Abstract
The interaction with nitric oxide (NO) is an important aspect of the biological activity of vitamin B12 (Cbl). Whereas the formation of nitroxylcobalamin (CblNO) via the binding of NO to reduced CblCo(II) has been studied in detail before, the possible intracellular formation of CblNO via reduction of nitrocobalamin (CblNO2) is still questionable. To study this further, spectroscopic and kinetic studies on the reaction of CblNO2 with the intracellular antioxidant ascorbic acid (Asc) were performed in aqueous solution at the physiological pH of 7.2. It was found that the redox pathway of this reaction requires anaerobic conditions as a result of the rapid re-oxidation of reduced CblCo(II). In the studied system, both CblOH2 and CblNO2 are reduced to CblCo(II) by ascorbate at pH 7.2, the CblOH2 complex being two orders of magnitude more reactive than CblNO2. Clear evidence for redox cycling between CblOH2/CblNO2 and CblCo(II) under aerobic conditions was observed as an induction period during which all oxygen was used prior to the formation of CblCo(II) in the presence of an excess of ascorbate. No evidence for the intermediate formation of CblNO or NO radicals during the reduction of CblNO2 could be found.
Graphical Abstract
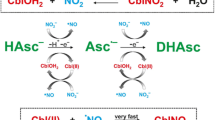
Nitrocob(III)alamin can be reduced by ascorbic acid under physiological conditions. The products of the reaction are cob(II)alamin and nitrite ion. This reaction is ca. 200 times slower than the one involving aquacob(III)alamin.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitric oxide (NO) is a free radical that plays a dual role in mammalian cells depending on its concentration. High concentrations of NO and its metabolites could cause harmful effects on an organism [1]. Low concentrations of NO regulate a wide range of biological processes, such as smooth-muscle relaxation, neurotransmission or immune response [2–4]. Numerous studies showed that reactions of NO with transition metal complexes such as iron porphyrins play an important role in biological and environmental processes [5, 6]. Another interesting aspect is the interaction between NO and the macrocyclic Co(III) complex Vitamin B12 and its derivatives [7].
Vitamin B12 derivatives, also known as Cobalamins (Cbl), are a group of cobalt containing complexes that play a role as cofactors for many enzymes [8]. Vitamin B12 is a six-coordinate complex of which the equatorial positions are occupied by the corrin ring. The upper (β) axial position can be occupied by various ligands, e.g., CN in cyanocobalamin, water in aquacob(III)alamin or NO2 − in nitrocob(III)alamin (Fig. 1) [9]. The lower (α) position is occupied by dimethylbenzimidazole indirectly linked to the corrin chelate. Reactions of vitamin B12 with NO play an important role in modifying the physiological action of vitamin B12. Up to now it was shown that there is no direct interaction between NO and aquacobalamin at physiological pH [10]. However, the reduced form of aquacobalamin binds NO rapidly and reversibly to form nitroxylcobalamin, CblNO: [7]
Nitroxylcobalamin is oxygen sensitive; in the presence of air it rapidly oxidizes to nitrocobalamin CblNO2 [11]. Nitrocobalamin is one of the naturally occurring forms of cobalamin and was isolated from biological samples, thus it is stable in biological systems [12, 13].
The goal of this study is to investigate the possible intracellular production of CblNO by reduction of CblNO2. So far the reaction of CblNO2 with the strong nucleophile and reducing agent glutathione (GSH) was studied [14]. According to the authors, the product of this reaction is glutathionylcobalamin CblSG, but not nitroxylcobalamin CblNO as expected. In this study, we used ascorbic acid as a strong reducing agent, known as intracellular antioxidant, to study the reduction of CblNO2 to CblNO (Scheme 1). We report here our findings on the kinetics and mechanism of the reaction between CblNO2 and ascorbate (Asc) at physiological pH.
Experimental section
Materials
Hydroxocobalamin hydrochloride (CblOH·HCl, ≥98 %) was obtained from Sigma. Sodium nitrite was purchased from LPPH (Poland). Ascorbic acid was obtained from Polfa Kraków (Poland). Tris buffer was obtained from Merck. All chemicals used throughout this study were of at least analytical reagent grade. Oxygen-sensitive solutions of Cbl(II) and NO were prepared and handled in gastight glassware or screw-cap cuvettes equipped with a silicone septum. Oxygen-free argon was used to deoxygenate the reactant solutions. Stock solutions of NO were prepared by saturation of a deoxygenated buffer solution with NO gas making use of vacuum line techniques. Oxygen-saturated solutions were prepared by bubbling oxygen through the solutions.
