Abstract
Introduction
Bisphosphonates are the standard treatment for glucocorticoid-induced osteoporosis (GIOP) with teriparatide being another option. While daily teriparatide has been shown to be effective in increasing bone mineral density (BMD), the efficacy of once-weekly teriparatide (56.5 µg) has not yet been evaluated. The TOWER-GO study, a 72-week, multicenter, open-label, randomized controlled trial, was conducted in patients with GIOP to compare the effects of once-weekly teriparatide and once-weekly alendronate 35 mg on BMD.
Materials and methods
Patients (N = 180) with GIOP for whom drug treatment was indicated according to the 2004 guidelines in Japan were randomized to receive once-weekly teriparatide (n = 89) or once-weekly alendronate (n = 91). The primary endpoint was the non-inferiority of percentage change in lumbar spine BMD at final follow-up. The secondary endpoints were the percentage change in BMD from baseline, incidence of bone fractures, and changes in bone turnover markers.
Results
While the non-inferiority of teriparatide to alendronate was not confirmed, BMD increased significantly from baseline with teriparatide and alendronate by 5.09% and 4.04%, respectively (both p < 0.05), at 72 weeks. The incidence of vertebral and non-vertebral fractures was similar in both groups. Bone formation markers increased in the teriparatide group and decreased in the alendronate group.
Conclusions
The non-inferiority of once-weekly teriparatide versus once-weekly alendronate in BMD change at 72 weeks was not shown, but the increase in bone formation markers over time and the increase of BMD in GIOP patients treated with once-weekly teriparatide were confirmed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the National Institutes of Health (NIH), osteoporosis is defined as “a skeletal disease which is characterized by decreased bone strength and an increased risk of bone fractures” [1]. Osteoporosis has been further classified into primary osteoporosis, which includes postmenopausal, male, and idiopathic osteoporosis, and secondary osteoporosis, which includes endocrine, nutritional, drug-induced, immobility, and congenital osteoporosis [2]. Glucocorticoid-induced osteoporosis (GIOP) is the most common type of secondary osteoporosis, and it can occur in patients of any age, from children to the elderly [3].
In 2004 [4], the Japanese Society for Bone and Mineral Research (JSBMR) released guidelines (Guidelines on the Management and Treatment of Glucocorticoid-induced Osteoporosis) for the management and treatment of GIOP, and these guidelines were updated in 2014 [5]. The primary objectives of both guidelines were to provide advice on the prevention and treatment of osteoporosis in patients who take or are expected to take oral glucocorticoids for 3 months or longer. Regarding to dosages of glucocorticoids, the 2004 guidelines [4] recommended that all patients on ≥ 5 mg of daily prednisolone be on drug treatment. In the 2014 guidelines where a scoring system was introduced for medications with four risk factors including glucocorticoids, the dose of daily prednisolone was graded into three levels (< 5 mg, ≥ 5 and < 7.5 mg, ≥ 7.5 mg) with each score, and with a total score of three or higher, drug treatment was recommended. The medications recommended as the first choice by both guidelines are bisphosphonates. However, teriparatide therapies are recommended as alternatives in the 2014 guidelines, since teriparatide therapy in the primary prevention of osteoporosis had not yet been confirmed when the guidelines were published [5]. Teriparatide is approved in Japan for the treatment of osteoporosis with a high risk of fracture.
Daily teriparatide therapy is recommended in the guidelines of other countries (e.g., American College of Rheumatology [ACR] 2017 guidelines) [6] based on the results of a study comparing the efficacy of daily administration of teriparatide versus alendronate in the treatment of GIOP [7]. That study found that the increase in lumbar spine bone mineral density (BMD) from baseline to 18 months was significantly higher in the teriparatide group (7.2%) than in the alendronate group (3.4%), and that the rate of new vertebral fracture was significantly lower in the teriparatide group than in the alendronate group. However, no studies have specifically evaluated the efficacy of once-weekly teriparatide in patients with GIOP.
