined on fish populations at different sites along the Fountain Creek Watershed. Based on the hypothesis that high levels of Se present in the Creek and resident bryophytes should be an indicator of diversity in the river fish we explored the possibility that the low toxicity of the selenium could be due to speciation. A speciation analysis was conducted to determine the selenium(IV) and selenium(VI). Our results show that sites with higher ratios of the more toxic Se(IV) relative to total selenium exhibit lower fish diversity and number of fish. Our results indicate that factors, other than total Se, such as Se speciation may be involved in controlling the bioavailability and toxicity of this element to aquatic organisms in Fountain Creek.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an element that has both beneficial and toxic effects [1,2,3,4,5,6] and as a result its environmental levels are regulated and strictly enforced by the EPA in the USA [7,8,9,10]. Selenium toxicity in fish is primarily acquired through the consumed diet [11] and is not the result of passive absorption from water. However, Se is known to be transferred from adults to the eggs [11,12,13,14] and thus can act as a reproductive toxicant [11]. This toxicity on fish reproduction has caused the EPA to develop a chronic exposure criterion for aquatic life [15]. The elevated levels of Se in the Lower Fountain Creek Watershed (LFCW) in southeastern Colorado have a significant environmental impact as the Creek empties into the Arkansas River, on the east side of the city of Pueblo, Colorado, and is a major source of the Se in the Arkansas River [9]. The geological sources of the Se are the shale deposits that underlie the waterways in various areas in Colorado [9]. We have carried out studies, characterizing and monitoring sites along the Lower Fountain Creek for Se content arriving from the Pierre Shale in the LFCW [16] during 2007–2009. The reported Se levels in Fountain Creek for total and dissolved Se ranged from 1.3 to 64.4 µg/l, with mean values of 5.4 µg/l [9]. The mean value exceeds the EPA’s past (5.0 µg/l) and new recommended values of 1.2 µg/l for Se in water [7, 9] and also exceeds the State of Colorado’s recommended water level of 4.6 µg/l [8].
Se bioaccumulation has been reported in birds in the Kesterson National Wildlife Refuge (KWF) in California underlining the importance of reports of elevated levels of Se in fish consumed by resident birds [17]. The EPA’s current fish tissue exposure limits are given as 15.8 mg Se/kg for egg/ovary, 8.0 mg Se/kg whole body and 1.2 µg Se/L for water [15]. The Arkansas River in southeastern part of the State of Colorado has been identified as an area of concern to the Central Flyway [17]. For example, the eastern Colorado Lower Arkansas River portion of the Central Flyway contains approximately 400 species of birds [18, 19]. The Se levels vary dramatically, and accordingly, reports have been prepared to describe some of the inhospitable environments in these rivers. Indeed, these reports detail the teratogenic deformities or birth defects in fish as a result of Se in the eggs or chronic exposure to high Se levels [12,13,14, 17, 20,21,22]. Observed deformities in these studies and others typically include lordosis, scoliosis, kyphosis, missing or deformed fins, missing or deformed gills or opercula, abnormally shaped head, missing or deformed eyes, and deformed mouths [12,13,14, 20,21,22]. Acute toxic Se exposure in fish results in edema, exophthalmos, and cataracts which was observed in Red Shiners (Notropis lutrensis) [20]. However, other fish species are found to tolerate higher levels of Se and show no adverse effects with a key example being the Cutthroat Trout (Oncorhynchus clarki lewisi) [21]. The reported literature speaks to different chemical forms being toxic with species-dependent effects [1, 12,13,14, 21, 22]. Furthermore, high levels of Se accumulation have been reported in apparently healthy fish species [21, 23,24,25,26].
Se is directly below sulfur in the periodic table and as a result many of its properties are similar to sulfur’s [27, 28]. The oxidized forms of Se, selenite (\({\text{SeO}}_{ 3}^{ 2- }\)) and selenate (\({\text{SeO}}_{ 4}^{ 2- }\)), are the most common forms of Se in environmental settings although the reduced form H2Se and its salts exist in the earth’s crust and is co-located with sulfur [4]. Se levels in aqueous environments are generally between 1 and 5000 nmol/L. Se is most soluble in aqueous solution under oxidizing conditions and such conditions can enhance its solubilization from rock [29]. A number of methods have been reported describing the analytical methods available to carry out speciation studies [1, 2, 6, 30, 31]. Pourbaix diagrams suggest that selenous acid (H2SeO3) is favored at the conditions that are normally found in oxidizing natural stream waters (pE ≈ 13.5) at a pH 3.0 [28] Hydrogen selenite (\({\text{HSeO}}_{3}^{ - }\)) is favored in the same oxidizing conditions up to about pH 5.0. Above pH 5.0, selenate (\({\text{SeO}}_{ 4}^{ 2- }\)) is the most favored Se species. Using the information in Pourbaix diagrams, it would follow that in less aerated waters (pE ≈ 13.5) at pH values between 3 and 8, hydrogen selenite, \({\text{HSeO}}_{3}^{ - }\) is the favored species [32]. Because the redox potential determines whether \({\text{HSeO}}_{3}^{ - }\) or \({\text{SeO}}_{ 4}^{ 2- }\) forms, which species predominates between pH 3–8 depends on the pE. The pE measurement reported often assumes the water is aerated because the water is in contact with air. However, in waters that are not surface waters the amount of aeration is less, therefore, the assignment of Se in this pH range should be in the form of Se(IV). In naturally aerated waters, the conversion of Se(IV) (selenite, \({\text{SeO}}_{ 3}^{ 2- }\)) to Se(VI) (selenate, \({\text{SeO}}_{ 4}^{ 2- }\)) is kinetically slow and non-equilibrium conditions predominate [33]. Other dissolved metals can also affect the process of oxidation and highlight the need to experimentally measure the Se species present in environmental samples under consideration [32].