General methods
All solutions were prepared in de-ionized water (18.2 Ω Millipore). Tris buffers (0.01 and 0.1 M) were used to control the pH (adjusted with HClO4). Solutions of reduced cobalamin, Cbl(II), were prepared by chemical reduction of aquacobalamin with ascorbic acid or sodium formate under argon atmosphere. The identity and concentration of the reduced cobalamin Cbl(II) were determined by UV–Vis spectroscopy.
Measurements
UV–Vis spectra and kinetic data for slow reactions were recorded on Perkin Elmer Lambda 25 or Shimadzu UV 3600 spectrophotometers equipped with a thermostatted (25.0 ± 0.1 °C) cell holder (Perkin Elmer PTP-6 Peltier System). UV/Vis spectra and kinetic data for rapid reactions were recorded on an Applied Photophysics SX20 stopped-flow spectrophotometer equipped with a photodiode array detector in addition to a single wavelength detector. All kinetic experiments were performed under pseudo-first-order conditions at 25.0 ± 0.1 °C. All data were analyzed using Origin Lab version 9.0 software.
Kinetic measurements
The rates of the reactions between CblOH2 (8.5 × 10−5 M) and NO2 − were determined under pseudo-first-order conditions with excess of NaNO2 (8.5 × 10−4–8.5 × 10−3 M). Kinetic data for this reaction were obtained at pH 7.2 in Tris buffer (0.1 M) and recorded at 354 and 532 nm.
The rates of the reaction between CblNO2 (8.5 × 10−5 M) and Asc were determined under pseudo-first-order conditions with excess of Asc (8.5 × 10−4–2.6 × 10−2 M). Kinetic data for the reaction were obtained at pH 7.20 in Tris buffer (0.1 M). A small aliquot of concentrated NaNO2 (8.5 × 10−4 M) was added to the solution of CblOH2, followed by a small aliquot of concentrated ascorbate added to the solution of CblNO2. The absorbances at 354 and 532 nm were recorded as a function of time.
The rates of the reaction between CblNO2 (8.5 × 10−5 M) and Asc for varying concentrations of NO2 − at pH 7.20 (Tris buffer, 0.1 M) were determined under pseudo-first-order conditions with excesses of Asc and NO2 −. A small aliquot of concentrated NaNO2 (1.7 × 10−4–4.3 × 10−3 M) was added to the solution of CblOH2 (8.5 × 10−5 M), followed by a small aliquot of concentrated ascorbate (2.2 × 10−2 M) added to the solution of CblNO2. The absorbances at 354 and 532 nm were recorded as a function of time.
pH measurements were carried out using a HI 221 (Hanna Instruments) pH meter equipped with an AmpHel glass electrode. The electrode was filled with a 3.5 M KCl + AgCl solution.
Results and discussion
Studies on the reaction between CblOH2 and NO2 −
The formation of nitrocobalamin (CblNO2) was initiated by addition of NaNO2 to a solution of aquacobalamin (CblOH2) at pH = 7.2 (25 °C, 0.1 M Tris buffer) under aerobic conditions. Rapid changes in the UV–Vis spectrum were observed and the reaction solution changed color from red to pink, indicating the formation of nitrocobalamin (CblNO2) according to the reaction shown in Scheme 2.
Figure S1 (Supporting Information) shows typical UV–Vis spectra recorded for the formation of CblNO2 in the reaction between CblOH2 and excess NO2 −. Five isosbestic points were observed at 345, 353, 422, 472 and 555 nm. The inset in Figure S1 shows the initial (λ max = 275, 351, 412, 497 and 525 nm) and final spectra (λ max = 272, 354, 413, 502 and 532 nm) of CblOH2 and CblNO2, respectively. Figure S2 (Supporting Information) shows the plot of absorbance at 310 nm as a function of time and the best fit to a first-order rate equation, giving the observed rate constant k obs = 1.126 ± 0.001 s−1.