We, therefore, decided to conduct the teriparatide once-weekly efficacy research for GIOP (TOWER-GO) trial to investigate whether once-weekly teriparatide 56.5 µg is non-inferior to once-weekly alendronate 35 mg for the treatment of GIOP in Japan. The dosages of 56.5 µg once-weekly teriparatide [8] and 35 mg once-weekly alendronate [9] were approved by the regulatory authorities in Japan. In the present study, the primary endpoint was the comparison of the percentage changes in lumbar spine BMD after 72 weeks between the two treatments. The time-dependent percentage change in lumbar spine BMD with each treatment, vertebral fracture/non-vertebral fracture occurrence rate, rate of change in bone turnover markers, and rate of quality of life (QOL) improvement were the secondary endpoints.
Materials and methods
Study design
This was a 72-week, open-label, randomized controlled, non-inferiority trial in Japanese patients with GIOP at 43 study sites. The study was conducted in compliance with the ethical principles that have their origin in the Declaration of Helsinki and the Ethical Guidelines for Clinical Research and was approved by the Mizuo Clinic institutional review board (Yokohama, Japan), the central IRB in this study. Where necessary, it was approved by the IRB at each site. All patients provided written, informed consent before enrollment in the study. This study was registered with the UMIN Clinical Trials Registry (UMIN000011419). Enrollment of subjects took place from November 2013 to December 2016, with the last patient observation completed in June 2018.
Subjects
The GIOP patients (age ≥ 20 years) were recruited (N = 180) based on guidelines released by the JSBMR (Guidelines on the Management and Treatment of Glucocorticoid-induced Osteoporosis) [4] in 2004. The 2004 guidelines recommended that treatment simultaneously with or in the very early stage of glucocorticoid administration is important based on the evidence from GIOP studies [10,11,12,13], epidemiological data [14], and domestic data. Therefore, based on the above guidelines, patients who were receiving or were going to receive ≥ 5 mg of prednisolone daily for 3 or more months were included in the present study [4].
The exclusion criteria were as follows: patients who had used bisphosphonates in the past except for ≤ 2 weeks more than 6 months before enrollment; patients previously treated with teriparatide; patients in whom teriparatide and alendronate were contraindicated; patients treated with denosumab for up to 6 months before enrollment; patients in whom three or more lumbar vertebral bodies (L1—L4) could not be evaluated using dual-energy X-ray absorptiometry (DXA); and other patients the investigators considered ineligible to participate in the study.
The included patients (N = 180) were randomly allocated to the once-weekly teriparatide 56.5 µg group (n = 89) (approved in Japan and Korea) or the weekly oral alendronate 35 mg group (n = 91) for 72 weeks by central stratified randomization using a dynamic allocation (minimization) method to balance age, sex, previous glucocorticoid use, prevalent vertebral fractures, lumbar spine BMD, and primary disease between the two groups. All patients received daily oral supplements of calcium 610 mg, vitamin D 400 IU, and magnesium 30 mg (Calcichew D3; Takeda Consumer Healthcare, Tokyo, Japan).
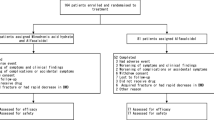
Of the 180 enrolled subjects, 81 in the teriparatide group and 90 in the alendronate group were included in the safety analysis set (Fig. 1). The decrease in the number of subjects in the safety analysis set was due to withdrawal of consent. The subjects who received the study drug at least once and were evaluated for efficacy at least once were included in the efficacy analysis set using the statistical analysis [last observation carried forward (LOCF) method] described below. These included 58 subjects in the teriparatide group and 79 subjects in the alendronate group (Fig. 1). The decrease in the number of subjects in the efficacy analysis set was due to withdrawal of consent after the baseline evaluation or discontinuation due to adverse events or interruption of hospital visits. In this study, if a prescribed evaluation was performed at least once by week 72, the patient was not treated as a dropout.