The Simpson Species Richness Index (SSRI) [34,35,36,37,38] is used to characterize the sites sampled in Fountain Creek. The SSRI is an index measure based on the abundance of fish species present at a given site as well as the number of individuals of a given fish species at a site compared to the probability of encountering the species at any site in the study area [34,35,36,37]. Se(II), Se(IV) and Se(VI) are known to facilitate several modes of action including forming adducts with cysteine residues, selenoproteins, and serving as cofactors for the reduction of antioxidant enzymes [39,40,41,42,43] and function of other critical enzymes such as MAP kinase [44] and protein phosphatases [45, 46]. Indeed, differential action of selenite and selenate have been reported with regard to cellular uptake by phosphate transporters [47], effect on tumor progression [48] and in general impact on fresh water organisms [38]. Knowing the oxidation state of Se is very important in beginning to elucidate the mode of action of the Se compound.
In this manuscript, we analyze the effects of Se on the diversity of fish populations in a series of sites along the Fountain Creek. The analysis shows that Se content can be important for diversity in fish species although other variables play a role as well. The analysis underlines the importance of measuring the oxidation states of the environmental Se if a full understanding of the watershed sites is desired. When speciation measurements were done, a distinct variance in Se(IV) and Se(VI) levels in different sites was observed. Importantly, the low Se(IV) levels are consistent with low toxicity at sites where total Se content was much higher than the EPA-recommended level. These studies demonstrate the importance of determining both Se(IV) and Se(VI) in the aqueous samples, and illustrate the need for such analysis should a complete understanding of the system be desired.
Experimental
Materials
The chemicals were purchased from Sigma-Aldrich unless specified otherwise. The HPLC solvents were HLPC grade and purchased from Fisher Scientific. The chemicals were also purchased ultra-pure grade and used without purifications.
Sample collection
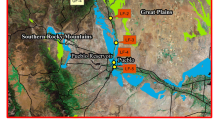
Water samples were collected from Lower Fountain Creek, near Pueblo, Colorado see Fig. 1. Sites for sample collections were selected based on map locations and information reported previously on the sites UF-1 to UF-3, MC-1 to MC-5 and LF-1 to LF-5 [16]. Water samples were collected according to United States Geological Survey (USGS) water sampling protocols [46] with some modifications [51]. The samples were placed on ice in the field to prevent selenite oxidation to selenate. After returning from the field the samples were split into 5 ml of aliquots with the first aliquot analyzed immediately upon returning to the lab. The remaining aliquots were stored at −20 °C under nitrogen for future use.
Methods for sample analysis
Total water Se was measured by ICPMS using EPA Method 200.8 on an Agilent 7500ce Inductively Coupled Plasma Mass Spectrometry (ICPMS) [52]. The chromatographic separation of the selenium species was carried out on a Thermo–Dionex ICS-5000 coupled to an Agilent 7500ce ICPMS. The analytical column used was a Dionex AS-7 anion exchange column. Separation of hydrogen selenite [Se(IV)) and selenate (Se(VI)] was adapted from Ge et al. [53] and Bednar et al. [54]. Improved chromatographic separation was accomplished using a 17.5 mM citrate buffer at pH 5.2 as the mobile phase. Furthermore, methanol (2% v/v) was used in the mobile phase to enhance the Se signal [55]. Mobile phase was isocratically delivered at 300 µl/min during the HPLC separation. After separation, the species was identified using Agilent 7500ce ICPMS.
Electrochemistry
Redox (pE) measurements of collected water samples were carried out on a Microlab F-522 system using the electrochemical interface and pH probe reference. The redox data obtained from the Microlab interface were normalized for temperature and to the E 0 of a standard hydrogen electrode (SHE) hydrogen electrode standard since the Microlab uses a Ag/AgCl electrode. The E 0 data were then converted to pE [56, 57]. pH measurements were obtained using the Microlab F-522 pH interface with a Microlab electrode.
Statistical analysis
All samples were prepared in triplicate and averages are reported. These averages are reported with their standard errors shown. This was done both for the data previously reported and the new data obtained as a result of the analysis carried out here. The Simpson Species Richness Index (SSRI) was used to compare the diversity of fish and relative number of each species at each site [38].
Simple canonical correlation analysis (SCCA) [58] was used to examine the possibility of a relationship between dissolved Se, biologically available Se as bryophyte Se, fish species number and fish diversity [59,60,61]. The variable silt/clay percentage was also added as the silt/clay content could have a significant effect on fish reproduction.
Results and discussion
Data analysis
In Table 1, we list the Se content in Fountain Creek Watershed [16] and compare the Se content with the number of fish species at these sites, the number of fish species found at a site divided by the probability of finding a fish at this site (PI) and the SSRI factor PI2. It is an objective to use the SSRI index and the SSRI factor PI2 to derive conclusions regarding fish diversity. The data from the previous studies [16] along the Fountain Creek in Colorado was subjected to this analysis. The calculated PI2 values are listed in Table 1 with the data used for these calculations [16]. The use of the SSRI index for the three reaches of Fountain Creek shown below Table 1 indicate that conclusions regarding fish diversity cannot be made for the data in Table 1 with respect to dissolved Se water levels. The idea that high Se levels should be detrimental to fish diversity and health is not reflected in the SSRI values for each reach as evidenced by the medial value of SSRI in the high Se reach of Lower Fountain Creek. The higher SSRI values of Monument Creek and Lower Fountain Creek suggest the need for additional information that is speciation analysis, which was therefore carried out in this manuscript.