The kinetics of the reaction in Scheme 2 was studied under pseudo-first-order conditions with NO2 − in at least tenfold excess using stopped-flow spectroscopy. A plot of k obs versus the total nitrite concentration at pH 7.20 is linear with a zero intercept (Figure S3, Supporting Information). A linear fit of the data gives a slope (k 1) of (1.29 ± 0.01) × 103 M−1 s−1 at 25 °C. The value of k 1 is in good agreement with values reported in the literature, viz. (1.20 ± 0.02) × 103 and 1.0 × 103 M−1 s−1, respectively [14, 15].
To obtain the equilibrium constant K 1 (=k 1/k −1) from kinetic data for the reversible formation of CblNO2, k −1 was determined independently from the reaction between CblNO2 and H+ as a function of H+ concentration, i.e., via an acid-catalyzed aquation reaction of CblNO2. The reactions were performed at equimolar concentrations of NO2 − and CblOH2 to protonate free nitrite at pH < 2 (formation of HONO, pK a ≈ 3.2) and induce the back reaction in Scheme 2. Typical spectral changes and a kinetic trace for this reaction are shown in Figure S4 (Supporting Information). The spectral changes observed are exactly the opposite than those observed for the forward reaction in Figure S1. A plot of the observed first-order rate constant as a function of H+ concentration (Figure S5, Supporting Information) shows that k obs increases linearly with increasing H+ concentration and exhibits a significant intercept, i.e., k obs = k a + k b(H+). This behavior indicates that both free and coordinated nitrite can be protonated to induce an acid-catalyzed aquation of Cbl of which the intercept (k a) presents the spontaneous process, i.e., k −1 in Scheme 2. A linear fit of the data in Figure S5 gave k a (=k −1) = (1.42 ± 0.03) × 10−2 s−1 and k b = 0.53 ± 0.01 M−1 s−1 at 25 °C. The value of k −1 is very close to that reported recently by Brasch and co-workers [14], who obtained the value in an indirect manner from the reaction of CblNO2 with glutathione, and from the spontaneous aquation of CblNO2. Their values are in the range (1.48–1.75) × 10−2 s−1 at 25 °C depending on the way the rate constant was determined. Based on our data, K 1 = (1.29 ± 0.01) × 103/(1.42 ± 0.03) × 10−2 = (9.1 ± 0.3) × 104 M−1 at 25 °C, which is close to the different values reported in the literature for the complex-formation constant of CblNO2, viz. 8.5 × 104 M−1 according to Walker et al. [14] and 2.2 × 105 M−1 as reported by Marques et al. [11].
It is also possible to determine the thermodynamic value of K 1 using a spectrophotometric technique as described by Grynkiewicz et al. [16]. The reaction between CblOH2 and NaNO2 at concentration ratios between 0.1 and 5 at pH = 7.2 was studied and the UV–Vis spectra before and after the reaction were recorded as a function of nitrite concentration. Figure S6 (Supporting Information) shows the UV–Vis spectra of the reaction product for different concentrations of NO2 −.
The spectra reported in Figure S6 exhibit clean isosbestic points. Two wavelengths were selected where the absorbance of the product of the reaction increases (505 nm) and decreases (520 nm). From the corresponding absorbance values, the parameters R min, R max and R were calculated using Eqs. (2)–(4).
In principle, K 1 can be estimated from the intercept of a plot of ln(NO2 −)free versus ln \( \frac{{R - R_{ {\rm min} } }}{{R_{ {\rm max} } - R}} \) according to Eq. (5) and shown in Figure S7 (Supporting Information). The free nitrite in solution at equilibrium was calculated from the recorded spectra which allowed the estimation of the concentrations of CblOH2 and CblNO2 at equilibrium. Due to the relatively high value of K 1, this method could only be applied to the data points related to the concentration ratio of NaNO2 and CblOH2 ranging from 1.5 to 4.0, i.e., close to the optimal formation of CblNO2 where measurable spectral changes occur and some free nitrite is still present in solution to maintain the equilibrium.
