Abstract
Introduction
Although teriparatide (TPTD) and exercise may improve osteoporosis, muscle atrophy, and fat metabolism during ageing, the effects of treatment with a combination of TPTD and exercise on these factors remain unclear. Therefore, this study examined the effects of TPTD and exercise on bone, skeletal muscle, and fat in ovariectomized and tail-suspended rats.
Materials and methods
Seven-month-old female Wistar rats were ovariectomized and subjected to tail suspension. The rats were then randomized into one of the following four groups (n = 20/group) after 4 weeks: control group, treated with TPTD vehicle and no exercise; TPTD group (30 µg/kg TPTD, 3 days/week); Exercise group (treadmill at 12 m/min, 60 min/day, 5 days/week); and Combined group treated with TPTD and treadmill exercise. After 1 and 8 weeks of treatment, bone, skeletal muscle, and fat tissue parameters were evaluated.
Results
TPTD improved bone mineral density (BMD), bone structure, bone strength at the femoral metaphysis, and the percentage of skeletal muscle mass, and decreased the percentage of fat mass and the adipose volume in the bone marrow. Treadmill exercise increased BMD, bone strength of cancellous bone, and the percentage of skeletal muscle mass, and decreased the percentage of fat mass as seen on dual-energy X-ray absorptiometry. Furthermore, combined treatment significantly affected BMD, bone structure, and bone strength of cortical bone at the femoral diaphysis.
Conclusion
TPTD or treadmill exercise improved bone, skeletal muscle, and fat mass. Combination therapy with TPTD and exercise had synergistic effects on BMD, structure, and bone strength in ovariectomized, tail-suspended rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased risks of falls and fragility fractures are multifactorial problems in the elderly population. Osteoporosis is one factor that is known to be associated with a higher risk of fragility fractures due to a loss of bone mass, microarchitecture, and strength [1]. Muscle atrophy or weakness is another musculoskeletal condition that develops during aging and is known to cause falls and fragility fractures in the elderly [2, 3]. It was recently shown that bone and muscle are interconnected, both chemically and metabolically [4]. Furthermore, it has been reported that marrow adipose tissue plays an important role in affecting the bone quantity and quality [5, 6]. Wong et al. reported that bone marrow adiposity and muscle adiposity are related in postmenopausal women with osteoporosis [7]. Thus, in elderly adults who have higher risks of falls and fragility fractures, it is important to prevent and treat osteoporosis, muscle atrophy, and fatty infiltration to bone marrow and skeletal muscle. In addition, fat infiltration and alterations in stem cell differentiation are common to osteoporosis and muscle weakness.
Effectively preventing or treating osteoporosis and muscle atrophy, as well as fatty infiltration to bone marrow and skeletal muscle, requires several types of interventions, such as combined treatment with pharmacotherapy and exercise. Teriparatide (TPTD) is a medication used to treat osteoporosis through the stimulation of bone formation. We previously reported that TPTD exerts anabolic effects on bone formation via insulin-like growth factor-I (IGF-I) [8]. IGF-I promotes satellite cell growth in skeletal muscle and restores muscle atrophy [9]. However, the effects of TPTD on skeletal muscle have yet to be elucidated. TPTD reduces fat mass in the bone marrow of ovariectomized (OVX) rats [10] and decreases triglyceride levels and lipid droplets of the fatty liver in diabetic osteoporosis model rats [11]. These findings suggest that TPTD may have anabolic effects on not only bone but also skeletal muscle and fat tissue.
Exercise is important in the management of osteoporosis, and exercise has been shown to increase bone mineral density (BMD) [12, 13], improve body balance [14, 15], and reduce the number of falls [16]. IGF-I is associated with the effects of exercise on BMD [17]. Skeletal muscle secretes myokines through muscle contraction due to exercise, and myokines mediate cross-talk between bone and body fat [18]. Exercise has an anabolic effect on bone through the secretion of IGF-I, and it improves lipid metabolism by inhibiting the action of inflammatory adipokines [19, 20].
Based on these findings, we hypothesized that combined therapy with TPTD and exercise can synergistically improve bone, skeletal muscle, and fat tissue in the bone marrow and body; however, no study to date has examined the combined effects of TPTD and exercise on these factors. Therefore, the purpose of the present study was to investigate the effects of TPTD and/or exercise on bone and skeletal muscle, as well as fat tissue, in OVX, tail-suspended rats as a model of postmenopausal osteoporosis and muscle atrophy.
Materials and methods
Animals and experimental protocol
Seven-month-old female Wistar rats (Japan SLC, Shizuoka, Japan) were housed in a controlled environment (temperature 23 ± 2 °C, humidity 40% ± 20%) with a 12-h light/dark cycle. Rats were allowed ad libitum access to tap water and commercial standard rodent chow.