Simpson Species Richness Index (SSRI)
Our previous study identified some sites to contain high concentrations of Se in the Fountain Creek shown in Table 1 [16]. High levels of Se in creek water generally correspond to high level of Se in the aquatic (bryophyte) plant [16]. However, there is a surprising difference in water Se content between some sites with similar Se in the plants (UF-4, LF-1 and LF-5). These results support the interpretation that the varying Se levels are real, and point to the need for additional data analysis and possibly a new analytical approach. The Simpson Species Richness Index (SSRI) [37] is a weighted index measure based on the abundance of fish species present at a given site as well as the number of individuals of a given fish species at a site compared to the probability of encountering the species at any site in the study area. The SSRI value increases when both species number and population of a given species increase [38]. In the following, we will include the fish diversity in the Fountain Creek data in the analysis.
The SSRI for the sites sampled in Fountain Creek provide a new aspect of the observations regarding Se content in aqueous samples and in the plants. The table indicates what is expected at the Monument Creek sites (MC) which are among the lower containing Se sites in the aqueous samples series. The Se content in the water and in the native bryophyte species Hygrohypnum ochraceum is relatively low. The lower Se levels would suggest an area of favorable fish health in a reach of stream where fish habitat is similar. The SSRI values for MC (SSRI = 0.054), LF (SSRI = 0.025) and UF (SSRI = 0.010) demonstrate that the greatest diversity of fish is in Monument Creek followed by Lower Fountain Creek.
The PI2 values were used as a predictor of diversity at each site for statistical analysis. The MC-1–MC-3 PI2 values of 0.011, 0.015, and 0.022, respectively, are accurate predictors of the higher SSRI value of MC.
The Lower Fountain Creek sites (LF) also share similar habitats and are more sand-based than Monument Creek and Upper Fountain Creek [10]. The unexpected high PI2 value at LF-4 (PI2 = 0.011) is interesting since it is the highest value in the LF reach and it is equivalent to the third highest PI2 value in the entire Fountain Creek Watershed. This high PI2 level is unexpected in an area where the water Se is dramatically above EPA and CDPHE water standards. In addition, this reach of the creek also has the second highest SSRI level (0.025) in a reach that routinely posts Se values above EPA and CDPHE water standards. At these sites (LF), the concentration of Se measured in H. ochraceum are the highest of all sampled sites, indicating that Se was readily internalized by plants and was thus available to the food web. The aforementioned reported levels of water Se [16] are well below the 10 µg/kg level reported as teratogenic [20]. These observations do have precedent in a report in 2009, where the highest fish whole body Se values of 3393 µg/kg dry weight and 906 µg/kg wet weight were found and there was no evidence of teratogenic effects on any fish sampled [16]. These observations point to an interesting paradox between water and fish Se levels and the expected outcome on fish health and diversity. Further analysis was thus carried out to include the fish diversity in the SSCA analysis.
Simple Canonical Correlation Analysis (SCCA)
A simple canonical correlation analysis (SCCA) [58] was used to examine the possibility of a relationship between dissolved Se, biologically available Se as bryophyte Se, fish species number and fish diversity. The variable silt/clay percentage was also added as the silt/clay content could have a significant effect on fish reproduction. The results are summarized in Table 2. These results, based on previous data, statistically support the conclusion that two main components contribute to fish diversity in Fountain Creek. Component 1 is described as the available selenium in water and is indicated by the high correlation of Se in water and Se in bryophyte shown in Table 2 (correlation 0.993, 0.748). The second component (Component 2) describes the fish species diversity and is indicated by the strong correlation between fish species number and diversity index in Table 2 (correlation 0.798, 0.322). This SCCA analysis provides two parameters, Component 1 and Component 2, which can be used to further understand the nature of the Se levels and the Fountain Creek sites.
The SCCA results presented in Table 3 indicate that Component 1 in these results is similar to the results presented in Table 2. There is a high degree of correlation between dissolved Se in water, Se in bryophyte and the Se species Se(IV) and Se(VI). There is also a small correlation with the silt/clay content which is probably related to the silt/clay content affecting the dissolution of the Se forms into the water. The major contributions to Component 1 come from dissolved Se in water (0.41) and the predominant Se(VI) (0.547). Component 1 in this table can be thought of as Se in water.
Component 2 in these results shows a high correlation of fish species number (0.798) to silt/clay content (0.27) in the creek bed and provides the greatest contributions to this component.
The Row plot shown in Fig. 2a has Component 1 and Component 2 on the X- and Y- axis. This plot splits the sites into four distinct quadrants each reflecting the similarity of the diversity in fish species by site. The four regions also correspond to the three areas of Pierre shale found in the Fountain Creek Watershed (FCW). There exist hydrological and geochemical similarities between the Lower Arkansas River (LAR) and the Fountain Creek (FC) [17]. The source of the LAR Se is Se-rich tributaries with high Se effluent such as FC [17]. The Se source in FC is Pierre Shale, Se-rich shale that was deposited in the late Cretaceous period [62]. This shale is also exposed in the Lower Fountain Creek Watershed (LFCW) in southeastern Colorado and results in elevated levels of Se in Lower Fountain Creek water and in natural springs that feed the Creek [63]. The two left quadrants describe sites with generally higher levels of Se content in the aqueous samples and in the biosamples. The two right quadrants separate out the samples with the greater PI2, and are predominantly populated by the samples from the Upper Fountain Creek. The elevated levels of Se in the LFCW have a significant environmental impact as the creek empties into the Arkansas River on the east side of the city of Pueblo, Colorado, and is a major source of Se in the Arkansas River [9].