The intercept of the plot in Figure S7 presents lnK 1 = −12.0 ± 0.5 according to Eq. (5), from which it follows that K 1 = (2 ± 1) × 105 M−1. This value is in close agreement with the values reported in the literature [14, 15], viz., 2.2 × 105 and 8.4 × 104 M−1, respectively, and especially with the kinetic value of (9.1 ± 0.3) × 104 M−1 reported above as found in this study.
Studies on the reaction between CblNO2 and ascorbate at pH = 7.2
Spectroscopic studies
To initiate the reaction, the solution of ascorbate (Asc) was added to the solution of CblNO2 at pH = 7.20 (25 °C, 0.1 M Tris buffer) under anaerobic conditions. CblNO2 was prepared as described in the previous section. Note that the abbreviation for ascorbate (Asc) refers to a mixture of the ascorbate mono-anion (HAsc−) and ascorbate di-anion (Asc2−), for which the pK a2 value is 11.3. At the selected pH, the strongest reducing species is Asc2−, it reacts five orders of magnitude faster than the HAsc− ion [17]. Changes in the UV–Vis spectrum were observed while the reaction solution changed color from red to orange. Figure 2 shows the UV–Vis spectral changes observed upon addition of Asc to CblNO2 (λ max = 354, 413 and 532 nm), indicating that CblNO2 is converted to reduced cobalamin, CblCo(II) (λ max = 312, 407 and 476 nm), with isosbestic points at 335, 372, 487 and 571 nm. The insets in Fig. 2 show the corresponding absorbance vs. time traces at 354 and 532 nm.
Spectral changes observed for the reaction between CblNO2 (7.6 × 10−5 M, obtained by mixing CblOH2 and NO2 −, concentration ratio NO2 −/CblOH2 = 40) and Asc (2.10 × 10−2 M) at pH = 7.2 (25 °C, 0.1 M Tris buffer, Ar atmosphere). Spectra were recorded every 2 min. Inset: plots of absorbance at 354 (left) and 532 nm (right) versus time
The kinetic traces showed a short induction period during the reduction of CblNO2 to CblCo(II), which could be related to the presence of traces of oxygen present in solution (see next section). The data fitted well to a first-order rate equation with observed rate constants, k obs = (1.00 ± 0.02) × 10−3 and (0.95 ± 0.02) × 10−3 s−1, respectively. A possible influence of the Tris buffer on the reaction was checked by repeating the reaction shown in Fig. 2 at 1 M instead of 0.1 M Tris buffer. The spectral changes and calculated rate constant (see Figure S8 Supporting Information) were found to be identical to that shown and obtained from Fig. 2.
Role of oxygen
To obtain further information on the influence of oxygen on the reaction between CblNO2 and Asc, the reaction was performed under 50 and 100 % oxygen-saturated conditions. Reactions were initiated by addition of Asc to a solution of CblNO2 at pH = 7.20 (25 °C, 0.1 M Tris buffer). Spectral changes observed for these reactions are shown in Figs. 3 and 5a. Figures 4a and 5b show plots of the absorbance at 532 nm as a function of time.
UV–Vis spectra recorded for the reaction between CblNO2 and Asc at pH = 7.2 between 85 and 130 min (a) and between 160 and 250 min (b) after initiation of the reaction. Experimental conditions: CblNO2 (7.6 × 10−5 M) was obtained by mixing CblOH2 with NaNO2 solution (concentration ratio NO2 −/CblOH2 = 40), 2.1 × 10−2 M Asc, 25 °C, 0.1 M Tris buffer, 50 % saturated O2. Spectra were recorded every 2 min
UV–Vis spectra (a) and the kinetic plot at 532 nm (b) for the reaction between CblNO2 and Asc. Conditions are as given for Fig. 3 except for 100 % oxygen saturation. Note the expanded absorbance scale used in the plot (b)!
Spectral changes observed under 50 % oxygen-saturated conditions (Figs. 3, 4a) suggest that after addition of Asc to the solution of CblNO2, several steps are observed. During the first 85 min no spectral changes occur, suggesting an induction period for the studied reaction. This suggests that CblNO2 is reduced to Co(II)Cbl and/or CblNO, but since these species are very oxygen sensitive they are rapidly re-oxidized to CblNO2. In addition, oxygen can also be consumed by the oxidation of ascorbate to dehydroascorbate (DHA) as indicated in reaction (6) [13].