Enrollment and outcomes. Of the allocated cases, those who received the study drug at least once were included in the safety analysis sets. The subjects who received the study drug more than once and were evaluated for efficacy at least once were included in the efficacy analysis set. SP safety population, ES efficacy set
Efficacy endpoints and measures
The primary endpoint was confirmation of non-inferiority of teriparatide to alendronate in the percentage change of lumbar spine (L2—L4) BMD at final follow-up (72 weeks). Lumbar spine BMD was measured using DXA at baseline, 24, 48, and 72 weeks. Missing BMD values were imputed by the LOCF method.
The secondary endpoints were the time-dependent percentage change in lumbar spine BMD from baseline to 72 weeks in each group, incidences of vertebral and non-vertebral fractures, changes from baseline in bone turnover markers, and the degree of improvement in QOL in the teriparatide and alendronate groups. The incidence of vertebral fractures was assessed at baseline, 24, 48, and 72 weeks using a semi-quantitative methodology [15, 16]. In the present study, the definition of non-vertebral fractures was the same as in the TOWER study [8]; that is, fragility fractures excluded fractures caused by a traffic accident or a fall from a height higher than standing height. Bone turnover markers were assessed at baseline, 4, 24, 48, and 72 weeks. Type I procollagen N-terminal propeptide (P1NP), osteocalcin (OC), C-terminal telopeptide of type I collagen (CTX), and tartrate-resistant acid phosphatase-5b (TRACP-5b) were analyzed as markers of bone turnover. P1NP and OC were classified as bone formation markers, and CTX and TRACP-5b were classified as bone resorption markers. QOL (5-Dimension EuroQol questionnaire [EQ-5D]) was measured at baseline and at 72 weeks. Serum samples were obtained under non-fasting conditions before administration of the study drugs.
Adverse events
Data on adverse events (AEs) and serious AEs (SAEs) were collected throughout the study for the safety analysis. AEs and SAEs were coded using the Medical Dictionary for Regulatory Activities/Japanese (MedDRA/J) version 21.0. Safety results are presented as the numbers and percentages of AEs for each AE, along with causality and severity.
Statistical analyses
The non-inferiority test for the primary endpoint was set at a one-sided significance level of α = 2.5%, and the two-tailed tests for the secondary endpoints were set at a significance level of α = 5%, with a two-sided confidence interval (CI) of 95%. To assess whether the result demonstrated non-inferiority for the primary endpoint, the coefficient of the CI and the lower limit of the CI value were set in keeping with a non-inferiority margin of 1.5%. It was known that the accuracy of bone mass measurement by the lumbar frontal DXA method was around 1–1.5%, and if there was no change of at least 3%, it was difficult to call the change significant. Based on the above and the study conducted by Kishimoto et al. [17], the non-inferiority margin was set to 1.5%, which was half the amount of change of 3%.
The intragroup and intergroup comparisons of the changes in BMD in the teriparatide and alendronate groups were performed by applying the paired t test and the unpaired t test to the percentage changes from baseline, respectively. For the bone turnover markers, the intragroup and intergroup comparisons were performed by applying the paired t test and the unpaired t test to the percentage changes from baseline, respectively. The incidences of new vertebral and non-vertebral fractures were calculated using the Kaplan–Meier method, and the log-rank test was used for the intergroup comparisons of the incidences of vertebral and non-vertebral fractures. Other categorical variables were compared between study groups using Fisher’s exact test.
All analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 180 subjects were enrolled, and 81 in the teriparatide group and 90 in the alendronate group were included in the safety analysis set. Of the safety analysis set, 58 subjects in the teriparatide group and 79 subjects in the alendronate group were included in the efficacy analysis set (Fig. 1). The background characteristics of the subjects in the efficacy analysis set are summarized in Table 1. No significant differences were observed in the baseline background characteristics between the two groups.