a Row plot showing the Se content in water and bryophyte (Component 1) compared to fish diversity (Component 2) highlight the relationship between the different shale types at sites; b Row plot showing the Se(IV) species content in water and Se content in bryophyte (Component 1) compared to fish diversity and Se(VI) content in the water (Component 2) highlights the relationship between the different shale types at sites
Figure 2b separates the Se species from total dissolved Se in the water in the FCW. The figure clearly indicates that predominant Se species found in the Lower Pierre Shale have little to no effect on fish species diversity while the Upper Pierre Shale has a positive effect on fish species diversity. The continuous Pierre Shale has a negative effect on fish species diversity. These effects on fish species diversity could be due to the form of Se dissolving from the parent rock material and the dissolved Se interactions that depend on local environment and water chemistry.
Plotting the results of the SCCA in a loading plot (Fig. 3a) with Component 1 on one axis and Component 2 on the other axis, the relationship between different parameters used in the simple canonical correlation analysis is illustrated. Group 1 is Se found in H. ochraceum (abbreviated Se Bry) and number of fish species (Num Spp). Group 2 is a correlation between PI2, Se (IV) (SeO3) and total dissolved Se (Se D Water). The results show that the variable of silt/clay percentage which was thought to be a major factor in fish diversity in Fountain Creek is nearly orthogonal in relation to the other lines. The interpretation of this result is that the silt/clay variable is independent of fish diversity while the other variables are correlated in two groups.
a Loading plot illustrating the relationship between First and Second Components describing the relationship between the different variables at the Fountain Creek sites; b Loading plot, including Se(IV) and (VI), illustrating the relationship between First and Second Components describing the relationship between the different variables at the Fountain Creek sites
In contrast, the Se levels in the H. ochraceum (Se Bry) are responding similarly as the number of fish species (Num. Spp.) at each site. These two variables are different from the correlation between PI2, Se (IV) levels (\({\text{HSeO}}_{3}^{- }\)) and total dissolved Se (Se D Water), which all respond similarly. Together, analysis of this data demonstrates that fish species diversity is related to Se levels with the Se levels in the bryophytes related more closely to fish species number, the Se(IV) in the aqueous phase and the total dissolved Se. This correlation follows the mapping of Pierre Shale deposits found in this watershed. The source of the Se in the watershed comes from three Pierre Shale deposits known as upper, lower and continuous. Each of these deposits is represented in the Row plot (Fig. 2) along with the associated level of fish species diversity. The Se does increase in the fish with increasing dissolved Se water levels. No teratogenic effects were found in these high Se areas (LF-4 and LF-5) even though the Se levels are more than double the EPA-recommended water limit of 5.0 ppb.
Plotting the results of the SCCA Se speciation analysis in a loading plot (Fig. 3b) with Component 1 on one axis and Component 2 on the other axis the relationship between different parameters used in the simple canonical correlation analysis is illustrated. Group 1 is Se found in H. ochraceum (abbreviated Se Bry), the number of fish species and Se(IV). Group 2 is a correlation between PI2, Se (VI) and total dissolved Se (Se D Water). The indicates that the variable of silt/clay percentage is independent of the other groups shown by nearly orthogonal position in relation to the other lines. The interpretation of this plot infers that Se species are important in determining fish health and diversity in the watershed. This statement is supported by the grouping of Se(IV) with fish species number and Se(VI) with the SSRI predictor (PI2).
In Fig. 4, the PI2 factor is shown as a function of the Se concentration for Monument Creek and Lower Fountain Creek sites. As shown in Table 1, the Upper Fountain Creek sites show little change. Monument Creek shows an upward trend in diversity except for the sites with Se >0.005 nM, Fig. 4a. When the Se concentration is higher than 0.005 nM, the sites have higher Se(IV) concentrations and fish diversity is lower. In contrast, the sites along the Lower Fountain Creek show a different pattern with diversity increasing as a function of total Se concentration. As shown in Fig. 4b, there is a linear relationship with R 2 = 0.743. At these sites there is a higher Se(VI) concentration.
Speciation has been reported to be important in interactions with other elements and influences not only uptake but also biological responses to different species that may differ in geometry, charge and oxidation state for elements [64,65,66]. It has been reported that the toxicity effects of Se are dependent on a compound’s oxidation state and presumably on the bioavailability of the chemical species [67]. That is, the Se(IV) has been reported to be more toxic than Se(VI), which may be surprising since many beneficial effects particularly related to the antioxidant effects are mainly observed with Se(IV) [39,40,41,42,43]. The Se availability from the aqueous samples varies with the pH and concentration according to the speciation diagrams [28]. Thus, the observed toxicity is likely site-specific and may be related to the specific Se speciation chemistry occurring at each site. Because some of the differences in fish diversity may be related to the uptake and nature of the internalized species, more information on Se speciation at each sample collection site is desirable. Given the potential relationship between Se species, uptake, and toxicity, we subsequently investigated the oxidation state of the Se in water samples recently collected at each site to determine whether site-specific speciation of Se in the Fountain Creek samples factors in the observed variation in fish diversity between sites.
