Abstract
While trivalent chromium has been shown at high doses to have pharmacological effects improving insulin resistance in rodent models of insulin resistance, the mechanism of action of chromium at a molecular level is not known. The chromium-binding and transport agent low-molecular-weight chromium-binding substance (LMWCr) has been proposed to be the biologically active form of chromium. LMWCr has recently been shown to be comprised of a heptapeptide of the sequence EEEEDGG. The binding of Cr3+ to this heptapeptide has been examined. Mass spectrometric and a variety of spectroscopic studies have shown that multiple chromic ions bind to the peptide in an octahedral fashion through carboxylate groups and potentially small anionic ligands such as oxide and hydroxide. A complex of Cr and the peptide when administered intravenously to mice is able to decrease area under the curve in intravenous glucose tolerance tests. It can also restore insulin-stimulated glucose uptake in myotubes rendered insulin resistant by treating them with a high-glucose media.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While chromium as the trivalent ion was proposed to be an essential trace element over half a century ago, a recent paradigm shift in understanding the effects of chromium has occurred [1–3]. The effects of Cr3+ in enhancing insulin sensitivity in rodents have been shown to be supra-nutritional [4]. No symptoms of chromium deficiency have been unambiguously established, and no biomarker of chromium status has been identified [1]. However, this shift raises questions about the role and mode of action of Cr3+ in increasing insulin sensitivity at a molecular level (at least in rodent models of diabetes and insulin insensitivity). It also may explain the lack of the demonstration of unambiguous benefits of chromium supplementation in clinical trials as the human subjects have failed to receive doses of Cr3+ proportionally as high as the doses administered to the rodents [1–3]. To date, several proposals for the mode of action of Cr3+ have been presented [1, 5], although none have been definitely established in vivo. Unraveling the mechanism and mode of action at a molecular level of the potentially beneficial action of Cr3+ would resolve over five decades of studies in the field of nutritional biochemistry.
Yet, much has been elucidated about the mechanism of transport and movement of chromium in the bodies of mammals. Chromium is passively absorbed from the gastrointestinal tract, transported by transferrin from the bloodstream to the tissues, and transported from the tissues to the urine (via the bloodstream) by the peptide low-molecular-weight chromium-binding substance (LMWCr); transferrin and LMWCr are currently the only two biomolecules known to specifically bind Cr3+ in vivo when Cr3+ is taken orally at nutritionally relevant doses [1]. Transferrin and LMWCr appear to form a detoxification pathway for passively absorbed Cr3+. Unlike other metal ions, Cr3+ binds first to the C-terminal metal-binding site of transferrin; given that transferrin is only approximately 30 % loaded with iron in the bloodstream, the protein appears to be readily available to bind and transport Cr and has been shown in vivo to be responsible for Cr transport (reviewed in Ref. [3]). Cr-containing LMWCr is rapidly cleared from tissues in vivo (although the mechanism is unknown) and has a very low tubular reabsorption rate so that the peptide in the blood is readily removed from the body via the kidneys and urine [1]. Increases in Cr intake result in increases in urinary Cr loss, presumably as the complex with LMWCr [1]. The movement of Cr(III)–LMWCr is stimulated by increases in blood insulin concentration; thus, increased movement of Cr from the bloodstream to the tissues by transferrin results in a subsequent elimination of Cr from the body bound to LMWCr. The peptide appears to be maintained in the tissue in the apo form; levels of the apo form potentially are under homeostatic control. At a minimum, LMWCr functions to rapidly clear Cr from tissues [1].
LMWCr is a peptide 10 or 11 amino acids in length; it is comprised of only the amino acids glycine, aspartate, glutamate, and cysteine and tightly binds four chromic ions [6, 7]. The oligopeptide has been isolated from the livers of mammals [6, 7], chicken [8], and alligator [8] and human urine [9] where all appear to contain the contiguous peptide of sequence Glu–Glu–Glu–Glu–Gly–Asp–Asp (EEEEGDD) [9]. Cr from oral administration has been found in the bloodstream to be bound to transferrin and a low-molar-mass peptide that co-elutes on size exclusion columns with LMWCr; similarly, injection of Cr-containing transferrin into the bloodstream results in the appearance of a low-molar-mass species that co-elutes with LMWCr (reviewed in Ref. [1]). Spectroscopic studies of LMWCr indicate that the chromic ions are bound to the peptide primarily via the carboxylate side chains of the aspartate and glutamate residues [10]. The chromic ions appear to be arranged in a multinuclear assembly [1].
The Cr(III)-containing form of LMWCr has been proposed to be the biologically active form of chromium. In vitro it has been found to activate the kinase activity of insulin receptor; this activation was proportional to the Cr content of LMWCr [11, 12]. In a proposed mechanistic scheme, at high intakes of Cr3+, LMWCr would accumulate to pharmacological levels, bind to insulin receptor (helping to maintain the receptor in its active conformation), and result in increased insulin sensitivity [13]. The validity of this proposal requires testing in vivo.
However, the discovery that LMWCr contains a heptapeptide that could readily be synthesized raises the question of whether this peptide might bind Cr similarly to LMWCr, have biological activity (i.e., increase insulin sensitivity), and thus have potential as a pharmaceutical agent to treat symptoms of diabetes and other conditions related to insulin insensitivity. Herein are reported initial studies to examine the binding of Cr3+ to the synthetic peptide EEEEGDD and the biological activity of the peptide and its complex with bound Cr.
Materials and methods
Materials
Deuterium oxide and sodium deuteroxide were obtained from Acros (Geel, Belgium). The heptapeptide EEEEGDD (purity >98 %) was synthesized by GenScript (Piscataway, NJ, USA); the sequence was confirmed by mass spectrometry as previously described [9]. Chromic chloride hexahydrate was from Fisher Scientific (Pittsburg, PA, USA). DE-52 cellulose and Sephadex G-10 were from Whatman (Florham Park, NJ, USA) and GE Healthcare (Uppsala, Sweden), respectively. MES (2-(N-morpholino)ethanesulfonic acid) was from Research Organics (Cleveland, OH, USA).