The unpaired t test between the teriparatide and alendronate groups supplemented by the LOCF method at 72 weeks showed no significant difference (Table 2). The lower limit of the CI value for the mean difference between the groups was below the non-inferiority margin. With these results, the present study could not confirm the non-inferiority of once-weekly teriparatide compared to once-weekly alendronate using the LOCF method. On the other hand, the percentage changes from baseline in lumbar spine BMD in the teriparatide and alendronate groups increased to 1.12% (p = 0.06) and 2.76% (p < 0.05) at 24 weeks, 2.6% (p < 0.05) and 3.07% (p < 0.05) at 48 weeks, and 5.09% (p < 0.05) and 4.04% (p < 0.05) at 72 weeks, respectively (Fig. 2).
Percentage change in lumbar spine BMD from baseline to 72 weeks. The circles with a solid line and rhombi with a dotted line indicate the teriparatide group and alendronate group, respectively. The error bars represent standard error. +Indicates p < 0.05 versus baseline by Student’s t test. The number of subjects at each evaluation is below the graph. BMD bone mineral density
Nine patients from the two study groups had a new vertebral fracture, and one patient in the alendronate group had a new non-vertebral (femoral neck) fracture during the observation period. The incidences of both vertebral (p = 0.29) and non-vertebral (p = 0.40) bone fractures did not differ significantly between the teriparatide and alendronate groups (Table 3).
Bone formation markers (P1NP (Fig. 3a) and OC (Fig. 3b)) increased with time after teriparatide administration and decreased after alendronate administration, whereas bone resorption markers [CTX (Fig. 3c), TRACP-5b (Fig. 3d)] decreased slightly after teriparatide administration and decreased after alendronate administration.
Percentage change in bone turnover markers from baseline to 72 weeks. The circles with a solid line and rhombi with a dotted line indicate the teriparatide group and alendronate group, respectively. The error bars represent standard error. +Indicates p < 0.05 versus baseline by Student’s t test. #indicates p < 0.05 versus between groups by Student’s t test. The number of subjects at each evaluation is shown below the graph. P1NP and OC are markers of bone formation. CTX and TRACP-5b are markers of bone resorption. a P1NP, b OC, c CTX, and d TRACP-5b
Compared with baseline, there was a small but not significant improvement in QOL in both groups at 72 weeks, as shown in Table 4 (within the teriparatide group p = 0.14 and within the alendronate group p = 0.08). No significant difference in QOL was observed between the two groups (p = 0.38).
Approximately 50% of patients in the safety analysis set reported AEs; however, the severity and/or causal relationship with study treatment was not reported (Table 5). The case of septic shock that occurred in the teriparatide group was due to the subject’s underlying disease and was not associated with the study drug. No substantial differences were observed between the two groups.
Discussion
The primary endpoint of this study, the non-inferiority of once-weekly teriparatide determined by lumbar spine BMD analysis, could not be confirmed at the final follow-up by the last observation with missing data that were imputed using the LOCF method. However, the increases of BMD and bone formation markers over time in GIOP patients treated with once-weekly teriparatide were confirmed.
Results from the placebo arms of RCTs [18, 19] and several reviews [20, 21] showed that BMD definitely decreased early when glucocorticoid treatment was started. Therefore, it would be important to see the BMD increases (Fig. 2) in each group in the present study, and an increase in BMD could be said to be the effect of the study drug.
Alendronate is the drug of first choice in the GIOP guidelines [4, 5]. The dose of 35 mg alendronate once-weekly was approved by the regulatory authorities in Japan based on the equivalence of effects on BMD and bone turnover markers in a placebo-controlled trial in primary osteoporosis [9]. The effect of 35 mg alendronate once-weekly for GIOP has also been reported [22]. Based on the above, the comparative study of once-weekly (56.5 µg) teriparatide and once-weekly alendronate (35 mg) was conducted using BMD as an index.
A decrease in BMD [14] and an increase in bone fracture risk [23] are known to manifest early during the disease course after glucocorticoid therapy is started. Both an increase in bone resorption in the early phase and a decrease in bone formation over the entire period of glucocorticoid administration are involved in the pathogenesis of GIOP [3]. The present study showed that, in the alendronate group, both the bone resorption marker and the bone formation marker decreased significantly. Furthermore, it showed that, in the once-weekly teriparatide group, the bone resorption marker decreased slightly, but the bone formation marker increased, similar to the results in patients with primary osteoporosis [8]. These changes in bone markers of this group were called the ‘anabolic window’ [24]. In contrast, daily teriparatide administration increased both bone formation and resorption markers [25]. This difference appears to depend on the differences in the mechanisms of action and the pharmacokinetics between the two teriparatide regimens.