Sample collection from Fountain Creek
Water samples were collected at each of the sites labeled in Fig. 1 with the exception of MC-1–MC-3. The reason these sites were not collected was the inability to access the private property at MC-1 and the closure of the restricted areas of the United States Air Force Academy (MC-2 and MC-3). The water samples were collected according to USGS surface water sampling protocols [68]. Samples were collected in clean 250-ml high density polyethylene containers. The water samples were obtained by rinsing the containers at the site with creek water to equilibrate the container and the samples were taken with nonisokinetic sampling methods. The containers were immediately placed on ice for transport. In addition, pH, specific conductance and dissolved oxygen were measured on site. In the lab, the samples were fractionated into total, dissolved fractions. The dissolved fractions were filtered using a 0.45-micron syringe filter. The dissolved fractions were separated into 5 ml of speciation aliquots. One aliquot was used for total dissolved analysis and one was used for Se speciation. The remainder of aliquots were frozen for stability and future use. The total and dissolved fractions used for EPA 200.8 analysis were acidified with optima grade nitric acid. Hardness was calculated from ICPMS data.
Method for determining both Se(IV) and Se(VI) oxidation states
To explore Se speciation, a new study that would allow speciation to be determined was designed. Water samples containing Se were collected from the Lower Fountain Creek, near Pueblo, Colorado. The sites sampled have the highest levels of Se recorded in the Creek and have a high bryophyte Se concentration as shown in a previous study [69]. Although the samples were collected according to USGS water sampling protocols [46], recent reports suggest that these samples may not be as stable as previously anticipated [16, 70].
Due to the high levels of Se in the water and the fact that fish living in these waters seem to be unaffected, it is important to determine the speciation of the Se at each site. The chromatographic separation of the selenium species is readily accomplished using ICPMS after chromatographic separation of the Se(IV) and Se(VI). Using a Dionex AS-7 anion exchange column, the separation was readily accomplished using a mobile phase of 17.5 mM citrate buffer at pH 5.2. The buffer improved the separation presumably through the interaction between Se and citrate, and 1H NMR studies confirmed that complexes do form between selenate and citrate (unpublished). Furthermore, the Se signals were enhanced using 2% aqueous methanol in the mobile phase as reported previously for arsenic [55, 71].
Se(IV) and Se(VI) speciation of Se in Fountain Creek water samples
Samples were collected from 11 different sites along the Fountain Creek. Initially, we measured the speciation of samples along the entire Creek and in Table 4 we show the data for measurement of not only total Se, but the distribution of the Se as selenite, Se(IV), and selenate, Se(VI), and the pH of the respective samples. The Se content in the samples is very similar in the sites examined except for the MC-4 site; the amounts are higher in this recent study. Because the Se content varies with seasonal and weather/precipitation patterns, variations would have been anticipated from the previous study, however, very few are observed [16].
For the purposes of the SCCA, the readings for Se(IV) and Se(VI) at sites UF-1, UF-2, and UF-3 that went below the instrument detection limits were estimated using the regression with the total Se as a single predictor. Due to the uneven distribution of data in the range of available values, the readings had to be transformed to the logarithmic scale in the case of Se(IV) and square root scale for Se(VI). In two cases such estimates yielded values above the detection limit and were trimmed at the limit values: 0.023 and 0.034 µg/L for Se(IV) and Se(VI), respectively.
The Upper Fountain Creek sites were reported to have the lowest total Se, Se(IV) and Se(VI) and collectively the highest percentage of Se(IV). As seen from the data in Table 1, the Upper Fountain Creek sites also had a less diverse fish habitat (except for the UF-4). Once the water flows down to the Monument and Lower Fountain sites, the majority of the Se had oxidized to selenate. Since selenite at neutral pH readily oxidizes to selenate, and the oxidation is more likely to happen at lower concentrations and at higher salinity levels in the presence of nitrate, conversion of Se(IV) to Se(VI) is expected [32, 72]. The presence of a higher concentration of Se(VI) at the Lower Fountain sites was, therefore, anticipated although this could be due to additional oxidation of Se(IV) or dissolution of more Se(VI) from the shale or a combination of both oxidation and dissolution processes to form more Se(VI). Indeed, the data in Table 4 confirm that in all but one Lower Fountain sites more than 90% of the Se was in the form of selenate, Se(Vl).
The results for the LF-4 and UF-4 sites were different than that of the others in that the standard errors were a little higher for these sites. This increase in standard error at higher concentration is most likely due to the increase in dissolved solids and is the result of a matrix effect caused by a combination of easily ionized elements that are abundantly present and the more difficult to ionize Se. The Se species are measured after a column separation resulting in a cleaner sample entering the plasma of the ICPMS.
Using speciation analysis, we considered the speciation using the known constants reported for the distribution of species in aqueous solution [28]. In Fig. 5 we calculated the equilibrium concentrations of solutions at the concentrations measured at the sites we were investigating (UF, LF-1 and LF-4). As shown in Fig. 6 the equilibrium concentrations favor selenate as the major form because the pH was near 8 in these sites. As the concentration of the Se increased although more Se(IV) was present, the difference in the concentration between the Se(IV) and Se(VI) species was less. At the neutral to basic concentrations observed in the Fountain Creek sites; the deprotonated selenate and selenite were the major species. A small amount of monoprotonated selenate was found at the site with the higher Se(VI) concentration. This is interesting because the pKa values for selenite are higher, however, because the concentration of Se(IV) is 100-fold less there would be more \({\text{HSeO}}_{4}^{ - }\) than \({\text{HSeO}}_{3}^{ - }\) at this site if equilibrium conditions existed. Although the conversion of Se(IV) to Se(VI) may be slow, it is of interest to investigate the system further, and at least establish what is expected should the system be governed by the thermodynamic stability.