The spectral changes observed after 85 min (Fig. 3a) show an absorbance decrease at 354 and 532 nm and an increase at 476 nm, suggesting that no oxygen is left in the solution since these changes are characteristic for the formation of Cbl(II). After 160 min, the reaction is still not complete, but from this point on the absorbance decreases at 476 nm. These spectral changes observed from 160 to 250 min (Fig. 3b) suggest the partial destruction of the cobalamin derivatives, presumably caused by hydrogen peroxide formed in reaction (6) [13], and the formation of CblCo(II). When the reaction shown in Fig. 4a is stopped after 2.5 h and oxygen is bubbled through the solution, the induction period shows up again as shown in Fig. 4b due to the redox cycling described above. A similar observation was reported for a related system where oxygen also caused an apparent induction period [18]. Spectral changes observed for this reaction under 100 % oxygen-saturated conditions are shown in Fig. 5a. There are some spectral changes around 350 nm, but nothing significant in the visible range over a period of 260 min. Figure 5b shows that the absorbance change at 532 nm is practically zero during this time. These spectra again indicate that the reduced CblCo(II) is re-oxidized to CblNO2 as long as oxygen and an excess of nitrite are present in solution. The spectral changes observed around 350 nm are probably caused by the oxidation of Asc and the formation of H2O2 in the solution.
To summarize, these results imply that in the presence of oxygen, the reaction between CblNO2 and Asc shows an induction period during which CblCo(II) is re-oxidized to CblNO2 as long as oxygen is present in solution. Thus, the induction period depends on the oxygen concentration as seen from Figs. 4 and 5b. Once oxygen is used up, the same reaction as under anaerobic conditions is observed. This clearly demonstrates the redox cycling of the system in the presence of ascorbate and dioxygen as suggested in Scheme 1.
Kinetic studies
Kinetic data were collected for the reaction of CblNO2 with Asc as a function of the Asc and NO2 − concentrations. Experiments were initiated by addition of a small aliquot of concentrated NaNO2 (8.5 × 10−4 M) to a buffered CblOH2 solution (8.5 × 10−5 M) at pH = 7.20 (Tris buffer). When the reaction between CblOH2 and NaNO2 was complete, a small aliquot of concentrated Asc was added to the reaction solution. Control experiments showed that the observed rate constants in the absence and presence of oxygen differed significantly; the reaction in the presence of oxygen is significantly slower as the result of the redox-cycling referred to above. Hence, all experiments were carried out under anaerobic conditions (i.e., in Ar atmosphere).
A plot of k obs versus total Asc concentration at a fixed nitrite concentration is shown in Fig. 6. The results suggest that the observed rate constant reaches a limiting value at high Asc concentration. This is typical for an outer-sphere electron transfer reaction that proceeds via the rapid pre-equilibrium formation of an encounter complex, followed by the rate-determining electron transfer reaction that leads to the formation of CblCo(II) and dehydroascorbate (DHA) as shown in Eq. (7). The rate expression corresponding to the suggested reaction scheme is given in Eq. (8).
From a non-linear fit of the data in Fig. 6 to Eq. (8), k = (3.3 ± 0.3) × 10−3 s−1 and K = 106 ± 17 M−1 at 25 °C. At low concentration of Asc, Eq. (8) simplifies to k obs = kK(Asc), where kK represents the second-order rate constant for the overall electron transfer reaction, i.e., kK = 0.35 ± 0.09 M−1 s−1 at 25 °C. At high Asc concentration, Eq. (8) simplifies to k obs = k, i.e., the limiting value that can be reached in Fig. 6 at high Asc concentration is k = (3.3 ± 0.3) × 10−3 s−1 at 25 °C.