Analytical procedures
Infrared spectra were recorded on a Bruker Vertec 70 Fourier transform spectrometer equipped with a DTGS (deuterated triglycine sulfate) detector using sealed liquid cell (0.05 mm path length) with calcium fluoride crystal windows. Each single beam spectrum consisted of 500 scans. Electronic spectra were recorded on a Beckman Coulter DU 800 spectrometer. Electron paramagnetic resonance (EPR) spectra were recorded on a Bruker Elexsys E540 X-band spectrometer with an ER 4102 ST resonator. Spectra were collected at 77 K with a quartz insertion dewar. The heptapeptide concentration (amino terminus concentration) was determined by the fluorescamine method [14], as the only primary amine group on the peptide is the amino terminus. Fluorescence measurements were obtained on a Jovin Yvon Fluoro Max-3 fluorescence spectrometer. Cr content, except where noted, was determined by atomic absorption spectrometer using a PerkinElmer Analyst 400. Electrospray ionization (ESI) mass spectra were obtained on a Bruker HCT ultra high capacity quadrapole ion trap mass spectrometer following procedures described previously [15]. NMR experiments were performed on a Bruker AV-600 using a TXI probe equipped with a Z-gradient (1H NMR, 600.057 MHz; 13C, 150.88 MHz). The peptide was dissolved in one of three solvents: D2O, H2O/2H2O (90:10), or d6-DMSO. When the peptide was dissolved in H2O/2H2O (90:10) as solvent, excitation sculpting (es) was employed as the water suppression technique. Pulse programs used in the NMR experiment were zgesgp (1H NMR) cosydfsgpph (COSY) in H2O/2H2O (90:10) and zg30 (1H NMR), cosygdpf (COSY), hsqcetgpsi (heteronuclear single-quantum-coherence, HSQC; J constant, 150 Hz), and hmbcgpndgf (heteronuclear multiple-bond-coherence, HMBC; 8 Hz constant for long range coupling) in 2H2O and d6-DMSO.
Preparation of infrared samples
H/2H exchanged heptapeptide and CrCl3 were prepared by heating the EEEEGDD peptide and CrCl .3 6H2O, respectively, dissolved in 2H2O at 60 °C for 30 min followed by freeze drying. H/2H exchanged Cr(III)-heptapeptide was prepared by incubation of the H/2H exchanged peptide (8 mM), which had been adjusted to pH 4.3, 5.3 or 6.4 by addition of NaO2H, with the H/2H exchanged CrCl3 (32 mM) for 8 h. For solutions in 2H2O, p2H is calculated using pH = p2H − 0.4 where pH is the direct reading of a pH meter calibrated in H2O solutions [16].
Isolation of Cr-peptide complex at pH 5.6 for spectroscopic studies
Reaction mixtures containing 4 mM CrCl .3 6H2O, 1 mM heptapeptide, and 20 mM MES (pH 5.6) in a total volume of 0.5 ml were incubated for 7 h. The mixtures were applied to a Sephadex G-10 column (1.5 × 6.5 cm) equilibrated with 20 mM MES (pH 5.6). The column was eluted with the same buffer, and the fractions (0.7 ml) were collected. The Cr- and peptide-containing fractions, which eluted at the void volume, were pooled and concentrated by freeze drying to use for EPR and electronic spectroscopic analysis. Free Cr3+ was found to bind the column under these conditions. A DE-52 column could also be utilized for this separation. The DE-52 cellulose column (1.5 × 5 cm) equilibrated with 20 mM MES (pH 5.6). After the reaction mixture (≤1 mL) was applied to the column, the column was washed with the same buffer (14 mL) after which a linear gradient (21 mL) of NaCl from 0 to 1 M in 20 mM MES (pH 5.6) was applied to the column. Fractions (0.7 mL) containing Cr and the peptide were pooled and concentrated by freeze drying to use for subsequent analysis.
Miscellaneous
Doubly deionized water [Millipore (Billerica, MA, USA) Milli-Q] was used throughout the mass spectrometric and spectroscopic studies.
Intraperitoneal glucose tolerance test
This study was performed via a protocol approved by the Institutional Animal Care and Use Committee of the University of Wyoming in accordance with the Guide for the Care and Use of Laboratory Animals. Care was taken to minimize discomfort, distress, and pain to the animals. Six-month-old male FVB mice were bred in our research vivarium and housed in a controlled environment (12-h-daylight cycle) in groups of 5 mice per cage at 22 °C. The mice were allowed to feed on regular lab chow (Cat #5001 LabDiet, St. Louis, MO, USA), and water ad libitum. Intraperitoneal glucose tolerance test (IPGTT) was performed as described by this laboratory previously [17]. Briefly, food was withheld from the mice overnight (~12 h) and on the day of the test the mice were randomly assigned to the following four groups (8–10 mice per group): (1) vehicle (saline) (2) CrCl3 (50 µM) (3) heptapeptide (50 µM) and, (4) peptide + chromium chloride (50 µM each, premixed). All mice received a single intravenous dose (through tail-vein) of the test agents at the dose indicated, immediately followed by an intraperitoneal injection of glucose (2 g/kg). Glucose concentrations were measured in blood drops obtained by clipping the tail of the mice at 0-, 15-, 60-, and 120-min intervals. Blood glucose concentrations were measured using ACCU-CHEK Advantage Glucose Analyzer (Roche Diagnostics). Area under the curve (AUC) for each individual IPGTT-time curve was calculated based on the trapezoid rule [18], and the total area under the curve for each treatment group was reported as mean ± SEM and statistically evaluated using one-way analysis of variance (ANOVA) followed by multiple comparisons tests with Bonferroni correction using GraphPad Prism version 5.00 for Windows (GraphPad Software Inc., San Diego, CA, USA).