The Se Pourbaix diagram of Se in water reported previously [73] was added the specific conditions of the LF samples investigated in the Spring of 2016 where the crosshairs intercept. The Pourbaix diagram was reproduced with permission
The Pourbaix diagram describes the thermodynamically stable forms of Se in a graphical form shown in Fig. 6. In this figure, it is shown that the conditions controlling the major species of Se at neutral pH are complicated, and depending on the redox potential the thermodynamic stable form which may be Se(IV) or Se(VI). To investigate the nature of the aqueous redox environment, the electrochemical potential of samples, LF-4 and LF-5, which contained the highest observed levels of oxidized Se(VI), were measured. These studies were performed to determine whether the high levels of observed Se(VI) are due to oxidation reactions in the water, or to unchanged Se (VI) from the shale.
The E 0 data were converted to pE and these values are shown in Table 5. pH data were also obtained using the Microlab F-522 pH interface with a Microlab electrode. As shown in Table 5, sampling the Lower Fountain Creek sites LF-4 and LF-5 in April gave a pH value that was slightly above neutral. Determining the E 0/pE and pH data allows us to reference sample data with a Pourbaix diagram which describes the thermodynamically state species of the Se. This allows us to determine whether the sample contains Se(VI) or Se(IV). For low concentrations of Se at neutral pH and pE below 7/8, the Pourbaix diagram predicts that the thermodynamically stable form is Se(IV). It was surprising that the pE for the LF samples collected in the spring of 2016 had values of 6.35/6.37. This suggests that in the creek water samples the stable form of the Se in LF-4 and LF-5 sites is Se(IV). However, because it was experimentally determined that Se(VI) was the major component, it is likely that the predominance of the Se(VI) form is dependent on other environmental factors and supports the findings of Mast et al. [70].
Using the SSRI predictor data (PI2) data in Table 3 SCCA was carried out and the results show that a Se species effect on diversity exists and that effect is independent of the silt/clay content of the creek bed material present at the sites. The Se species effect can be positive or negative depending on the origin of the creek bed parent material. This is most notable between the LF-3, LF-4, and LF-5 sites compared to the UF-3 and UF-4 sites, the latter of which has a higher percentage composition of Se(IV). Thus, this analysis confirms that there is less observed toxicity at the LF-3–LF-5 sites despite the higher total Se-levels. Although this analysis does not prove that the Se(VI) is less toxic to the fish, the analysis does provide a statistical basis to suggest that the toxicity effect is correlated with observed oxidation state.
Previously, a report on Se-levels in the Fountain Creek fish study did indicate increased levels of Se in ovarian and liver tissue of the fish [16]. The Se level increase in these tissues may be a result of increasing levels of Se containing enzymes. The Se level increase in ovarian tissue may explain teratogenic effects in new hatchlings if the Se levels continue to rise. The most common mode of toxicity of Se is oxidative damage [38]. This type of toxicity requires that Se be internalized and assimilated into the organs and the fish. Se is generally nontoxic when it is stored in a “safe” form, and either because it cannot be absorbed or transported, it is rapidly excreted or is not metabolized. However, some transporters prefer Se(IV) over Se(VI) [47]. The high Se water levels at several sites and the lack of observation of teratogenic levels in the fish raise the question of how Se levels in this biota can be so high without causing toxic effects in fish. The data provided here show that there are significant differences in the speciation in the creek water and the lack of teratogenic response to the Se level may be because the Se is in the Se(VI) form which is less toxic than Se(IV).
In summary, current speciation studies with Se generally investigate only one oxidation state; however, as described in this manuscript, speciation studies of the water at representative Fountain Creek sites demonstrate that total Se levels cannot fully describe the interactions of Se in the complex environmental system and will require additional analyses in the future. Our experimental data show that a correlation exists between fish species diversity and dissolved Se in Fountain Creek water and this is backed up by statistical analyses. The increase in fish diversity with the dissolved Se may be a result of a limited amount of total Se or particular Se species in other areas of the watershed [16, 28]. Se is known to have protective effects such as reducing oxidative stress and is a required component of some enzymes [12,13,14, 21, 22]. The high diversity at the LF-4 site where the dissolved Se concentration is well above EPA limits may be consistent with a low amount of Se(IV) and high amount of Se(VI) observed at these sites. EPA and other studies have indicated that Se(IV) is the more toxic of the two forms [38].
Conclusion
Based on previous data on the Se levels along the Fountain Creek [16], a canonical correlation analysis was done to examine the possibility of a relationship between dissolved Se, biologically available Se as bryophyte Se, fish species number and fish diversity. The analysis revealed that two different components were involved and that diversity in fish species was related to the total dissolved Se levels in the creek water samples. Because some sites were found to contain high levels of Se and high fish diversity when the opposite was expected for some sites, we investigated the possibility that such differences between sites could arise due to Se speciation differences. As a result, we designed and performed studies where we measured the amounts of two different Se species [that is Se(IV) and Se(VI)] as well as total dissolved Se content at selected sites along the Fountain Creek, and determined the oxidation state of the Se. The speciation studies showed a distinct variance in Se(IV) and Se(VI) levels in the different sites consistent with the observations that low toxicity is observed when low levels of Se(IV) are present at the sites. Although these studies do not prove that the level of selenite in the water is the principal factor for toxicity they do provide the data consistent with this interpretation. These studies underline the importance of determining both Se(IV) and Se(VI) in the aqueous samples, and the need for such speciation analyses should a complete understanding of the complex system be desired.