As a model of estrogen deficiency, bilateral ovariectomies were performed under general anesthesia at 7 months of age. General anesthesia was induced by intraperitoneal injection of xylazine hydrochloride (Sederac; Nippon Zenyaku Kogyo, Fukushima, Japan) and ketamine hydrochloride (Ketalar; Daiichi Sankyo Propharma, Tokyo, Japan). Starting at 2 weeks after OVX, rats were tail-suspended for 4 weeks to create hindlimb unloading. Finally, at six weeks after OVX, rats were randomly assigned to one of the following four groups (n = 20/group): a control (Cont) group-administered TPTD vehicle without low-intensity aerobic exercise training; an exercise (Exe) group that performed low-intensity aerobic exercise on a treadmill; a TPTD group-administered TPTD; and a combination (Comb) group that was administered TPTD and performed low-intensity aerobic exercise. After 1 and 8 weeks of treatment, the following parameters were evaluated. The animals were anesthetized by intraperitoneal injection of xylazine hydrochloride and ketamine hydrochloride for measurement of the percentages of fat mass and muscle in the whole body and lower limbs only after 8 weeks of treatment. Rats were euthanized by injection of sodium pentobarbital (150 mg/kg body weight) (Dainippon Sumitomo Pharma, Osaka, Japan), and the bilateral femora, right tibia, lumbar vertebrae, and blood samples from the vena cava were harvested for evaluation after 1 week and 8 weeks of treatment.
The protocols for all animal experiments were approved in advance by the Animal Research Committee of our institute, and all subsequent animal experiments adhered to the “Guidelines for Animal Experimentation” of our university (approval number: a-1-2935).
Tail-suspension model
Hindlimb unloading was performed as described previously [21]. The tail was suspended to maintain the rats at a head-down tilt of 30° with the hindlimbs elevated above the floor of the cage for 28 days (Yamashita Technical Research Institute Co., Ltd., Tokushima, Japan). The rats were allowed a 360° range of movement to facilitate free movement about the cage.
TPTD administration and treadmill exercise
TPTD (Asahi Kasei Pharma, Tokyo, Japan) was dissolved in saline containing 0.1% rat serum albumin, and a dose of 30 μg/kg body weight was administered subcutaneously three times per week for 1 week or 8 weeks based on a previously reported protocol [22].
Low-intensity treadmill exercise was performed at a speed of 10 m/min at a 5% incline for 60 min/day, 5 days/week, for 1 week or 8 weeks (MK-680; Muromachi Kikai, Tokyo, Japan). The treadmill exercise conditions were determined based on our previous study [23]. All rats completed the treadmill exercise during the entire experimental period.
Bodyweight measurement
Bodyweight was measured at the beginning and end of the experiment and changes in body weight were compared among all groups.
Tissue preparation
After sacrifice, the right femur and lumbar vertebrae (L2-4) were fixed in 10% neutral-buffered formalin (Wako Chemical Industries, Osaka, Japan) until preparation for BMD and micro-computed tomography measurement. The left femur was dissected free of soft tissue, wrapped in gauze moistened with saline, and frozen at − 80 °C until biomechanical testing. The right proximal half of the tibia was decalcified with neutral 10% ethylene diamine tetraacetic acid for approximately 4 weeks, and then embedded in paraffin. Subsequently, 3-µm-thick mid-frontal slices were sectioned and stained with hematoxylin and eosin for histomorphometry of bone marrow fat.
Serum bone metabolic markers
The blood samples were centrifuged at 15,000 rpm for 20 min to separate serum after collecting the blood sample from the vena cava. Serum procollagen type 1N-terminal propeptide (P1NP) and tartrate-resistant acid phosphatase-5b (TRACP-5b) were measured using an enzyme immunoassay (EIA) kit (Rat/mouse P1NP EIA kit, Immunodiagnostic Systems Ltd., Boldons, United Kingdom) and a solid phase immunofixed enzyme activity assay (RatTRAP test, Immunodiagnostic Systems Ltd.).
BMD measurement
BMDs of the femur and lumbar spine (L2-L4) were measured using dual-energy X-ray absorptiometry (DXA) (QDR-4500 Delphi; Hologic, Bedford, MA). Each region was scanned in “small animal” mode, with the “regional high-resolution” scan option. Femoral BMD was measured at the proximal, middle, and distal thirds of the femora, as well as the total femur.
Percentages of fat and skeletal muscle mass in the pelvis and hindlimbs
Body composition, including lean mass (g), fat mass (g), bone mass (g), and body fat percentage (%), was also assessed with DXA (QDR-4500 Delphi; Hologic) only at 8 weeks of treatment. Each region was scanned in the “small animal” mode using the “rat whole body” scan option.
After scanning, the area distal from the pelvis was selected as the measurement range. The percentage of skeletal mass (muscle percentage, %) in the pelvis and hindlimbs was calculated by summing the muscle masses of the lower limbs, assuming that all non-fat and non-bone mass is skeletal muscle.
Biomechanical testing
Mechanical testing of the left femoral shaft was performed at room temperature using a material testing machine (MZ500S; Maruto, Tokyo, Japan) only at 8 weeks of treatment. The mid-diaphysis of the femur was stabilized by placing it on two supports of the test apparatus placed 20 mm apart. The load of a three-point bending test was applied in the anteroposterior direction midway between the two supports.