Cell culture
Mouse C2C12 myoblasts (American Type Culture Collection, Manassas, VA, USA) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal bovine serum and 1 % penicillin–streptomycin under a humidified atmosphere of 5 % CO2 in air and maintained at low confluence. To induce differentiation, the culture medium was changed to 1 % fetal bovine serum instead of 10 % fetal bovine serum when cells reached 70–80 % confluence. Once differentiated into myotubes, the cells were rendered quiescent by incubating them with serum-free medium for 24 h [19]. Quiescent cells were rendered insulin resistant by treating them for 24 h with 25 mM glucose [20].
Cellular glucose uptake assay
Glucose uptake activity was analyzed by measuring the uptake of 2-deoxy-d-[3H]glucose as described previously [21]. Briefly, C2C12 myotubes were incubated with normal (5 mM) or high (25 mM) glucose for 24 h in the presence of test compounds (10 µM) and challenged with insulin 5 nM for 10 min, following which the cells were washed with Krebs–Ringer phosphate HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer and incubated for a further 30 min at 37 °C in the presence of 2-deoxy-[3H]glucose (0.2 mCi). At the end of the incubation period, the cells were washed three times with ice-cold phosphate-buffered saline (PBS). The cells were lysed overnight with 1 ml 0.5 M NaOH and 0.1 % sodium dodecyl sulfate (w/v). The radioactivity retained by the cell lysates was determined by a scintillation counter (Beckmann LC 6000IC) and normalized to protein amount measured with a Micro BCA Protein Assay Kit (Pierce Chemical, Rockford, IL, USA).
Results and discussion
Stoichiometry of Cr3+ binding
The addition of four equivalents of Cr3+ as CrCl3 to the EEEEDGG peptide in 20 mM HEPES buffer, pH 7.4, results in the rapid formation of a light purple precipitate. Centrifuging the sample results in a light purple pellet containing Cr and peptide and in a clear, colorless supernatant. The lack of solubility of the Cr-peptide complex limits the ability to perform spectroscopic studies on this complex. This behavior unfortunately is in contrast to that when Cr3+ is added to the peptide pEEEEGDD (where pE is pyroglutamate) [9]. The precipitate removes nearly all the peptide and chromic ions from solution, suggesting the peptide binds four equivalents of Cr3+ forming a neutral species that readily precipitates from aqueous solution. This was confirmed by Cr analysis of the product. The binding of 4 equivalents of Cr would be consistent with 4 equivalents of Cr binding to the peptide pEEEEGDD [9]. After centrifuging the product of the addition of four equivalents of Cr3+ to the peptide, the Cr and peptide concentrations of the supernatant have been measured allowing the determination of the Cr:peptide ratio of the precipitate; the Cr concentration was determined using the diphenylcarbazide method [7], while peptide concentration was determined as described above [14]. The Cr:peptide ratio of the precipitate was 3.2:1. In addition to this representing binding of Cr to form a neutral complex, aggregation of the anionic peptide with bridging chromic ions would also be consistent with the results. However, when 4 equivalents of CrCl3 are added to 5 mg of the peptide dissolved in 1 L of HEPES buffer, precipitation occurs again, suggesting that the effect is not simply the result of aggregation of the peptide. Increasing the pH above 8 or below 6 results in the precipitate dissolving.
Thus, the addition of Cr3+ to the EEEEDGG peptide was repeated in 20 mM MES buffer, pH 5.6, which does not result in the rapid formation of a precipitate. The resulting Cr-peptide complex can be isolated by either size exclusion or ion exchange chromatography. Surprisingly, the complex at pH 5.6 is not cationic, but anionic as it adheres to the anion exchange resin DE-52 cellulose, but not the cation exchange resin CM-52 cellulose. As shown in Fig. 1, DE-52 chromatography can readily be used to separate the Cr-peptide complex from free Cr3+ and peptide. Free Cr3+ elutes quickly from the column while the Cr-peptide complex elutes much later (as indicated by the co-elution of Cr3+ and peptide). A small amount of apopeptide elutes just before the Cr-peptide complex. The Cr-peptide complex binds approximately 2 Cr3+ per peptide; the loss of 2 Cr3+ upon the lowering of pH explains the negative charge of the Cr-peptide complex at pH 5.6. Charge balance considerations are now in order. The EEEEGDD peptide at pH 5.6 or 7.4 should have a −6 charge (six negative charges from the aspartate and glutamate side chains and another negative charge from the carboxy terminus and one positive charge from the amino terminus). Addition of 4 Cr3+ ions should make the Cr-peptide complex at pH 7.4 have a positive charge. Assuming the Cr-peptide is neutral requires the presence of small anionic ligands. At pH 5.6 where only 2 Cr3+ are bound, the Cr-peptide complex should be neutral; however, as it is anionic, anionic ligands must be bound to the Cr centers. Given the preference of Cr3+ for hard ligands and the limited ligands available for binding to the Cr3+ centers under the experimental conditions, hydroxide ions would be an excellent candidate for the potential anionic ligands. Precipitation occurs even if Cr3+ is added to 5 mg of peptide in 1 L of buffer; given this occurs even at this dilute concentration suggests that the Cr-peptide complex is quite insoluble, opposed to Cr3+ and the peptide forming Cr-bridged aggregates that precipitate from solution.