In the study conducted by Saag et al. [7], the incidence of vertebral fractures (secondary endpoint) was significantly higher in the alendronate group than in the daily teriparatide group. However, this study found that there was no significant difference in the incidence of non-vertebral fractures. In the present study, no difference was found in fracture incidence between once-weekly teriparatide and once-weekly alendronate. The present study was likely unable to confirm any difference between the drugs because of the small number of subjects and fractures.
Patients reported slight improvements in QOL, suggesting an increase in the degree of satisfaction of patients in both groups. However, there may be a placebo effect, so that larger clinical trials may be needed. On the other hand, no severe AEs were reported in the once-weekly teriparatide group during the entire administration period (72 weeks), indicating that there are no safety concerns associated with its administration.
Bisphosphonate formulations such as alendronate suppress bone resorption and formation, whereas once-weekly teriparatide formulations act to promote bone formation. This difference in mechanism of action suggests that the once-weekly teriparatide formulation is an alternative therapy for patients with suppressed bone turnover and at risk of adverse events such as atypical femoral fracture (AFF) and bisphosphonate-related osteonecrosis of the jaw (BRONJ) associated with long-term administration of bisphosphonate formulations.
One of the limitations of this study was the relatively high dropout rate in the once-weekly teriparatide group (Fig. 2). The inconvenience of subcutaneous injection every week at a medical institution for a prolonged duration (72 weeks) and the occurrence of AEs were seen as the reasons for dropout in the post-marketing observational study [26]. This high dropout rate might contribute to the higher incorporation rate of 24-week BMD with the LOCF method in the once-weekly teriparatide group compared to that in the once-weekly alendronate group (17.2% vs 5.1%) (Table 2), and this might subsequently affect the statistical estimation of the true values.
In conclusion, the non-inferiority of once-weekly teriparatide administration could not be confirmed at the final follow-up, but the once-weekly teriparatide regimen increased the bone formation markers of GIOP patients over time, which resulted in an increase in lumbar spine BMD. This suggested that once-weekly teriparatide administration could be an alternative treatment option for GIOP patients.
References
NIH-National Institutes of Health (2000) Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 17:1–45
Akkawi I, Zmerly H (2018) Osteoporosis: current concepts. Joints 6:122–127
Weinstein RS (2011) Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med 365:62–70
Nawata H, Soen S, Takayanagi R, Tanaka I, Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S, Miki T, Sagawa A, Nishizawa Y, Seino Y, Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2005) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2004). J Bone Miner Metab 23:105–109
Suzuki Y, Nawata H, Soen S, Fujiwara S, Nakayama H, Tanaka I, Ozono K, Sagawa A, Takayanagi R, Tanaka H, Miki T, Masunari N, Tanaka Y (2014) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 32:337–350
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J et al (2017) 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol 69:1521–1537
Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, Dalsky GP, Marcus R (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357:2028–2039
Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H, Nishizawa Y, Fujita T, Shiraki M (2012) Randomized Teriparatide [human parathyroid hormone (PTH) 1–34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab 97:3097–3106
Uchida S, Taniguchi T, Shimizu T, Kakikawa T, Okuyama K, Okaniwa M, Arizono H, Nagata K, Santora AC, Shiraki M, Fukunaga M, Tomomitsu T, Ohashi Y, Nakamura T (2005) Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab 23:382–388
Adachi JD, Olszynski WP, Hanley DA, Hodsman AB, Kendler DL, Siminoski KG, Brown J, Cowden EA, Goltzman D, Ioammidis G, Josse RG, Ste-Marie LG, Tenenhouse AM, Davison KS, Blocka KL, Pollock AP, Sibley J (2000) Management of corticosteroid-induced osteoporosis. Semin Arthritis Rheum 29:228–251
