Abstract
Introduction
Osteoporosis is a common disorder characterized by decreased bone mineral density (BMD). Interestingly, osteoporosis and obesity have several similar features, including a genetic predisposition and a common bone marrow stem cell. With aging, the composition of bone marrow shifts to adipocytes, osteoclast activity increases, and osteoblast function declines, resulting in osteoporosis.
Materials and methods
We performed a genome-wide association study (GWAS) analysis with osteoporosis and body mass index (BMI) and did identify an association in 349 and 384 SNPs by filtering with the significant p values (p < 0.001) of BMI and osteoporosis, respectively.
Results
Only three of those SNPs were common (rs2326365, rs7097028, and rs11000205) between the SNPs significantly associated with BMI and/or osteoporosis in Korean Association REsource (KARE) females. Two of the three SNPs belonged to the ASCC1 gene and one to the FAM50B gene. We carried out a minor allele frequency (MAF) analysis of the rs7097028 and rs11000205 SNPs in the ASCC1 gene with a geographic genome variant browser. Both rs7097028 and rs11000205 in the ASCC1 gene were seen mostly in African and Southeast Asian populations.
Conclusions
Our results suggest that the ASCC1 gene is a significant genetic factor for determining the risk for both osteoporosis and obesity in KARE postmenopausal females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is an increasing problem in our elderly society [1], which can increase the risk of fractures due to decreased bone mineral density (BMD) [2, 3]. BMD is affected by both environmental and genetic factors. Both adipocytes and bone cells originate from the same bone marrow stem cells [4] and the cells responsible for new bone formation, osteoblasts, reside in the bone marrow. Thus, if more of the marrow is taken over by fat cells, this will weaken the bones. These two disorders synergistically induce functional impairments and physical disorders [5], suggesting a complication of obesity on bone health. Also, processes that accelerate osteoclast activation, such as menopause, can further increase the risk of fragility and fractures. Moreover, the function of osteoblasts is reduced late in life, further amplifying the imbalance between bone resorption and formation [6].
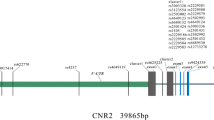
Activation signal cointegrator complex (ASCC, also known as ASC-1), which plays a key role in repairing alkylated DNA in cell lines, is composed of three proteins: ASCC1, ASCC2 and ASCC3 (also known as p50, p100, and p200) [7, 8]. The ASCC1 gene is located on human chromosome 10q22. Very little is known about the association between other members of the ASCC1-containing complex and disease [9]. A recent study described a germline missense mutation in exon 8 of ASCC1 in patients with Barrett’s esophagus and esophageal adenocarcinoma [10]. Recently, recessive mutations in ASCC1 were reported to cause hypotonia, arthrogryposis, and bone fractures in neonates with poor prognosis [11].
However, osteoporosis is a complex, multifactorial disorder, resulting from lifestyle, as well as gene–gene and environment interactions. It is often due to the loss of ovarian hormones, especially estrogen; associated with a certain race (Asian); inadequate calcium and vitamin d in the diet; smoking; high coffee consumption; some hormonal disorders, such as hyperthyroidism and parathyroidism; and sexual gland deficiency [12]. Aging, body composition, metabolic factors, and postmenopausal hormone levels, along with decreased physical activity, may provide conditions for weight gain, especially fat mass [13].
However, to our knowledge, no study has focused on the impact of ASCC1 gene variants and osteoporosis and obesity risk, and the impact of gene–environmental interaction on osteoporosis in Korean postmenopausal females. We aimed to identify the SNPs from our genome-wide association study (GWAS) that simultaneously increased the risk of osteoporosis and obesity. Our study identified 3 SNPs (rs2326365, rs7097028, and rs11000205) that were associated with both osteoporosis and obesity.
Materials and methods
Subjects
The subjects of the Korean Association REsource (KARE) included in this study were recruited from the Ansung and Ansan cohorts of the Korean Genome and Epidemiology Study (KoGES), which represents rural and urban communities in Korea, respectively. A total of 10,038 people were recruited and originated from the Korean Health and Genome Study. Among them, 1196 were excluded after genotype quality control, following which 8842 genotypes (4183 men and 4659 women; age 40–69 years) were released into the public domain. The detailed analysis of the Ansung and Ansan cohorts was described in a previous report [14].