Purification of the Cr-peptide by DE-52 chromatography. Reaction mixture containing 4 mM CrCl3, 1 mM peptide, and 20 mM MES (pH 5.6) in a total volume of 1.0 ml was incubated for 7 h. The mixture was applied to a column of DE-52 cellulose (1.5 × 5 cm) pre-equilibrated with 20 mM MES (pH 5.6). The column was eluted with the same buffer (14 ml) and then eluted with linear gradient of NaCl from 0 to 1.0 M in 20 mM MES pH 5.6. Fractions (0.7 ml) were collected, and Cr (closed circle) and peptide (open square) concentrations were measured
Spectroscopic studies
Infrared spectroscopy
In the present experiment, the H/2H exchanged heptapeptide was dissolved in 2H2O. For all but one sample, the pH of the solutions was adjusted to 4.3 by the addition of NaO2H. The solutions were incubated for 4 h and were then allowed to incubate another further 4 h with and without 4 equivalents of Cr3+ as CrCl3. IR spectra were collected for the peptide in 2H2O, peptide in 2H2O (pH 4.3), and peptide and CrCl3 in 2H2O (pH 4.3). Figure 2a shows spectrum of H/2H exchanged peptide in 2H2O solution. When the peptide is simply dissolved in 2H2O, the resulting pH is well less than 3. Absorption peaks were observed at approximately 1715, 1673, 1654, 1460, and 1402 cm−1. The band at ~1715 cm−1 is assigned to carbonyl stretch (ν C=O) of deuterated carboxyl groups. The band at 1402 cm−1 is the symmetric carboxyl stretch. The difference in wavenumbers between the symmetric and asymmetric stretches (~310 cm−1) is as expected for carboxylic acids. Thus, all the carboxylate groups exist as the carboxylic acids. This can be confirmed using the previously published extinction coefficients for vibrations for the protonated amino- and carboxyl-terminal groups and protonated carboxyl groups of aspartate and glutamate over the range 1450–1800 cm−1 [22], the calculated spectrum has an intense feature at ~1705 cm−1 resulting from the summation of the intensities of the asymmetric C=O stretches of the aspartic acid and glutamic acid side chains and carboxy-terminal carboxylic acid. Prominent amide I and amide II′ bands were observed at 1673 and 1654 cm−1 and 1460 cm−1, respectively. As 4/7ths of the peptide is comprised of consecutive glutamic acid residues, a comparison of these features with random coil and alpha-helical polyglutamate would appear in order. The amide I and II′ vibrations for alpha-helical polyglutamate are at 1649 cm−1 and 1553 and 1520 cm−1, respectively, while these bands for random coil polyglutamate are at 1651 and 1551 cm−1, respectively [23]. Thus, the peptide conformation appears to be distinctly different from either polyglutamate conformation, particularly as the position of the amide II bands (1460 cm−1) differs substantially from those of polyglutamate.
Figure 2b shows spectra for H/2H exchanged peptide adjusted to pH 4.3 by NaO2H and incubated with or without CrCl3. The peptide incubated without Cr3+ displayed absorption peaks at 1568 and 1406 cm−1 that can be assigned as to the antisymmetric and symmetric carboxyl stretch, respectively, of the dissociated carboxylate form of carboxyl groups. The major contributor to the peak at 1568 cm−1 is the glutamate carboxylate whose asymmetric stretch is at ~1567 cm−1 in 2H2O and in the range 1557–1575 in proteins [22]. The difference in wavenumbers between the asymmetric and symmetric stretches (~162 cm−1) is as expected for ionic carboxylates [24]. This was again confirmed using the previously published extinction coefficients for vibrations for the protonated amino-terminal group and deprotonated carboxyl-terminal group and deprotonated carboxyl groups of aspartate and glutamate over the range 1450–1800 cm−1 [22]; the calculated spectrum has an intense feature at ~1565 cm−1 resulting from the summation of the intensities of the asymmetric C=O stretches of the aspartate and glutamate acid side chains and the carboxy-terminal carboxylate. The spectrum for peptide incubated with Cr3+ showed large decrease in the intensity of the band at 1568 cm−1 and a new band at ~1711 cm−1. The band at 1711 is, thus, assigned to the asymmetric group of a carboxylate group bound to a Cr3+ ion. Clearly, the carboxylate groups of aspartate, glutamate, and potentially the N-terminus are binding Cr; in fact, the almost complete disappearance of the band at 1568 cm−1 suggests nearly all (if not all) the carboxylate moieties are involved in Cr binding. The symmetric carboxylate stretch from the peptide also decreased in intensity, indicating the binding of Cr3+ to the carboxylate groups. The bands from amide I and II’ were little affected by binding of Cr3+. A more specific assignment can be made. At this pH, protonation of the carboxylate groups would be extremely unlikely; consequently, the only type of asymmetric carboxylate stretch at this high energy would be from a carboxylate bound to Cr3+ in a unidentate fashion (i.e., bound via a single oxygen of the carboxylate moiety), predicted to be at ~1700 cm−1 [25]. A broad increase in absorbance is found between 1300 and 1400 cm−1. Given the weak intensity of the band at ~1710 cm−1, bands from carboxylate moieties bound in other modes may be present, but overlapping to such an extent that they cannot be distinctly observed. Surprisingly the amide stretches are virtually unaffected by the addition of Cr.
When the pH of the peptide solutions was further adjusted to 5.3 and 6.4, the intensity of the asymmetric carboxylate stretch at ~1568 cm−1 for the peptide increases in intensity. Additionally, the intensity of the amide I band at ~1460 cm−1 increases at pH 5.3, but decreases at pH 6.4. The addition of Cr3+ again results in the disappearance of the free carboxylate asymmetric and symmetric stretches from the free peptide and the appearance of a new asymmetric carboxylate stretch at ~1710 cm−1. Thus, Cr3+ binds to most, if not all of the carboxylates; some of the carboxylate are bound in a unidentate fashion as described above for pH 4.3. The intensity of the amide band at 1460 cm−1 increases upon Cr binding at pH 6.4. The relatively small changes in the amide bands are surprising. One would have anticipated that the free peptide with seven negatively charged carboxylate ligands would have a very fluxional and random structure from the repulsion of the negatively charged groups, while binding of 2–4 equivalents of substitutionally inert Cr3+ ions would lock the peptide into a rigid structure.