References
Bird SM, Ge H, Uden PC, Tyson JF, Block E, Denoyer E (1997) J Chromatogr A 789:349–359
Bird SM, Tyson JF (1997) J Anal At Spectrom 12:785–788
Block E, Booker SJ, Flores-Penalba S, George GN, Gundala S, Landgraf BJ, Liu J, Lodge SN, Pushie MJ, Rozovsky S, Vattekkatte A, Yaghi R, Zeng H (2016) ChemBioChem 17:1738–1751
Combs GF Jr, Gray WP (1998) Pharmacol Ther 79:179–192
Mousa SA, O’Connor L, Rossman TG, Block E (2007) Carcinogenesis 28:962–967
Weekley CM, Harris HH (2013) Chem Soc Rev 42:8870–8894
United States Environmental Protection Agency (2016) National recommended water quality criteria—aquatic life criteria table. https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table. Cited 4/18/2016 2016
Colorado Department of Public Health and Environment (2013) In: The basic standards and methodologies for surface water 5. Colorado Department of Public Health and Environment Water Quality Control Commission, Denver, Colorado, pp 218
Divine CE, Gates TK (2006). Prepared by Arcadis G&M, Inc., 630 Plaza Drive, Suite 100, Highlands Ranch, Colorado 80129, pp. 1–41
Edelmann, P, Ferguson SA, Stogner Sr. RW, August M, Payne WF, Bruce JF (2005) In: Water Resources Investigations Report #02-4104 Survey US Geological Survey (ed). Evaluation of water quality, suspended sediment, and stream morphology with an emphasis on effects of stormflow on Fountain and Monument Creek Basins, Colorado Spring and vicinity, Colorado, 1981 through 2001, Denver
Janz DM, DeForest DK, Brooks ML, Chapman PM, Cilron C, Hon D, Hopkins WA, Mcintyre DO, Mebane CA, Palace VP, Skorupa JP, Wayland M (2010) In: Chapman PM, Adams WJ, Brooks ML, Delos CG, Luoma SN, Maher WA, Ohlendorf HM, Presser TS, Shaw DP (eds) Ecological assessment of selenium in the aquatic environment. CRC Press, Pensacola, pp 151–155
Hamilton SJ, Holley KM, Buhl KJ, Bullard FA (2005) Ecotoxicol Environ Saf 61:168–189
Hamilton SJ, Holley KM, Buhl KJ, Bullard FA (2005) Ecotoxicol Environ Saf 61:32–43
Hamilton SJ, Holley KM, Buhl KJ, Bullard FA, Ken Weston L, McDonald SF (2005) Ecotoxicol Environ Saf 61:7–31
United States Environmental Protection Agency (2015) In: Office of Water OOSAT (ed) Draft Aquatic Life Ambient Water Quality Criterion for Selenium – Freshwater. United States Environmental Protection Agency, Washington, DC pp 1–740
Nimmo DR, Herrmann SJ, Carsella JS, McGarvy CM, Foutz HP, Herrmann-Hoesing LM, Gregorich JM, Turner JA, Vanden Heuvel BD (2016) SpringerPlus 5:437
Presser TS, Sylvester MA, Low WH (1994) Environ Manag 18:423–436
Colorado Parks and WIldlife (2016) Birding locations. http://cpw.state.co.us/thingstodo/Pages/Birding-Locations.aspx. Cited 04/06/2016 2016
Andersson K, Davis CA, Harris G, Haukos DA (2015) Wildl Soc Bull 39:79–86
Lemly AD (1997) Ecotoxicol Environ Saf 37:259–266
Kennedy CJ, McDonald LE, Loveridge R, Strosher MM (2000) Arch Environ Contam Toxicol 39:46–52
Mathews TJ, Fortner AM, Jett RT, Morris J, Gable J, Peterson MJ, Carriker N (2014) Environ Toxicol Chem 33:2273–2279
da Silva EG, Mataveli LR, Arruda MA (2013) Talanta 110:53–57
Maseko T, Callahan DL, Dunshea FR, Doronila A, Kolev SD, Ng K (2013) Food Chem 141:3681–3687
Thosaikham W, Jitmanee K, Sittipout R, Maneetong S, Chantiratikul A, Chantiratikul P (2014) Food Chem 145:736–742
Maneetong S, Chookhampaeng S, Chantiratikul A, Chinrasri O, Thosaikham W, Sittipout R, Chantiratikul P (2013) Microchem J 108:87–91
Block E (2013) J Sulfur Chem 34:158–207
Baes CF, Mesmer RE (1976) Hydrolysis of cations. A Wiley-Interscience Publication, John Wiley & Sons, New York.