Load–displacement curves were recorded at a crosshead speed of 5 mm/min. Breaking force (N), breaking energy (N mm), maximum load (N), and breaking time (s) were calculated using software for measuring bone strength (CTR win. Version 1.05; System Supply, Nagano, Japan), as previously described [24, 25].
Following the three-point bending test, the distal part of the femur was evaluated using a compression test, as previously described [26]. Load–displacement curves were recorded, and the breaking force (N), breaking energy (N mm), maximum load (N), and stiffness (N/mm) were calculated using the same software.
Fat histomorphometry
Fat histomorphometric analysis at the proximal tibia with 200× magnification was performed using a semiautomatic graphic system (Histometry RT CAMERA; System Supply). Measurements were obtained at 400 μm caudally from the lowest point of the growth plate and 100 μm medially from the endosteal surface. The histomorphometric adipocyte volume per total bone marrow volume (AV/MV, %), number of adipocytes per unit area of marrow volume (N.A/MV, number of cells/mm2), and volume of each adipocyte per number of adipocytes (AV/N.A, μm2) were evaluated as parameters of fat histomorphometry in the bone marrow of the proximal tibia only at 8 weeks of treatment, as described previously [10].
Micro-computed tomography examination
The excised right femur and lumbar spine from rats in the four groups that were treated only for 8 weeks (n = 4–5 each) were secured in a sample holder. Micro-computed tomography was performed with CosmoScan GX II (Rigaku Corporation, Tokyo, Japan), according to the manufacturer’s instructions, with an isotropic voxel size of 36 μm, energy of 90 kVp, and current of 88 μA. Captured images were rendered using TRI/3D BON (Ratoc System Engineering Co., Ltd., Tokyo, Japan) software. Evaluation of osteoporosis was performed based on the bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), cortical thickness (Ct.Th), and cortical area (Ct.Ar).
Statistical analysis
All data are expressed as means ± standard deviation (SD). A Kolmogorov–Smirnov test showed that all data were normally distributed. Differences between groups were evaluated using one-way analysis of variance (ANOVA) and analyzed using Scheffe’s methods as post hoc tests. All statistical analyses were performed using the Statistical Package for the Biosciences (SPBS) version 9.6 (Akita University, Akita, Japan) [27]. Values of p < 0.05 were considered significant.
Results
Bodyweight
Body weights at the beginning of the experiment and at 1 week of treatment were not significantly different among the groups (Table 1). The body weight was significantly lower in the Exe and Comb groups than in the Cont group (p < 0.05) after 8 weeks of treatment.
BMD
The BMDs of the femur and lumbar spine at 1 week and 8 weeks after treatment are shown in Table 2. At 1 week of treatment, the BMDs of the femur and lumbar spine did not show significant differences among the four groups.
After 8 weeks of treatment, the Exe and TPTD groups showed significantly higher BMDs in the total, proximal, and distal femur than the Cont group (p < 0.01 or p < 0.01). The combined treatment with exercise and TPTD (Comb group) significantly increased the BMDs in the total, proximal, middle, and distal femur compared with that of the Cont, Exe, and TPTD groups (p < 0.05, p < 0.01).
In the lumbar spine, the Exe, TPTD, and Comb groups showed significantly higher BMDs than the Cont group (p < 0.05, p < 0.01, and p < 0.01, respectively). Furthermore, combined treatment with exercise and TPTD led to a significant increase in the BMD of the lumbar spine compared with that of the Exe alone group (p < 0.01).
Bone metabolic markers
The serum TRACP-5b level was significantly different among the four groups at 1 week, but not at 8 weeks, after treatment by ANOVA (p = 0.036) (Supplementary Table 1). The TRACP-5b level was significantly lower in the Comb group than in the Exe group at 1 week after treatment (p < 0.05). There were no significant differences of P1NP among the four groups at 1 week and 8 weeks after treatment.
Bone strength as determined by biomechanical testing
In the femoral diaphysis, a cortical bone-rich site, the Comb group showed a significantly higher breaking force and maximum load than the Cont group (p < 0.01 and p < 0.01, respectively) (Fig. 1a). In addition, the Comb group showed a significantly higher maximum load than the Exe and TPTD groups (p < 0.01 and p < 0.01, respectively). Breaking energy and breaking time of the femoral diaphysis showed no significant differences among the groups.
Bone strength of the femoral mid diaphysis (a) and distal metaphysis (b) (n = 9–10 per group). Values represent the means ± SD. *p < 0.05, **p < 0.01 with Scheffe’s method. Cont control group, administered vehicle without aerobic exercise; Exe exercise group, performed aerobic exercise training; TPTD teriparatide group, administered teriparatide at 30 µg/kg, three times/week; Comb combined group, administered teriparatide and performed aerobic exercise training
In the femoral distal metaphysis, a cancellous bone-rich site, the intervention groups showed significantly higher breaking energy and maximum load than the Cont group (p < 0.05, p < 0.01) (Fig. 1b). The Comb group showed a significantly higher breaking force than the Cont and Exe groups (p < 0.01 and p < 0.05, respectively). The Exe and TPTD groups showed significantly longer breaking times than the Cont group (p < 0.01).