Electronic spectroscopy
The ultraviolet spectrum of the EEEEDGG peptide is essentially featureless between 250 and 350 nm (Fig. 3). The UV spectrum of LMWCr contains a band at ~260 nm [7], suggesting that this feature does not arise from the contiguous peptide component of LMWCr. This feature had been tentatively assigned to a disulfide bond [26], which would be consistent with the absence of such a band in the EEEEGDD peptide.
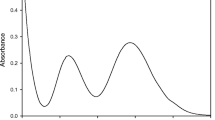
The electronic spectrum of a mixture containing 4 equivalents of CrCl3 per EEEEGDD peptide in 20 mM MES buffer (pH 5.6) was collected at several time points over a 7 h period (Fig. 4). Similar experiments were performed in the absence of peptide (Figure S1). When dissolved in water, CrCl3 (actually trans-[CrCl2(H2O)4]+) hydrolyzes via [CrCl(H2O)5]2+ to the hexaaquo species that can subsequently undergo slow hydrolysis to hydroxo-bridged multinuclear species. The ultraviolet/visible spectrum of a solution of CrCl3 in water 30 min after dissolution has maxima at approximately 420 and 587 nm; based on the reported spectra of [CrCl2(H2O)4]+, [CrCl(H2O)5]2+, and [Cr(H2O)6]3+ [27], this solution is a mixture of the last two species with [Cr(H2O)6]3+ being the predominate species. The presence of three isobestic points in the spectra over time from 30 min to 6 h (Supplemental Fig. 1) indicates that only two major species, [CrCl(H2O)5]2+ and [Cr(H2O)6]3+ are probably present over this time window (hydrolysis to multinuclear hydroxo species can also be occurring). After 6 h, the spectrum is that of [Cr(H2O)6]3+, with maxima at 407 and 573 nm; continued hydrolysis to hydroxide-containing species would result in shifts of the maxima to higher wavelengths.
These spectra allow the effects of the hydrolysis of CrCl3 to be contrasted to those from the binding of chromic ions to the peptide. In the presence of the peptide as a function of time, the visible absorption bands at ~420 and 580 nm shift slightly to lower wavelengths to 414 and to 574 nm, respectively, representing a mixture of bound and unbound Cr. The higher energy band decreases slightly in intensity with time, while the lower energy band increases appreciably in intensity. This behavior differs appreciably from that associated with the hydrolysis of [CrCl2−x (H2O)4+x ]+, indicating that Cr3+ is binding to the peptide. The presence of two isobestic points in the spectra suggests that primarily two species, [Cr(H2O)6]3+ and peptide bound Cr, are present and that [Cr(H2O)6]3+ is probably the species that initially binds to the peptide under these conditions. The spectra suggest that Cr binding to the peptide is complete after 7 h; at later time points changes from the hydrolysis of the free Cr3+ can be observed (as well as in the spectra in the absence of peptide), and the resulting spectra no longer cross at the isobestic points.
Figure 5 displays the electronic spectrum for the Cr(III)-peptide isolated by size exclusion chromatography at pH 5.6. The spectrum is comprised of two distinct features with visible maxima at 412 nm and 566 nm, which are readily assigned to the d → d transitions for an octahedral ligand field 4A2g → 4T1g and 4A2g → 4T2g, respectively. The ε values for the two maxima, 34 and 39 M−1 cm−1, respectively, are consistent with assignment to d → d transitions. The lower energy transition corresponds to the ligand field splitting parameter 10Dq and has an energy of 1.77 × 104 cm−1, suggesting all or nearly all oxygen coordination about the chromic centers. The Racah parameter B can be calculated from the energies of the two maxima to be 675 cm−1 [28]. In the region 670–710 nm, a broad but weak shoulder is present in the spectrum; this feature is composed of several very weak absorptions (~3 to 6 M−1 cm−1) resulting from formally forbidden spin-flip transitions. These transitions can gain intensity from exchange coupling in multinuclear Cr3+ assemblies, particularly when bridged by oxo or hydroxo ligands [28–32]; however, the intensities of the transitions for this species do not provide conclusive evidence for the existence of coupling between the chromic centers.
The visible spectrum of the Cr-peptide complex at pH 5.6 is not surprisingly relatively similar to the spectrum of LMWCr (at pH 6.5) [7] as both possess pseudo-octahedral fields of predominately, if not solely oxygen-based, ligands about their chromic centers. The spectrum of LMWCr possesses maxima corresponding to the two d → d transitions at 576 and 414 nm, giving a B value of 847 cm−1, and possesses a shoulder corresponding to the spin-flip transitions at 680–700 nm [27].
NMR spectroscopy
Using a combination of one- and two-dimensional NMR experiments and multiple solvents (2H2O, H2O/2H2O (90:10), and d6-DMSO), all the 1H signals from the peptide can be assigned (Scheme 1). The addition of paramagnetic Cr3+ ions (S = 3/2) should result in dramatic shifts and broadening of signals arising from atoms binding to or in close proximity to the chromic centers. This could allow moieties involved in Cr3+ binding to be identified. However, the addition of Cr3+ to the peptide between pH 5.6 and 7.4 results in the nearly complete loss of intensity from paramagnetic broadening of all the 1H signals from the free peptide. Only the signals from a trace of free peptide (that are broadened in the presence of Cr3+ ions in the solution) can be observed, even on instruments with magnetic fields as low as 360 MHz.
EPR spectroscopy
EPR spectra were collected on the Cr-peptide complex formed at pH 5.6 after isolation by size exclusion chromatography. Figure 6 displays the spectrum of the Cr-peptide at 77 K. The spectrum is composed of a broad signal at g = 1.98 (ΔH pp = 36.2 mT) and weak signal at g = 5.17. For comparison, the spectrum for CrCl3 under these conditions (Figure S2) shows an isotropic and sharp signal at g = 1.98 (ΔH pp = 15.5 mT). The spectrum clearly indicates that Cr binds to the peptide and that the environment about the Cr centers is less symmetric in the Cr-peptide complex. At pH 6.4, the EPR of the Cr-peptide complex is qualitatively similar to that at pH 5.6; however, the width of the signal at g = 2 (ΔH pp = 52.0 mT) increases, perhaps reflecting the binding of 4 rather than 2 Cr3+. The spectra at both pH’s are somewhat similar to that of bovine liver LMWCr, which binds 4 Cr3+ in an asymmetric multinuclear assembly, in that the latter has broad signal at g ~2 (ΔH pp = 80 mT) and weaker signal(s) at g ~5.2 [10].