Further experiments were performed to study both the influence of free nitrite in the equilibrated solution on the observed rate constant and the mechanism of the reaction between CblNO2 and Asc. Thus, kinetic data were collected for the reaction of CblNO2 with Asc as a function of NO2 − concentration. Experiments were initiated by adding a small aliquot of concentrated NaNO2 solution (8.5 × 10−5–4.3 × 10−3 M) to a buffered CblOH2 solution (8.5 × 10−5 M) at pH = 7.20 (Tris buffer) under anaerobic conditions (Ar atmosphere). When the formation of CblNO2 was complete, a small aliquot of concentrated Asc (2.1 × 10−3 M) was added to the reaction solution. A low ascorbate concentration was selected to ensure that the reactions were run under conditions where k obs depends linearly on the ascorbate concentration, i.e., k obs = kK(Asc) as seen from Fig. 6 in the lower ascorbate concentration range.
In the proposed reaction Scheme 3, CblOH2 is in rapid equilibrium with CblNO2. On addition of Asc to the equilibrium mixture, both complexes can be reduced by Asc to form CblCo(II) and DHA. The overall rate of the process will depend on the Asc and nitrite concentrations. The nitrite concentration will control the contributions of CblOH2 and CblNO2 to the overall reduction process.
The experimental data are summarized in Fig. 7 and were fitted to Eq. (9). This equation is based on assigning different rate constants to the reduction of CblOH2 and CblNO2 by Asc and can be rewritten as shown in Eq. (9) and (10).
The free nitrite concentration in solution was estimated from the spectrum of the CblOH2/CblNO2 solution at low nitrite concentrations prior to the addition of ascorbate. A non-linear fit of the data in Fig. 7, keeping K 1 constant at 2 × 105 M−1 based on our spectrophotometric measurements, resulted in k 2 = 17.3 ± 0.4 M−1 s−1 and k 3 = 0.13 ± 0.01 M−1 s−1 at 25 °C. The value of k 2 strongly depended on the pre-selected value of K 1 since it presents the extrapolated value of k obs/Asc at the free nitrite concentration going to zero. For instance when K 1 = 3 × 105 M−1, a fit of the data in Fig. 7 results in k 2 = 25.0 ± 0.6 M−1 s−1 and k 3 = 0.13 ± 0.02 M−1 s−1. This value of k 2 indeed agrees exactly with the value determined directly for this reaction (see next section). Furthermore, the data clearly show that the reduction of CblNO2 by Asc is controlled by the free NO2 − concentration in solution. The reaction pathways shown in Scheme 3 correspond to the rate equation given in Eqs. (9) and (10).
These data indicate that at a low concentration of NO2 −, k obs(2)/(Asc) = k 2 and only the reduction of CblOH2 is observed. At high concentration of NO2 −, k obs(3)/(Asc) = k 3 and only the reduction of CblNO2 is observed. Under these conditions, k 3 = 0.13 ± 0.01 M−1 s−1 is approx. 150 (or 200) times smaller than k 2 = 17.3 ± 0.4 (or 25.0 ± 0.6) M−1 s−1, from which it follows that the reduction of CblNO2 by Asc contributes not more than 5–7 % to the overall process. The main reduction process proceeds via aquacobalamin and represents ca. 95 % of the reaction. A similar conclusion was reached by Brasch and co-workers for the substitution of nitrocobalamin by glutathione which clearly proceeded through the CblOH2 intermediate [14].
In summary, in the system described above, independent of the concentration of NO2 −, the reaction is dominated by reduction of CblOH2 by ascorbate. However, the value of k3 shows that the reduction of the CblNO2 complex is not negligible and can be of significant meaning. The question arose to which extent the spontaneous reduction of nitrite by ascorbate can contribute to the observations under the selected conditions. We, therefore, studied the spontaneous reduction of nitrite by ascorbate under the selected conditions using CblOH2 as a kinetic indicator for the concentration of nitrite in solution as a function of the reaction time. The selected concentrations were such that the formation kinetics of CblNO2 was two orders of magnitude faster than the reduction of CblOH2 by ascorbate based on the kinetic data reported in this study. The results in Fig. 8 indicate that the nitrite concentration did not decrease at all over a period of 3 h, which is much longer than any kinetic measurements performed on the reactions involving the reduction of CblNO2 by ascorbate.