Fat histomorphometry
AV/MV and N.A/MV were significantly lower in the TPTD and Comb groups than in the Cont and Exe groups (p < 0.05, p < 0.01) (Table 3). However, no significant differences in AV/N.A were observed among the groups. The number of adipocytes was greater in the Cont group (Fig. 2a) than in the other groups (Fig. 2b–d). Cancellous bone was greater in the Exe, TPTD, and Comb groups (Fig. 2b–d) than in the Cont group (Fig. 2a).
Histological sections stained with hematoxylin and eosin at the metaphyseal tibia; magnification ×10. Cont: control group, administered vehicle without aerobic exercise (a); Exe: exercise group, performed aerobic exercise training (b); TPTD: teriparatide group, administered teriparatide at 30 µg/kg, three times/week (c); Comb: combined group, administered teriparatide and performed aerobic exercise training (d)
Percentages of fat and skeletal muscle of the pelvis and lower limbs
The percentages of fat and skeletal muscle of the pelvis and lower limbs are shown in Fig. 3. Hindlimb weights were significantly lower in the Exe and Comb groups than in the Cont group (p < 0.01). Exercise, TPTD, and combined treatment with exercise and TPTD significantly decreased the percentage of fat mass in each respective group when compared with the Cont group (p < 0.01). The combined treatment with exercise and TPTD also significantly decreased the percentage of fat mass in the Comb group when compared with the Exe group (p < 0.05). The percentages of muscle mass were significantly higher in the Exe, TPTD, and Comb groups than in the Cont group (p < 0.01).
Pelvis and hindlimb weight and percentages of fat and skeletal muscle mass (n = 7 per group). Measurement of the percentages of fat and skeletal muscle mass was performed using DXA. Values represent the means ± SD. *p < 0.05, **p < 0.01 with Scheffe’s method. Cont control group, administered vehicle without aerobic exercise; Exe exercise group, performed aerobic exercise training; TPTD teriparatide group, administered teriparatide at 30 µg/kg, three times/week; Comb combined group, administered teriparatide and performed aerobic exercise training
Micro-computed tomography
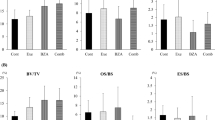
Table 4 shows the results of micro-computed tomography. At the femoral neck, BV/TV was significantly higher in the Comb group than in the other groups (p < 0.01). Tb.Th values were significantly higher in the Exe, TPTD, and Comb groups than in the Cont group (p < 0.05, p < 0.05, and p < 0.01, respectively), and in addition, Tb.Th was significantly higher in the Comb group than in the Exe or TPTD groups (p < 0.01). Tb.N was significantly higher in the Comb group than in the Cont and Exe groups (p < 0.05).
At the distal metaphysis, all parameters were significantly higher in the TPTD and Comb groups than in the Cont group, and additional increases in BV/TV, Tb.Th, and Tb.N were observed in the Comb group compared with the Exe group (p < 0.01, p < 0.05, and p < 0.01, respectively).
At the femoral midshaft, Ct.Th and Ct.Ar were significantly higher only in the Comb group compared to the other groups (p < 0.05, p < 0.01, respectively). The axial images of micro-CT at the femoral midshaft showed that the cortical bone was thicker in the Comb group (supplementary Figure 1d) than in the Cont group (supplementary Figure 1a).
At the lumbar spine, TPTD monotherapy significantly increased BV/TV compared with that in the Cont group (p < 0.05). The combined treatment with exercise and TPTD significantly increased BV/TV and Tb.Th compared with those in the Cont, Exe, and TPTD groups (p < 0.01, p < 0.05, and p < 0.05, respectively). No significant difference in Tb.N was seen among the groups.
Discussion
Exercise effects on bone, skeletal muscle, and fat
Treadmill exercise for OVX, tail-suspended rats significantly increased BMD, except at the middle region of the femur and in the lumbar spine, bone strength at the distal metaphysis of the femur, and the percentage of skeletal muscle at the pelvis and hindlimbs. Exercise did not affect bone metabolic markers in this study. Treadmill exercise only decreased the percentage of fat as seen on DXA, but not the adipose tissue in the bone marrow. It was previously demonstrated that exercise stimulates the proliferation of mesenchymal stem cells, as well as osteogenesis, in addition to suppressing adipocyte diameter [28]. Furthermore, a study also showed that treadmill training prevents bone loss by inhibition of peroxisome proliferator-activated receptor gamma (PPARγ) expression rather than by promoting the osteogenic factor, runt-related transcription factor 2 [29]. In OVX rats, treadmill training activates glycogen synthase kinase-3β/β-catenin signaling and inhibits the production of PPARγ in lumbar vertebrae [30]. Treadmill exercise also increases soleus and plantar muscle mass in OVX rats [31]. These mechanisms act to increase BMD and decrease the percentage of fat. The effects of treadmill exercise on the BMD and bone strength of cancellous bone, skeletal muscle mass, and fat mass in the OVX tail-suspended rats were consistent with the results of previous studies in OVX rats.