Mass spectrometry
ESI mass spectra were obtained on aqueous solutions of the peptide to which 10 equivalents of Mn2+ (as MnCl .2 4 H2O), Fe2+ (as FeSO .4 7 H2O) and Fe3+ (as FeCl .3 6 H2O), Co2+ (as Co(NO3) .2 6 H2O), Cu2+ (as CuCl .2 2 H2O), Zn2+ (as Zn(NO3) .2 6 H2O), and Cr3+ (as Cr(NO3) .3 9 H2O) were added. Other than Cr, these represent the first row transition elements known to be essential for humans. As shown in Table 1, the peptide binds 1 or 2 equivalents of the metal ions under these conditions. For Fe3+, no features were observed in the mass spectrum. This has been observed previously in ESI MS for the addition of Fe3+ to peptides [15]; Signal intensities can fluctuate, and signals can be lacking. For the addition of trivalent chromic ions, a unique feature is observed corresponding to the addition of 2 Cr3+ and 2 O2− (Table 1; Fig. 7). Given the propensity of Cr3+ to form di-hydroxo-bridged dinuclear complexes, this suggests that this signal corresponds to a Cr-peptide complex containing a Cr(III)2(μ-OH)2 unit with the two hydrogen ions coming from deprotonation of carboxylate groups on the peptide. This is consistent with the results described above where the peptide bound two chromic ions at low pH.
Biological activity studies
Next, the biological properties of the heptapeptide were evaluated using both in vitro and in vivo models. For the in vivo model, the whole-body glucose tolerance test in wild-type FVB mice, wherein mice are challenged with a relatively high dose of glucose and the post-glucose challenge blood glucose levels are assessed, was used. For these studies the peptide was injected via the tail-vein just before intraperitoneal injection of glucose. This was a single dose study; and for these preliminary studies, the dose was chosen based on this laboratory’s previous studies using Cr [19, 20]. This dose was also expected to provide a supra-nutritional quantity of Cr. Given the concentration of Cr in the bloodstream is ~1 ppb and ~80 % of this Cr is bound to transferrin [33], then ~0.8 ppb is Cr associated with LMWCr, corresponding to a concentration of ~15 nM. Assuming Cr is evenly distributed in the body, the injected dose would give a concentration of ~60 nM, about 4 times greater than normal blood concentrations.
As shown in Fig. 8a, following glucose challenge in the vehicle-treated mice, blood glucose levels peaked at 15 min and returned to near-normal levels within 120 min. In contrast, in the absence of glucose challenge blood glucose remained stable over the period of the study. Interestingly, in the group of mice that received the peptide-Cr injection an enhancement in glucose tolerance was observed, with glucose disposal curves showing significantly lower blood glucose levels at 15, 30 and 60 min. The total AUC for blood glucose was significantly attenuated by the peptide treatment compared to the vehicle treatment (Fig. 8b). It is important to note that in these studies peptide was premixed with equimolar concentrations of CrCl3 prior to administration. Control experiments were also performed with CrCl3 and the peptide separately. As shown in Fig. 8b, both Cr and the peptide alone did also significantly reduce the post-glucose challenge AUC compared to vehicle treatment. However, the reduction in AUC accorded by the peptide-Cr combination was significantly greater than that observed with either the peptide or Cr. Again, there was no means to ascertain whether premixing equimolar concentrations of Cr with the peptide assures the formation of 1:1 complex without any spare reactants in the mixture. For these preliminary studies, the use of the 1:1 ratio was utilized to minimize precipitation of the Cr-peptide complex. In addition, it is of interest that the peptide by itself reduced the post-glucose challenge AUC to a significant extent compared to the vehicle treatment, suggesting that the peptide by itself may facilitate glucose clearance and/or do so by chelating with endogenous Cr. Further studies are necessary to address these questions.
Heptapeptide enhances glucose disposal in mice subjected to an intraperitoneal glucose tolerance test (IPGTT). a The peptide (50 µM), either alone or premixed with equimolar concentrations of Cr (as CrCl3) or CrCl3 was injected (100 µL) via tail-vein, immediately following which glucose (2 g/kg body mass) was administered intraperitoneally. Post-injection blood glucose levels were monitored (*p < 0.05 compared to the respective time points from the other two groups represented in the figure, n = 8–10 per group). b Area under curve ± SEM (*p < 0.05 compared to all other groups; # p < 0.05 compared to glucose + vehicle group; and @ p < 0.05 compared to peptide and Cr treated group
In addition, notable is that the peptide and Cr have beneficial effects while the intravenous administration of isolated LMWCr to rats has no effect on blood insulin, glucose, or cholesterol levels [34]. This is not surprising as LMWCr is responsible for clearing Cr from the body. LMWCr has a mean low tubular reabsorption rate and is thus rapidly cleared from the bloodstream by the kidneys for clearance in the urine [35]; thus, the synthetic peptide-Cr appears to be a better candidate for a potential pharmaceutical than the biological material.
To further assess the insulin-sensitizing effect of the peptide and the functional consequence of such effect cellular glucose uptake was assessed in myotubes rendered insulin resistant. Cultured myotubes were rendered insulin resistant by subjecting them to high-glucose media and then challenging them transiently with insulin (Fig. 9). High-glucose conditions significantly blunted insulin-stimulated myotube glucose uptake, which was partly reconciled by treating the cells with the peptide-chromium complex. As illustrated in the figure, even under low-glucose conditions the peptide complex augmented insulin-stimulated glucose uptake. Interestingly, neither chromium nor the peptide alone altered cellular glucose uptake in this model, suggesting the Cr-peptide complex is required. The molecular mechanisms by which chromium-peptide augments insulin-dependent glucose uptake is yet unclear.