The possible reduction of coordinated nitrite in CblNO2 by Asc was tested using FeII(edta) as a trap for NO, a likely reduction product of coordinated nitrite. In this reaction, the colorless FeII(edta) solution (under strict anaerobic conditions), black line in Fig. 9, reacts very rapidly with NO to form the green–brown FeII(edta)NO complex, red line in Fig. 9. The reaction of CblNO2 with Asc was carried out in the presence of an excess nitrite (6 mM) and 1 mM Fe(edta) [19]. The lack of spectral changes similar to those shown in Fig. 9 excluded the possible generation of NO as a reduction product of coordinated nitrite in the system. Additional negative evidence for the formation of NO was provided by EPR spectra recorded during the reaction, which showed no signal characteristic for the formation of NO radicals.
The NO2 − concentration in solution was also monitored during the reduction of CblNO2 by Asc as another way to gain information on the possible reduction of coordinated nitrite. The reaction was carried out for 3 h in the presence of an excess of nitrite, followed by the addition of a fresh portion of CblOH2 to the reaction mixture. The recorded spectra showed the reformation of CblNO2 but did not reveal clear evidence for a decrease in the concentration of nitrite in solution. Thus, all efforts to study the possible reduction of coordinated nitrite in CblNO2 by Asc failed. Future work will focus on studying this reaction under other reaction conditions, especially at lower pH.
To check the value of k 2 in a direct manner, the reaction between CblOH2 and Asc in the absence of nitrite was studied under the selected experimental conditions.
Studies on the reduction of CblOH2 by ascorbate at pH = 7.2
The reaction was initiated by the addition of Asc to a solution of CblOH2 at pH = 7.20 (25 °C, 0.1 M Tris buffer) under anaerobic conditions. Rapid changes in the UV–Vis spectrum were observed and the reaction solution changed color from red to orange. Figure 10a shows typical UV–Vis spectra recorded for the formation of CblCo(II) during the reaction between CblOH2 (4.3 × 10−5 M) and Asc (4.3 × 10−5 M). Isosbestic points were observed at 287, 334, 372, 487 and 570 nm. The kinetics of the reduction of CblOH2 was studied under pseudo-first-order conditions with Asc in at least tenfold excess using stopped-flow spectrophotometry. Figure 10b shows a plot of absorbance at 351 nm versus time, and the best fit to the first-order rate equation, which resulted in the observed rate constant, k obs = (1.32 ± 0.01) × 10−2 s−1.
a UV–Vis spectra recorded for the reaction between CblOH2 (4.3 × 10−5 M) and Asc (4.3 × 10−5 M) at pH = 7.2 (25 °C, 0.10 M Tris buffer). Spectra were recorded every 2 min. Solid black line spectrum of CblOH2 before addition of Asc. b Typical kinetic trace recorded at 351 nm for the reaction between CblOH2 (4.3 × 10−5 M) and Asc (4.3 × 10−4 M) at pH = 7.2 (25 °C, 0.10 M Tris buffer). Experimental data—black curve; first-order fit—red curve. The data show some deviations during the first 20 s of the reaction as a result of redox-cycling with traces of oxygen present in the system. This effect becomes more visible when aerated solutions are used (see Figs. 4, 5)
A plot of k obs versus the total Asc concentration at pH 7.20 is linear with an almost zero intercept (2 ± 6) × 10−3 s−1 (Fig. 11). A linear fit of the data gives a slope of 27 ± 1 M−1 s−1 (k 2) which is in close agreement with the indirect value obtained in the previous section and the reported value [20] of 24 M−1 s−1. Kinetic studies on the reaction between CblOH2 and Asc [20, 21], reported that CblOH2 reacts rapidly with Asc to form CblCo(II) under the conditions of our study.
Conclusions
The reaction of CblNO2, one of the natural occurring forms of cobalamin, with intracellular ascorbate has been studied by UV–Vis and stopped-flow spectrophotometry. The present study provides kinetic and mechanistic information on this reaction at pH ~7. Under this condition, the only product of the reaction is CblCo(II). Rate constants for the reduction of CblOH2 and CblNO2 by Asc were determined as a function of nitrite concentration. The CblOH2 species reacted with ascorbate ca. 200 times faster than CblNO2. Thus, on addition of the reductant to the equilibrium mixture of CblOH2 and CblNO2, it reacts rapidly with CblOH2 which in turn is reformed by the aquation of CblNO2 (k −1 = 1.4 × 10−2 s−1 at 25 °C) and so represents the major reactive species in solution. For the reduction of CblNO2 by ascorbate, no direct evidence for the reduction of coordinated nitrite could be found.