Because osteoporosis is more common in postmenopausal females, we studied only females aged ≥ 40 years (n = 3,013). Females (855) who were taking medications that could affect bone density and 791 females who did not have bone density measurements were excluded. We categorized the participants as two groups of subjects: the ≥ 40-year-old women and ≥ 50-year-old women. There were 2223 normal controls and 443 osteoporosis cases in ≥ 40-year-old women, and 722 normal controls and 405 osteoporosis cases in 50-year-old women (Table 1).
Height and weight were measured and body mass index [BMI = weight (kg)/height (m2)] was calculated. Obesity (cases) was defined by a BMI equal to or greater than 30 kg/m2 and non-obesity (controls) was defined by a BMI equal to or less than 25 kg/m2. Osteoporosis is normally diagnosed by dual-energy X-ray absorptiometry (DXA), fracture history, and FRAX worldwide and these remain the standard for osteoporosis assessment, but there are several advantages, such as devices are portable, costs are lower, no ionizing radiation exposure; therefore, quantitative ultrasound (QUS) is suitable for large population cohort study. So, the bone speed of sound (SOS) value was measured at the distal radius and midshaft tibia using an Omnisense 7000P QUS device (Sunlight Medical Ltd, Tel-Aviv, Israel) [15]. The T score was calculated by dividing the difference between the measured SOS and the mean SOS in a healthy adult population by the standard deviation (SD) of the SOS in an adult population. Subjects with T scores at either the distal radius SOS (DR-SOS) or the midshaft tibia SOS (MT-SOS) < − 2.6 SD and − 3.0 SD, respectively, were classified as case subjects according to the diagnostic categories established for adult females [16, 17]. Subjects in whom both the DR-SOS and MT-SOS T scores were > − 1.4 SD and − 1.6 SD, respectively, were classified as control subjects [17].
The genetic information used in this study was distributed by the Human Resources Bank of Korea Centers for Disease Control and Prevention (KBN-2017-046) and analyzed after approval by the Research Ethics Committee of the Korea National Institute of Health (KNIH) and Hoeso University (1041231170418-HR-056-02). Written informed consent was obtained from all subjects.
Genotyping and selection of SNPs
Genotype data were obtained by the Center for Genome Science, Korea National Institute of Health. DNA isolated from participants’ peripheral blood was genotyped with an Affymetrix Genome-Wide Human SNP array 5.0 (Affymetrix, Inc., Santa Clara, CA, USA). Subjects with a genotyping accuracy ≤ 98%, high missing genotype call rates (≥ 4%) and heterozygosity (> 30%), or gender mismatch were excluded. We performed GWAS and selected three SNPs in two genes (ASCC1 and FAM50B). The location of the genes and SNPs on the chromosome was based on the NCBI human genome build 36 (hg18) assembly.
Statistical analysis
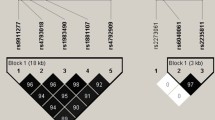
In most statistical analyses, PLINK version 1.07 (https://pngu.mgh.harvard.edu/–purcell/plink) was used. Linear regression analysis was used for examining the association with BMI, which was an obesity-related quantitative trait, based on the additive genetic model. The osteoporosis and obesity case–control association analysis was performed using logistic regression models based on the additive genetic model for the estimation of odds ratios (OR) [95% confidence interval (CI)]. Age, residential area, smoking, alcohol habit and exercising were used as covariates in both analyses. Statistical significance was analyzed by the two-tailed Student’s t test, and p values of < 0.05 were considered significant. The Haploview version 4.1 (Whitehead Institute for Biomedical Research, Cambridge, MA, USA) program was used to examine the linkage disequilibrium (LD) block based on the data from the KARE database. The Geography of Genetic Variants (GGV) browser (https://popgen.uchicago.edu/ggv), which analyzes based on 1000 Genome database, was used to show the distribution of the SNPs in various countries.