Heptapeptide enhances insulin-stimulated glucose uptake under basal and high-glucose conditions. Quiescent myotubes were incubated with media containing normal (5 mM) or high (25 mM) glucose concentrations for 24 h in the presence or absence of the heptapeptide, Cr, or Cr-peptide complex at a concentration of 10 µM. After incubation cells were challenged with 5 nM insulin. Myotubes were incubated for 30 min with 2-deoxy-[3H]-glucose (0.2 μCi), and glucose uptake was assessed by scintillation counting. Data represent mean ± SEM (n = 3). *p < 0.01 compared with basal low-glucose conditions; † p < 0.01 compared with insulin-stimulated cells form low-glucose conditions; ‡ p < 0.01 compared with insulin-stimulated cells under with high-glucose conditions
LMWCr has previously been shown in the presence of insulin to increase the metabolism of glucose by isolated rat adipocytes without a change in the insulin requirement for half-maximal activity [36–38]; This lack of change in half-maximal insulin concentration suggests a role for LMWCr inside the insulin sensitive cells after insulin binds externally to the receptor [34]. The stimulation of glucose metabolism by LMWCr is proportional to the Cr content of the oligopeptide [39]. Thus, the ability of the Cr-heptapeptide complex to increase glucose uptake in response to insulin is consistent with previous studies on the biological material. The mechanism of action of the Cr-synthetic peptide complex and the biological material, hence, could potentially be the same. In vitro studies have suggested that LMWCr may bind to insulin-activated insulin receptor stimulating its kinase activity [11, 12]; while these results are consistent with those of the current study, the interaction of the Cr-peptide complex with other molecules involved in the insulin signaling cascade and related systems could also be consistent with the results of the current study. More definite conclusions will require further studies.
Conclusions
A variety of spectroscopic techniques and mass spectrometry reveal that the heptapeptide EEEEDGG, corresponding to the contiguous peptide component of LMWCr, binds multiple Cr3+ ions in octahedral environments; the carboxylate groups of the peptide are involved in Cr binding. Small anionic ligands, such as hydroxide and oxide that bridge the Cr centers probably also serve as ligands. Preliminary in vivo and in vitro studies suggest that the heptapeptide-chromium may enhance glucose disposal by augmenting insulin signaling and enhancing cellular glucose uptake. Further in-depth studies with various concentrations of peptide and Cr are necessary to fully characterize the biological role of the peptide.
Currently studies are underway to examine the ability of the heptapeptide when titrated with varying amounts of Cr3+ and other biologically relevant metal ions to activate members of the insulin signaling pathway in vitro and in vivo and to characterize the resulting metal-peptide complexes and establish whether Cr is unique in its interaction with the peptide. Additionally, efforts to solubilize the Cr-peptide complex at neutral pH so that its structure can be determined are also underway.
Abbreviations
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under curve
- DTGS:
-
Deuterated triglycine sulfate
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- IPGTT:
-
Intravenous glucose tolerance test
- LMWCr:
-
Low-molecular-weight chromium-binding substance
- MES:
-
2-(N-morpholino)ethanesulfonic acid
- PMSF:
-
Phenylmmethylsulfonyl fluoride
- RIPA:
-
Radioimmunoprecipitation assay
References
Vincent JB (2003) The bioinorganic chemistry of chromium. Wiley, Chichester
Vincent JB (2010) Chromium: celebrating 50 years as an essential element? Dalton Trans 39:3787–3794
Vincent JB, Love S (2012) The binding and transport of alternative metals by transferrin. Biochim Biophys Acta 1820:361–378
Di Bona KR, Love S, Rhodes NR, McAdory D, Halder Sinha S, Kern N, Kent J, Strickland J, Wilson A, Beaird J, Ramage J, Rasco J, Vincent JB (2011) Chromium is not an essential element for mammals: effects of a “low-chromium” diet. J Biol Inorg Chem 16:381–390
Hua Y, Clark S, Ren J, Sreejayan N (2012) Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem 23:313–319
Yamamoto A, Wada O, Ono T (1987) Isolation of a biologically active form of chromium low-molecular-mass chromium compound from rabbit liver. Eur J Biochem 165:627–631
Davis CM, Vincent JB (1997) Isolation and characterization of a biologically active form of chromium oligopeptide from bovine liver. Arch Biochem Biophys 339:335–343
Hatfield MJ, Gillispie S, Chen Y, Li Z, Cassady C, Vincent JB (2006) Low-molecular-weight chromium-binding substance from chicken liver and American alligator liver. Comp Biochem Physiol Part B 144:423–431
Chen Y, Watson HM, Gao J, Shinha SH, Cassady CJ, Vincent JB (2011) Characterizing the organic component of low-molecular-weight chromium-binding substance and its binding of chromium. J Nutr 141:1225–1232
Jacquamet L, Sun Y, Hatfield J, Gu W, Cramer S, Crowder MW, Lorigan GA, Vincent JB, Latour J-M (2003) Characterization of chromodulin by X-ray absorption and electron paramagnetic spectroscopies and magnetic susceptibility measurements. J Am Chem Soc 125:774–780
Davis CM, Vincent JB (1997) Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry 36:4382–4385