Our results show that the studied reactions are extremely oxygen sensitive due to the reverse oxidation of CblCo(II) back to CblOH2 and CblNO2, in the presence of nitrite. When the reaction is performed under aerobic or oxygen-saturated conditions, it is significantly slower due to an induction period that involves redox cycling of CblOH2 and CblCo(II) coupled to the oxidation of ascorbate as long as oxygen is present in solution.
In terms of the biological relevance of this work, it has recently been shown that nitrite plays an important role in heme proteins-dependent redox reactions [22], and as such can also control the redox behavior of cobalamin as shown in this study. Our observation that nitrite can slow down the reduction of aquacobalamin by ascorbate by a factor of 200 at pH 7.2, due to the efficient formation of nitrocobalamin, is really impressive and demonstrates the important role of nitrite in controlling biological redox processes.
References
Kröncke K-D, Fehsel K, Kolb-Bachofen V (1997) Nitric Oxide 1:107–120
Ignarro LJ, Cirino G, Casini A, Napoli C (1999) J Cardiovasc Pharmacol 34:879–886
Snyder S (1992) Science 257:494–496
Moncada S, Palmer RM, Higgs EA (1991) Pharmacol Rev 43:109–142
Henry Y, Guissani A (1999) Cel Mol Life Sci 55:1003–1014
Mannick JB, Schonhoff CM (2004) Free Radical Res 38:1–7
Wolak M, Zahl A, Schneppensieper T, Stochel G, van Eldik R (2001) J Am Chem Soc 123:9780–9791
Goulding CW, Postigo D, Matthews RG (1997) Biochemistry 36:8082–8091
Banerjee R (ed) (1999) Chemistry and biochemistry of B12. Wiley, New York
Wolak M, Stochel G, Hamza M, van Eldik R (2000) Inorg Chem 39:2018–2019
Hannibal L, Smith CA, Jacobsen DW, Brasch NE (2007) Angew Chem Int Ed 46:5140–5143
Anes JM, Beck RA, Brink JJ, Goldberg RJ (1994) J Chromatogr B 660:180–185
Smith EL, Fantes KH, Ball S, Waller JG, Emery WB, Anslow WK, Walker AD (1952) Biochem J 52:389–395
Walker DT, Dassanayake RS, Garcia KA, Mukherjee R, Brasch NE (2013) Eur J Inorg Chem 2013:3049–3053
Marques HM, Knapton L (1997) J Chem Soc Dalton Trans. doi:10.1039/A703139D:3827-3834
Grynkiewicz G, Poenie M, Tsien RY (1985) J Biol Chem 260:3440–3450
Katafias A, Limpert O, Kita P, Fenska J, Koter S, Kaczmarek-Kedziera A, Rozycki H, Bajek A, Uzarska M, van Eldik R (2014) Eur J Inorg Chem 2529–2535
Lente G, Fabian I (2004) Inorg Chem 43:4019–4025
Schneppensieper T, Wanat A, Stochel G, Goldstein S, Meyerstein D, van Eldik R (2001) Eur J Inorg Chem 2001:2317–2325
Nazhat NB, Golding BT, Johnson GRA, Jones P (1989) J Inorg Biochem 36:75–81
Beaven GH, Johnson EA (1955) Nature 176:1264–1265
Lu N, Chen C, He Y, Tian R, Xiao Q, Peng Y-Y (2014) Nitric Oxide 40:1–9
Acknowledgments
The work was supported by the National Science Centre in Poland (Grant No. DEC-2012/05/B/ST5/00389). The Faculty of Chemistry of the Jagiellonian University is the beneficiary of the structural funds from the European Union, Grant No. POIG. 02.01.00-12-023/08 “Atomic Scale Science for Innovative Economy (ATOMIN)”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Polaczek, J., Orzeł, Ł., Stochel, G. et al. Mechanistic information on the nitrite-controlled reduction of aquacob(III)alamin by ascorbate at physiological pH. J Biol Inorg Chem 20, 1069–1078 (2015). https://doi.org/10.1007/s00775-015-1288-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-015-1288-9