Drever JL, Holland HD, Turekian KK (2004) Treatise Geochem 5:583–625
Jagtap R, Maher W (2016) Microchem J 124:422–529
Narasaki H, Mayumi K (2000) Anal Sci 16:65–68
Torres J, Pintos V, Domínguez S, Kremer C, Kremer E (2010) J Solut Chem 39:1–10
Bodek I, Lyman WJ, Reehl WF, Rosenblatt DH (1988) Environmental inorganic chemistry - properties, processes, and estimation methods. Pergamon press, New York
Gray JS (2000) J Exp Mar Bio Ecol 250:23–49
Korb JE, Daniels ML, Laughlin DC, Fulé PZ (2007) Southwest Nat 52:493–503
Lande R (1996) Oikos 76:5–13
Smith B, Wilson JB (1996) Oikos 76:70–82
Simpson EH (1949) Nature 163:688
Doyotte A, Cossu C, Jacquin M-C, Babut M, Vasseur P (1997) Aquat Toxicol 39:93–110
Glass RS, Berry MJ, Block E, Boakye HT, Carlson BA, Gailer J, George GN, Gladyshev VN, Hatfield DL, Jacobsen NE (2008) Phosphorus Sulfur Silicon 183:924–930
Ip C, Birringer M, Block E, Kotrebai M, Tyson JF, Uden PC, Lisk DJ (2000) J Agric Food Chem 48:2062–2070
Moon HJ, Ko WK, Han SW, Kim DS, Hwang YS, Park HK, Kwon IK (2012) Biochem Biophys Res Commun 418:247–253
Zhang S, Rocourt C, Cheng WH (2010) Mech Ageing Dev 131:253–260
Hei YJ, Farahbakhshian S, Chen X, Battell ML, McNeill JH (1998) Mol Cell Biochem 178:367–375
Mueller AS, Bosse AC, Most E, Klomann SD, Schneider S, Pallauf J (2009) J Nutr Biochem 20:235–247
Shelton LR, Capel PD (1994) In: Survey USG (ed) Guidelines for collecting and processing samples of stream bed sediment for analysis of trace elements and organic contaminates for the National Water-Quality Assessment Program. U.S. Geological Survey, Sacramento
Zhang L, Hu B, Li W, Che R, Deng K, Li H, Yu F, Ling H, Li Y, Chu C (2014) New Phytol 201:1183–1191
Yoon SO, Kim MM, Chung AS (2001) J Biol Chem 276:20085–20092
Green GN (1992) In: United States Geological Survey (ed) United States Geological Survey Open Report 92–507, The digital geologic map of Colorado in ARC/INFO format. United States Geological Survey, Denver, Colorado
National Aeronautics and Space Administration Goddard Space Flight Center Rapid Response (2013). National Aeronautics and Space Administration
Bednar AJ, Chappell MA, Seiter JM, Stanley JK, Averett DE, Jones WT, Pettway BA, Kennedy AJ, Hendrix SH, Steevens JA (2010) Chemosphere 81:1393–1400
United States Environmental Protection Agency (1994) SW-846, 200 Series, rev. 5.4 Method 200.8 Determination of Trace Elements in Water and Wastes by Inductively Coupled Plasma - Mass Spectrometry, pp 1–57
Ge H, Cai X-J, Tyson JF, Uden PC, Denoyer ER, Block E (1996) Anal Commun 33:279–281
Bednar AJ, Kirgan RA, Jones WT (2009) Anal Chim Acta 632:27–34
Larsen EH, Stürup S (1994) J Anal At Spectrom 9:1099–1105
Suslow TV (2004) Oxidation-Reduction Potential (ORP) for Water Disinfection Monitoring. Division of Agriculture and National Resources, University of California, Davis
Duffy SJ (2011) Environmental chemistry: a global perspective. Oxford University Press, Oxford
Hotelling H (1936) Biometrika 28:321–377
Härdle W, Simar L (2007) Applied multivariate statistical analysis, Vol 22007. Springer, Berlin
Knapp TR (1978) Psychol Bull 85:410
Huang S-Y, Lee M-H, Hsiao CK (2009) J Stat Plan Inference 139:2162–2174
Tourtelot HA (1962) In: Survey Report 390 USG (ed) Preliminary Investigation of the Geologic Setting and Chemical Composition of the Pierre Shale Great Plains Region. United States Government Printing Office, Washington, DC, pp 1-81
Mau DP, Stogner RW Sr, Edelmann P (2007) In: Report 2007–5104: Characterization of Stormflows and Wastewater Treatment-Plant Effluent Discharges on Water Quality, Suspended Sediment, and Stream Morphology for Fountain and Monument Creek Watersheds, Colorado, 1981–2006: U.S. Geological Survey Scientific Investigations, p 76
Crans DC (2015) J Org Chem 80:11899–11915
Doucette KA, Hassell KN, Crans DC (2016) J Inorg Biochem 165:56–70
Willsky GR, Chi LH, Godzala M 3rd, Kostyniak PJ, Smee JJ, Trujillo AM, Alfano JA, Ding W, Hu Z, Crans DC (2011) Coord Chem Rev 255:2258–2269
Rosenfeld I, Beath OA (1964) Selenium: geobotany, biochemistry, toxicity, and nutrition. Academic Press, New York
United States Geological Survey (2006) Collection of water samples (ver. 2.0): United states geological survey techniques of water—resource investigations, book 9, c. https://water.usgs.gov/owq/FieldManual/chapter4/html/Ch4_contents.html
Herrmann SJ, Turner JA, Carsella JS, Lehmpuhl DW, Nimmo DR (2012) Environ Manage 50:1111–1124
Mast MA, Mills TJ, Paschke SS, Keith G, Linard JI (2014) Appl Geochem 48:16–27
Suzuki KT, Ogra Y (2002) Food Addit Contam 19:974–983
Torres J, Pintos V, Gonzatto L, Domínguez S, Kremer C, Kremer E (2011) Chem Geol 288:32–38
Drever JL (1997) The geochemistry of natural waters surface and grounwater environments. Prentice Hall, Upper Saddle River
Acknowledgements
JSC and SJB would like to thank the Board of Pueblo, Colorado, County Commissioners, the Lower Arkansas Valley Water Conservancy District, Colorado, and the Board of Water Works of Pueblo. JSC, SJB and DCC would like to thank Colorado State University—2014–2015 Faculty SEED Grant. DCC thanks the Arthur Cope Foundation for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carsella, J., Melnykov, I., Bonetti, S. et al. Selenium speciation in the Fountain Creek Watershed and its effects on fish diversity. J Biol Inorg Chem 22, 751–763 (2017). https://doi.org/10.1007/s00775-017-1457-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-017-1457-0