Davis CM, Royer AC, Vincent JB (1997) Synthetic multinuclear chromium assembly activates insulin receptor kinase activity: functional model for low-molecular-weight chromium-binding substance. Inorg Chem 36:5316–5320
Vincent JB (2000) Elucidating a biological role for chromium at a molecular level. Acc Chem Res 33:503–510
Undenfriend S, Stein S, Bohlen P, Dairman W, Leimgruber W, Weigele M (1972) Fluorescamine: a reagent for assay of amino acids, peptide, proteins and primary amines in the picomole range. Science 178:871–872
Feng C, Commodore JJ, Cassady CJ (2015) The use of chromium(III) to supercharge peptides by protonation at low basicity sites. J Am Soc Mass Spectrom 26:347–348
Krezel A, Bal W (2004) A formula for correlating pK a values determined in D2O and H2O. J Inorg Biochem 98:161–166
Hua Y, Zhang Y, Dolence J, Shi GP, Ren J, Nair S (2013) Cathepsin K knockout mitigates high-fat diet-induced cardiac hypertrophy and contractile dysfunction. Diabetes 62:498–509
Slowinski EW (1979) Calculus with analytical geometry, 2nd edn. Prindle, Weber, and Schmidt, Boston, pp 260–262
Sreejayan N, Dong F, Kandadi MR, Yang XP, Ren J (2008) Chromium alleviates glucose intolerance, insulin resistance, and hepatic ER stress in obese mice. Obesity 16:331–1337
Dong F, Kandadi MR, Ren J, Sreejayan N (2008) Chromium (d-phenylalanine)3 supplementation alters glucose disposal, insulin signaling, and glucose transporter-4 membrane translocation in insulin-resistant mice. J Nutr 138:1846–1851
Panzhinskiy E, Lapchak PA, Topchiy E, Lehmann TE, Ren J, Nair S (2014) Novel curcumin derivative CNB-001 mitigates obesity-associated insulin resistance. J Pharmacol Exp Ther 349:248–257
Venyaminov SY, Kalnin NN (1990) Qualitative IR spectrophotometry of peptide compounds in water (H2O) solutions. I. Spectral parameters of amino acid residue absorption bands. Biopolymers 30:1243–1257
Venyaminov SY, Kalnin NN (1990) Qualitative IR spectrophotometry of peptide compounds in water (H2O) solutions. II. Amide absorption bands of polypeptides and fibrous proteins in α-, β-, and random coil conformations. Biopolymers 30:1259–1271
Deacon GB, Phillips RJ (1980) Relationship between the carbon–oxygen stretching frequencies of carboxylate complexes and the type of carboxylate coordination. Coord Chem Rev 33:227–250
Tackett JE (1989) Characterization of chromium(III) acetate in aqueous solution. Appl Spectroscopy 43:490–499
Levina A, Codd R, Dillion CT, Lay PA (2003) Chromium in biology: toxicology and nutritional aspects. Prog Inorg Chem 51:145–644
Elving PJ, Zemel B (1957) Cr(III) aquo and chloride complexes. J Am Chem Soc 79:1281–1285
Blake AB, Yavari A, Hatfield WE, Sethulekshmi CN (1985) Magnetic and spectroscopic properties of some heterotrinuclear basic acetates of chromium(III), iron(III), and divalent metal ions. J Chem Soc Dalton Trans 2509–2520. doi:10.1039/DT9850002509
Dubicki L, Day P (1972) The electronic spectrum of trinuclear Cr(III) acetate. Inorg Chem 11:1868–1875
Harton A, Nagi MK, Glass MM, Junk PC, Atwood JL, Vincent JB (1994) Synthesis and characterization of symmetric and unsymmetric oxo-bridged trinuclear chromium benzoate complexes: crystal and molecular structure of [Cr3O(O2CPh)6(py)3]ClO4. Inorg Chim Acta 217:171–179
Ellis T, Glass MM, Harton A, Folting K, Huffman JC, Vincent JB (1994) Synthetic models for low-molecular-weight chromium-binding substance: synthesis and characterization of oxo-bridged tetranuclear chromium(III) assemblies. Inorg Chem 33:5522–5527
Chaudhuri P, Winter M, Kuppers H-J, Wieghardt K, Nuber B, Weiss J (1987) General route to μ-hydroxo-bis(μ-acetato)-bridged heterometal dinuclear complexes. Syntheses, magnetic and redox properties, electronic spectra, and molecular structures of the Cr(III)–Co(II) and Cr(III)–Fe(II) species. Inorg Chem 26:3302–3310
Hopkins LL Jr, Schwarz K (1964) Chromium(III) binding to serum proteins, specifically siderophilin. Biochim Biophys Acta 90:484–491
Sun Y, Mallya K, Ramirez J, Vincent JB (1999) The biomimetic [Cr3O(O2CCH2CH3)6(H2O)3]+ decreases plasma cholesterol and triglycerides in rats: towards chromium-containing therapeutics. J Biol Inorg Chem 4:838–875
Wada O, Wu GY, Yamamoto A, Manabe S, Ono T (1983) Purification and chromium-excretory function of low-molecular-weight chromium-binding substances from dog liver. Environ Res 32:228–239
Yamamoto A, Wada O, Suzuki H (1988) Purification and properties of biologically active chromium complex from bovine colostrum. J Nutr 118:39–45
Yamamoto A, Wada O, Suzuki H (1987) Separation of biologically active chromium complex from cow colostrum. Tohoku J Exp Med 152:211–219
Vincent JB (1994) Relationship between glucose tolerance factor and low-molecular-weight chromium-binding substance. J Nutr 124:117–118
Yamamoto A, Wada O, Manabe S (1989) Evidence that chromium is an essential factor for biological activity of low-molecular-weight Cr-binding substance. Biochem Biophys Res Commun 163:189–193
Acknowledgments
The authors would like to thank Alec A. Cruce and Prof. Michael K. Bowman for collecting EPR spectra. Funding was provided by the Research Grants Committee and Office of Research at The University of Alabama and the University of Wyoming.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arakawa, H., Kandadi, M.R., Panzhinskiy, E. et al. Spectroscopic and biological activity studies of the chromium-binding peptide EEEEGDD. J Biol Inorg Chem 21, 369–381 (2016). https://doi.org/10.1007/s00775-016-1347-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-016-1347-x