TPTD effects on bone, skeletal muscle, and fat
Several previous studies have mentioned the effects of the frequency and dose of TPTD on cortical BMD or the cortical porosity of the proximal or middle shaft of the femur. Takao-Kawabata et al. demonstrated that administration of TPTD three times per week significantly and dose-dependently increased the BMD of the proximal femur and the cortical thickness of the middle shaft of the femur compared with OVX control rats [32]. Takakura et al. also showed that cortical porosity develops as a result of an increased frequency of administration with a lower concentration of total TPTD administration [22]. In the present study, administration of TPTD three times per week increased the proximal and distal femoral BMD, but not the BMD of the middle shaft of the femur, increased the maximum load of the distal metaphysis, but not that of the middle shaft of the femur, and increased the Tb.Th of the proximal femur and the BV/TV of the distal femur, with no effect on the bone metabolic markers. The effects of TPTD on the proximal or distal femur were consistent with the results of previous studies. However, the effects of TPTD on the middle shaft of the femur were different from the results for OVX rats in previous studies compared with the results for OVX tail-suspended rats in the present study. The model of OVX, tail-suspended rats appears to cause more severe bone loss and bone fragility at the middle shaft of the femur, which is a cortical bone-rich site, than the OVX rats reported in the previous studies.
In the present study, TPTD monotherapy significantly increased the percentage of skeletal muscle and decreased the percentage of fat in the pelvis and hindlimbs as measured by DXA and decreased the adipose volume in bone marrow in the OVX, tail-suspended rats. This is the first study to demonstrate that TPTD treatment increased the percentage of skeletal muscle in osteoporosis and muscle atrophy model rats. A previous in vitro study reported that parathyroid hormone (PTH) modulates the uptake and retention of 25-hydroxyvitamin D in skeletal muscle cells [33]. Growth hormone (GH) is a potent anabolic agent that promotes skeletal muscle cell protein synthesis and growth [34]. GH exerts its effect either directly or indirectly by promoting IGF-I. We previously demonstrated that the anabolic effects of PTH on bone are exerted via IGF-I [8]; therefore, we hypothesized that TPTD may have anabolic effects on atrophied skeletal muscle via the 25-hydroxyvitamin D or the IGF-I/GH axis. Brent et al. reported that PTH (1–34) and GH prevent disuse osteopenia and sarcopenia in rats [35]. As a direct effect of TPTD on skeletal muscle, Kimura et al. reported that the differentiation of satellite cells into myotubes is accelerated by PTH and the expression of the PTH-1 receptor [36]. In addition, TPTD treatment significantly improves muscle weakness in dystrophin-deficient mdx mice [37]. Although the effects of TPTD on skeletal muscle remain unknown, TPTD may have an anabolic effect on muscle atrophy or muscle weakness.
We previously reported that TPTD increases cancellous bone volume by stimulating bone formation and suppresses adipocyte volume [10]. TPTD decreases triglyceride levels and lipid droplets of the fatty liver in diabetic osteoporosis model rats [11]. TPTD also decreases body weight and fat mass via an increase in undercarboxylated osteocalcin [38]. In the present study, TPTD monotherapy significantly decreased the percentage of fat in the pelvis and decreased the adipose volume in the bone marrow. These results were consistent with the results of previous studies. TPTD may have positive effects on fat metabolism in this model.
Effects of combined treatment with TPTD and exercise on bone, skeletal muscle, and fat
Compared with each monotherapy, combined treatment with treadmill exercise and TPTD significantly increased BMD at all sites of the femur and lumbar spine, the bone strength of a cortical bone site (middle femur) and a cancellous bone-rich site (distal metaphysis), BV/TV and the trabecular thickness of the proximal and distal femur and lumbar spine, and cortical thickness and area with suppression of bone resorption compared with exercise monotherapy at 1 week after treatment. Furthermore, combined therapy significantly decreased adipose volume in the bone marrow and the percentage of fat compared with treadmill exercise monotherapy. However, the combined treatment did not have a significant effect on the percentage of skeletal muscle in this model rat.
Exercise increases systemic PTH levels depending on the type, intensity, and duration of exercise [39,40,41], in addition to an effect of dynamic loading on bone tissue. Sugiyama et al. reported synergistic effects on the cortical bone volume of the proximal tibia and distal ulna with loading and high-dose intermittent PTH administration in mice [42]. PTH treatment can further increase cortical bone formation during direct loading [42, 43]. In a recent study, bone adaptation during exercise occurred not only due to dynamic loading, but also PTH release, and PTH signaling during exercise contributed to improvement in the structural-level mechanical properties of cortical bone [44]. Based on these results, combined treatment with TPTD and treadmill exercise showed synergistic effects, especially on cortical bone compared with TPTD monotherapy. However, the synergistic effects of TPTD and treadmill exercise on the percentage of fat mass or skeletal muscle mass compared with the effects of TPTD monotherapy were not observed in the OVX, tail-suspended rats. The mechanisms of the combined effects of TPTD and treadmill exercise on fat mass or skeletal muscle may be different from those on cortical bone.