Results
Participant characteristics
In this study, the KARE cohort included 3013 females (age 40–69 years) for the association studies. Age, weight, height, BMI, DR-SOS, MT-SOS, DR-SOS T score, and MT-SOS T score are shown in Table 1. As shown in Table 1, age in the case–control study was different between the two groups. The patients in the osteoporosis case in both groups were older than the patients in the control group. Significant differences in all main characteristics, including BMI, MT-SOS, DR-SOS, DR-SOS T score, and MT-SOS T score, were observed between the control and case subjects. In addition, lower DR-SOS, MT-SOS, DR-SOS T score, and MT-SOS T score, and higher age and BMI were seen in the women with osteoporosis, compared to those in the control group.
Selection of SNPs from the KARE data
In the GWAS, the SNPs (p < 10–3), were selected based on the KARE data. We found 349 and 384 SNPs (Supplement tables 1 and 2) that were related to BMI and osteoporosis, respectively, and only three of those SNPs were common (rs2326365, rs7097028, and rs11000205). Two of the three SNPs belonged to the ASCC1 gene and one to the FAM50B gene. Then we analyzed the association between BMI and osteoporosis for each gene and found 14 SNPs in the ASCC1 gene and seven SNPs in the FAM50B. Among the 14 SNPs in the ASCC1 gene, 10 and 11 SNPs were highly associated with BMI and osteoporosis, respectively, in the women aged ≥ 40. In contrast, only one of the seven SNPs of the FAM50B gene was associated with BMI and osteoporosis (Table 2).
Association of SNPs with BMI and bone mineral density
We examined the association of the three SNPs (rs2326365, rs7097028, and rs11000205) with the obesity-related quantitative trait (BMI) and osteoporosis in the KARE cohort females aged ≥ 40 and ≥ 50 based on the additive model. In the females aged ≥ 40, only SNP rs2326365 of the FAM50B gene was significantly associated with BMI (β = 0.32, p = 8.63 × 10–4), obesity (OR = 1.34, 95% CI 1.05–1.71, p = 0.017), and osteoporosis (OR = 1.48, 95% CI 1.18–1.85, p = 7.9 × 10–4) (Table 3). For the ASCC1 gene, SNPs rs7097028 and rs11000205 showed associations with BMI (β = 0.45, p = 1.77 × 10–3, β = 0.34, p = 1.29 × 10–3, respectively), obesity (OR = 1.46, 95% CI 1.03–2.01, p = 0.032, OR = 1.29, 95% CI 1.06–1.82, p = 0.017), and osteoporosis (OR = 1.70, 95% CI 1.23–2.36, p = 1.39 × 10–3, OR = 1.57, 95% CI 1.23–2.02, p = 3.60 × 10–4, respectively) (Table 3).
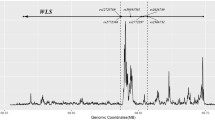
In case of the women aged ≥ 50, only rs11000205 of the ASCC1 gene was significantly associated with BMI (β = 0.66, p = 1.44 × 10–5), obesity (OR = 1.97, 95% CI 1.67–2.83, p = 2.55 × 10–4), and osteoporosis (OR = 1.52, 95% CI 1.16–1.99, p = 2.35 × 10–3) (Table 4). rs7079028 had no associations with females aged ≥ 50. For the FAM50B gene, rs2326356, which had association in females aged ≥ 40, showed associations with only BMI (β = 0.40, p = 3.27 × 10–3) and osteoporosis (OR = 1.66, 95% CI 1.29–2.12, p = 6.54 × 10–5) (Table 4). The other SNPs in ASCC1, rs7072673, rs1245516, rs7088203, rs12098424, rs11000212, rs11000213, rs11000214, and rs10823907, showed associations with obesity and osteoporosis (p < 0.05) in both groups. Additionally, when the association between the ASCC1 gene and obesity was analyzed, six SNPs reached genome-wide significance after Bonferroni corrections and eight SNPs in the ASCC1 gene reached genome-wide significance for an association with osteoporosis (Bonferroni-corrected p value threshold was 0.00357) (Fig. 1).