American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis (2001) Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Update Arthritis Rheum 44:1496–1503
Brown JP, Josse RG (2002) Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. Can Med Assoc J 167:1S-34S
Bone and Tooth Society, National Osteoporosis Society, Royal College of Physicians (2002) Glucocorticoid-induced osteoporosis: guidelines for prevention and treatment. Royal College of Physicians, London
Van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Genant HK, Jerges M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. The study of osteoporotic fractures research group. J Bone Miner Res 11:984–996
Kishimoto H, Fukunaga M, Kushida K, Shiraki M, Itabashi A, Nawata H, Nakamura T, Ohta H, Takaoka K, Ohashi Y, Phase III Research Group (2006) Efficacy and tolerability of once-weekly administration of 17.5 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with 2.5-mg once-daily dosage regimen. J Bone Miner Metab 24:405–413
Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 339:292–299
Stoch SA, Saag KG, Greenwald M, Sebba AI, Cohen S, Verbruggen N, Giezek H, West J, Schnitzer TJ (2009) Once-weekly oral alendronate 70 mg in patients with glucocorticoid-induced bone loss: a 12-month randomized, placebo-controlled clinical trial. J Rheumatol 36:1705–1714
Briot K, Roux C (2015) Glucocorticoid-induced osteoporosis RMD Open 1:e000014
Taylor AD, Saag KG (2019) Anabolics in the management of glucocorticoid-induced osteoporosis: an evidence-based review of long-term safety, efficacy and place in therapy. Core Evid 14:41–50
Okada Y, Nawata M, Nakayamada S, Saito K, Tanaka Y (2008) Alendronate protects premenopausal women from bone loss and fracture associated with high-dose glucocorticoid therapy. J Rheumatol 35:2249–2254
van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
Takeuchi Y (2019) How different is once-weekly teriparatide from the daily once or the same? Osteoporos Sarcopenia 5:27–28
McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768
Ifuku E, Yoshimura T, Uzawa T, Hokonohara T (2019) Safety and efficacy in actual clinical practice of once-weekly subcutaneous teriparatide for osteoporosis patients with a high fracture risk. Osteoporos Sarcopenia 5:44–50
Acknowledgements
The authors would like to thank the members of the GIOP (Glucocorticoid-induced Osteoporosis) study group for their contributions as co-investigators. This study was sponsored by Asahi Kasei Pharma Corporation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by IT and HO. Data analysis was performed by SS and HO. The draft of the manuscript was written by IT, SS, and HO. All authors reviewed and commented on previous versions of the manuscript. The final manuscript was edited by IT and HO, and all authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
I.Tanaka has received speaking fees, and/or honoraria from Asahi Kasei Pharma, Astellas Pharma, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan, Mitsubishi Tanabe Pharma, MSD, Ono Pharmaceutical, Pfizer Japan, Takeda Pharmaceutical, Taisho Pharma, and Teijin Pharma. Y. Tanaka has received speaking fees and/or honoraria from AbbVie, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan, Janssen Pharmaceutical, Mitsubishi Tanabe Pharma, Novartis Pharma, Pfizer Japan, Takeda Pharmaceutical, Teijin Pharma, and YL Biologics and has received research grants from Asahi Kasei Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, Sanofi, Takeda Pharmaceutical, and UCB Japan. S. Soen has received consulting fees, speaking fees, and/or honoraria from Asahi Kasei Pharma, Astellas Pharma, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan, MSD, Ono Pharmaceutical, Pfizer Japan, Takeda Pharmaceutical, and Teijin Pharma. H. Oshima has no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tanaka, I., Tanaka, Y., Soen, S. et al. Efficacy of once-weekly teriparatide in patients with glucocorticoid-induced osteoporosis: the TOWER-GO study. J Bone Miner Metab 39, 446–455 (2021). https://doi.org/10.1007/s00774-020-01171-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01171-5