Limitations
The results of the present study must be considered in light of several limitations. First, the percentage of skeletal muscle mass was evaluated only with DXA. Further investigation is needed to evaluate the effects of TPTD on skeletal muscle size, type of skeletal muscle, and its related gene expression during treatment. Second, only a single type or condition of the exercise was evaluated. Other types or conditions of exercise may have different effects on bone, skeletal muscle, and fat when combined with TPTD treatment. Third, there were no control groups with OVX or tail-suspension alone to evaluate their individual effects on bone, skeletal muscle mass, and fat mass. Finally, the mechanisms of the effects of combined therapy of TPTD and exercise were not investigated by analyzing gene expression in bone, muscle, and fat tissues in this study. Future studies are needed to address these points.
Conclusions
In the present study, TPTD and/or treadmill exercise for OVX, tail-suspended rats as a model of osteoporosis and muscle atrophy, representing immobilized, postmenopausal, osteoporotic women, demonstrated the following. TPTD monotherapy increased the BMD and structure of cancellous bone at the femur and lumbar spine, the bone strength at the femoral metaphysis, and the percentage of skeletal muscle mass, and it decreased the percentage of fat mass and adipose volume in the bone marrow. Treadmill exercise only increased the BMD and bone strength of cancellous bone, increased the percentage of skeletal muscle mass, and decreased the percentage of fat mass as measured by DXA. In addition to these effects of each treatment, the combined treatment with TPTD and treadmill exercise had synergistic effects on BMD, structure, and bone strength of cortical bone at the femoral diaphysis. These results suggest that the combination therapy of TPTD and treadmill exercise may be effective for improving bone, skeletal muscle, and fat in elderly patients with osteosarcopenia. In the clinical situation, treatment of elderly and immobilized, postmenopausal, osteoporotic women to prevent fragility fractures by TPTD alone may not be enough. In addition to treatment with TPTD, some kind of exercise, such as standing or walking with support, is needed to achieve a preventive effect on fragility fractures, especially at cortical bone-rich sites in elderly and immobilized, osteoporotic patients.
References
Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C (2015) Osteoporosis and sarcopenia in older age. Bone 80:126–130. https://doi.org/10.1016/j.bone.2015.04.016
Oliveira A, Vaz C (2015) The role of sarcopenia in the risk of osteoporotic hip fracture. Clin Rheumatol 34:1673–1680. https://doi.org/10.1007/s10067-015-2943-9
Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M (2019) Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 99:427–511. https://doi.org/10.1152/physrev.00061.2017
Kawao N, Kaji H (2015) Interactions between muscle tissues and bone metabolism. J Cell Biochem 116:687–695. https://doi.org/10.1002/jcb.25040
Schwartz AV (2015) Marrow fat and bone: review of clinical findings. Front Endocrinol (Lausanne) 30(6):40. https://doi.org/10.3389/fendo.2015.00040
Singhal V, Bredella MA (2019) Marrow adipose tissue imaging in humans. Bone 118:69–76. https://doi.org/10.1016/j.bone.2018.01.009
Wong AK, Chandrakumar A, Whyte R, Reitsma S, Gillick H, Pokhoy A, Papaioannou A, Adachi JD (2020) Bone marrow and muscle fat infiltration are correlated among postmenopausal women with osteoporosis: The AMBERS Cohort Study. J Bone Miner Res 35:516–527. https://doi.org/10.1002/jbmr.3910 (Epub 2019/11/20)
Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S (2001) Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology 142:4349–4356. https://doi.org/10.1210/endo.142.10.8436
Hawke TJ (1985) Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551. https://doi.org/10.1152/jappl.2001.91.2.534
Nozaka K, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Shimada Y (2008) Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone 42:90–97. https://doi.org/10.1016/j.bone.2007.08.041 (Epub 2007/09/14)
Nomura S, Kitami A, Takao-Kawabata R, Takakura A, Nakatsugawa M, Kono R, Maeno A, Tokuda A, Isogai Y, Ishizuya T, Utsunomiya H, Nakamura M (2019) Teriparatide improves bone and lipid metabolism in a male rat model of type 2 diabetes mellitus. Endocrinology 160:2339–2352. https://doi.org/10.1210/en.2019-00239
Bonaiuti D, Shea B, Iovine R, Negrini S, Robinson V, Kemper HC, Wells GA, Tugwell P, Cranney A (2002) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000333
Wallace BA, Cumming RG (2000) Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int 67:10–18. https://doi.org/10.1007/s00223001089
Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB (2004) Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA 292:837–846. https://doi.org/10.1001/jama.292.7.837
Papaioannou A, Adachi JD, Winegard K, Ferko N, Parkinson W, Cook RJ, Webber C, McCartney N (2003) Efficacy of home-based exercise for improving quality of life among elderly women with symptomatic osteoporosis-related vertebral fractures. Osteoporos Int 14:677–682. https://doi.org/10.1007/s00198-003-1423-2 (Epub 2003/07/22)
Sakamoto K, Endo N, Harada A, Sakada T, Tsushita K, Kita K, Hagino H, Sakai A, Yamamoto N, Okamoto T, Liu M, Kokaze A, Suzuki H (2013) Why not use your own body weight to prevent falls? A randomized, controlled trial of balance therapy to prevent falls and fractures for elderly people who can stand on one leg for ≤15 s. J Orthop Sci 18:110–120. https://doi.org/10.1007/s00776-012-0328-3 (Epub 2012/11/09)
Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM (2012) A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151:1319–1331. https://doi.org/10.1016/j.cell.2012.10.050
Kaji H (2016) Effects of myokines on bone. Bonekey Rep 5:826. https://doi.org/10.1038/bonekey.2016.48 (Epub 2016/07/20)
Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8:457–465. https://doi.org/10.1038/nrendo.2012.49 (Epub 2012/04/03)
Lombardi G, Sanchis-Gomar F, Perego S, Sansoni V, Banfi G (2016) Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine 54:284–305. https://doi.org/10.1007/s12020-015-0834-0 (Epub 2015/12/30)
Shimada Y, Sakuraba T, Matsunaga T, Misawa A, Kawatani M, Itoi E (2006) Effects of therapeutic magnetic stimulation on acute muscle atrophy in rats after hindlimb suspension. Biomed Res 27:23–27. https://doi.org/10.2220/biomedres.27.23
Takakura A, Lee JW, Hirano K, Isogai Y, Ishizuya T, Takano-Kawabata R, Iimura T (2017) Administration frequency as well as dosage of PTH are associated with development of cortical porosity in ovariectomized rats. Bone Res 5:17002. https://doi.org/10.1038/boneres.2017.2 (Epub 2017/04/25)
Akagawa M, Miyakoshi N, Kasukawa Y, Ono Y, Yuasa Y, Nagahata I, Sato C, Tsuchie H, Nagasawa H, Hongo M, Shimada Y (2018) Effects of activated vitamin D, alfacalcidol, and low-intensity aerobic exercise on osteopenia and muscle atrophy in type 2 diabetes mellitus model rats. PLoS ONE 13:e0204857. https://doi.org/10.1371/journal.pone.0204857 (Epub 2018/10/17)
Miyakoshi N, Fujii M, Kasukawa Y, Shimada Y (2019) Impact of vitamin C on teriparatide treatment in the improvement of bone mineral density, strength, and quality in vitamin C-deficient rats. J Bone Miner Metab 37:411–418. https://doi.org/10.1007/s00774-018-0941-0 (Epub 2018/07/16)
Segawa T, Miyakoshi N, Kasukawa Y, Aonuma H, Tsuchie H, Shimada Y (2016) Combined treatment with minodronate and vitamin C increases bone mineral density and strength in vitamin C-deficient rats. Osteoporos Sarcopenia 2:30–37. https://doi.org/10.1016/j.afos.2016.01.002 (Epub 2016/03/21)
Kasukawa Y, Miyakoshi N, Itoi E, Tsuchida T, Tamura Y, Kudo T, Suzuki K, Seki A, Sato K (2004) Effects of h-PTH on cancellous bone mass, connectivity, and bone strength in ovariectomized rats with and without sciatic-neurectomy. J Orthop Res 22:457–464. https://doi.org/10.1016/j.orthres.2003.08.017
Murata K, Yano E (2002) Medical statistics for evidence-based medicine with SPBS user’s guide. Nankodo, Tokyo
Marędziak M, Śmieszek A, Chrząstek K, Basinska K, Marycz K (2015) Physical activity increases the total number of bone-marrow-derived mesenchymal stem cells, enhances their osteogenic potential, and inhibits their adipogenic properties. Stem Cells Int 2015:379093. https://doi.org/10.1155/2015/379093 (Epub 2015/06/16)
Chen YJ, Huang CH, Lee IC, Lee YT, Chen MH, Young TH (2008) Effects of cyclic mechanical stretching on the mRNA expression of tendon/ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connect Tissue Res 49:7–14. https://doi.org/10.1080/03008200701818561
Bu S, Chen Y, Wang S, Zhang F, Ji G (2012) Treadmill training regulates β-catenin signaling through phosphorylation of GSK-3β in lumbar vertebrae of ovariectomized rats. Eur J Appl Physiol 112:3295–3304. https://doi.org/10.1007/s00421-011-2306-4
Shi R, Tian X, Feng Y, Cheng Z, Lu J, Brann DW, Zhang Q (2019) Expression of aromatase and synthesis of sex steroid hormones in skeletal muscle following exercise training in ovariectomized rats. Steroids 143:91–96. https://doi.org/10.1016/j.steroids.2019.01.00
Takao-Kawabata R, Isogai Y, Takakura A, Shimazu Y, Sugimoto E, Nakazono O, Ikegaki I, Kuriyama H, Tanaka S, Oda H, Ishizuka T (2015) Three-times-weekly administration of teriparatide improves vertebral and peripheral bone density, microarchitecture, and mechanical properties without accelerating bone resorption in ovariectomized rats. Calcif Tissue Int 97:156–168. https://doi.org/10.1007/s00223-015-9998-0 (Epub 2015/04/25)
Abboud M, Rybchyn MS, Liu J, Ning Y, Gordon-Thomson C, Brennan Speranza TC, Cole L, Greenfield H, Fraser DR, Mason RS (2017) The effect of parathyroid hormone on the uptake and retention of 25-hydroxyvitamin D in skeletal muscle cells. J Steroid Biochem Mol Biol 173:173–179. https://doi.org/10.1016/j.jsbmb.2017.01.001 (Epub 2017/01/16)
Ullman M, Oldfors A (1989) Effects of growth hormone on skeletal muscle. I. Studies on normal adult rats. Acta Physiol Scand 135:531–536. https://doi.org/10.1111/j.1748-1716.1989.tb08612.x
Brent MB, Brüel A, Thomsen JS (2018) PTH (1–34) and growth hormone in prevention of disuse osteopenia and sarcopenia in rats. Bone 110:244–253. https://doi.org/10.1016/j.bone.2018.02.017 (Epub 2018/02/20)
Kimura S, Yoshioka K (2014) Parathyroid hormone and parathyroid hormone type-1 receptor accelerate myocyte differentiation. Sci Rep 11(4):5066. https://doi.org/10.1038/srep05066
Yoon S, Grynpas M, Mitchell J (2019) Intermittent PTH treatment improves bone and muscle in glucocorticoid treated Mdx mice: A model of Duchenne Muscular Dystrophy. Bone 121:232–242
Schafer A, Sellmeyer D, Schwartz A, Rosen C, Vittinghoff E, Palermo L, Bilezikian J, Shoback D, Black D (2011) Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study). J Clin Endocrinol Metab 96:E1982-1989. https://doi.org/10.1210/jc.2011-0587
Brahm H, Piehl-Aulin K, Ljunghall S (1997) Bone metabolism during exercise and recovery: the influence of plasma volume and physical fitness. Calcif Tissue Int 61:192–198. https://doi.org/10.1007/s002239900322
Iwamoto J, Shimamura C, Takeda T, Abe H, Ichimura S, Sato Y, Toyama Y (2004) Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats. J Bone Miner Metab 22:26–31. https://doi.org/10.1007/s00774-003-0443-5
Scott JPR, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD (2011) The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J Appl Physiol 110:423–432. https://doi.org/10.1152/japplphysiol.00764.2010
Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE (2008) Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone 43:238–248. https://doi.org/10.1016/j.bone.2008.04.012 (Epub 2008/05/01)
Chow JW, Fox S, Jagger CJ, Chambers TJ (1998) Role for parathyroid hormone in mechanical responsiveness of rat bone. Am J Physiol 274:E146-154. https://doi.org/10.1152/ajpendo.1998.274.1.E146
Gardinier JD, Mohamed F, Kohn DH (2015) PTH Signaling during exercise contributes to bone adaptation. J Bone Miner Res 30:1053–1063. https://doi.org/10.1002/jbmr.2432
Acknowledgments
The authors would like to thank Asahi Kasei Pharma Corporation for providing TPTD and Ms. Matsuzawa and Ms. Kudo for their support of our experiments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Chiaki Sato: Investigation, Validation, Visualization. Writing—original draft Itsuki Nagahata: Investigation, Validation. Yusuke Yuasa: Investigation, Validation. Kazunobu Abe: Investigation. Hikaru Saito: Investigation. Ryo Shoji: Investigation. Hiroyuki Tsuchie: Investigation, Formal analysis. Koji Nozaka: Investigation, Formal analysis. Yuji Kasukawa: Conceptualization, Methodology, Writing—review & editing. Naohisa Miyakoshi: Conceptualization, Methodology, Project administration. Writing—review & editing. Yoichi Shimada: Conceptualization, Funding acquisition, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
Naohisa Miyakoshi has received payments for lectures from Asahi Kasei Pharma Corporation. The other authors declare that they have no conflicts of interest.
Ethical approval
The protocols for all animal experiments were approved in advance by the Animal Research Committee of our institute, and all subsequent animal experiments adhered to the “Guidelines for Animal Experimentation” of our university (approval number: a-1-2935).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sato, C., Miyakoshi, N., Kasukawa, Y. et al. Teriparatide and exercise improve bone, skeletal muscle, and fat parameters in ovariectomized and tail-suspended rats. J Bone Miner Metab 39, 385–395 (2021). https://doi.org/10.1007/s00774-020-01184-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01184-0