Plots for the association of the ASCC1 SNPs with osteoporosis and obesity in the KARE postmenopausal female subjects. Standard significant p value threshold (p = 0.05) and the Bonferroni-corrected p value threshold for the ASCC1 gene (p = 0.00357) are indicated by the dotted lines and the 14 SNPs analyzed is plotted. Haploview represents the level of linkage disequilibrium (r2)
Geography of genomic variants
We further analyzed the frequency of the rs11000205 SNPs in the ASCC1 gene with a geographic genome variants (GGV) browser. The GGV browser provides maps of allelic frequencies in populations distributed across the globe based on 1000 genomes (hg19) (Fig. 2). The rs11000205 was mostly seen in Africa and Southeast Asia. For SNP rs11000205, its minor allele frequency (MAF) was higher in African than in East Asian populations and was hardly seen in Caucasians (Han Chinese in Beijing, China (CHB): 0.165; Gambians in western divisions in the Gambia (GWD): 0.252; Mende people in Sierra Leone (MSL): 0.329; Americans of African ancestry in the southwestern USA (ASW): 0.265; Finnish people in Finland (FIN): 0; and people of Mexican ancestry in Los Angeles USA (MXL): 0.02). In our cohort, the minor allele frequencies of rs11000205 were 0.152, and were very similar to those of Chinese and Japanese populations.
Discussion
Recently, many GWAS have identified relationships between diseases and genetic variants. In this study, we performed an analysis of genetic variations in the ASCC1 and FAM50B genes, genes associated with both osteoporosis and BMI, using a Korean female cohort. Putative functional SNPs in the ASCC1 and FAM50B genes were genotyped to assess their relationship to osteoporosis and obesity. Only three SNPs, rs2326365, rs7097028, and rs11000205, were found to be associated with both osteoporosis and obesity in our cohort. As a result, we found that the C allele of rs2326365, and the G allele of rs11000205 were correlated with both increased osteoporosis and obesity risks in Korean postmenopausal women. Previous studies have been conducted to investigate the relationship between the ASCC1 gene and osteoporosis. However, to our knowledge, our research is the first case–control GWAS on the relationship between SNPs in ASCC1 and FAM50B genes and osteoporosis and obesity risk.
The FAM50B (family with sequence similarity 50 member B) gene, located on human chromosome 6p25, contains an intronless open reading frame (ORF) that arose from ancestral retroposition. The encoded protein is associated with a plant protein that regulates circadian rhythm. This gene is contiguous to a differentially methylated region and is engraved and paternally expressed in many tissues (provided by RefSeq, Nov 2015). There is little published research on the FAM50B gene and further studies need to be carried out in the future. Therefore, we decided to focus on the ASCC1 gene. The ASCC1 gene that encodes subunits of the nuclear activating signal cointegrator 1 (ASC-1) complex is well known to downregulate genes associated with neurogenesis, neuronal migration, and bone development [11]. The rs11000217 ASCC1 gene variant presented in this study was found in a Turkish family as indicated in the GGV and this mutation was also specific only to Africans and Asians (Supplement Fig. 1).
Although an ASCC1 variant was selected as a prospective candidate in our study, reports relating it to disease or phenotypes are scant. One of the previous studies suggested that the ASCC1 complex seems to be related to the highly regulated development of neuromuscular units and possibly bone structures in humans, as well as in mice and zebrafish [11]. In that study, gene expression knockdown models were mediated by antisense morpholino oligonucleotides and there was a severe impediment in neuromotor unit development. Silvia et al. found that an inactive variant of ASCC1 was associated with a disease, which had clinical value for observing the progression and prognosis of rheumatoid arthritis [9]. Oliveira et al. reported a case that derived from a genetic deficiency in ASCC1, which caused severe congenital neuromuscular disease, thereby confirming its involvement in bone fractures [18]. Our study showed results similar to those in previous in vivo experiments. In addition, we analyzed the recombination rate with the Haploview database but a high recombination rate among SNPs in the ASCC1 gene was not found in Korean women (Fig. 1). In addition, we used Regulome DB (https://www.reguloumdb.org/index) and HaploReg (https://archive.broadinstitute.org/mammals/haploreg/haploreg_v3.php) to perform in silico analysis to determine how the SNPs that were statistically significant in the ASCC1 and FAM50B genes, influenced gene or protein expression (Supplement table 3). Changes in rs7079028 and rs1100205 motifs were predicted by HaploReg, indicating that genotypes are likely to affect ASCC1 gene expression. However, neither of them showed a score in the Regulome DB. This means that the presence of the minor allele is not an expression quantitative trait loci (eQTL) region that indicates a difference in the expression of the ASCC1 gene. In addition, no features were found in the FAM50B gene variant.
Our hypothesis is that in Korean postmenopausal women carrying the minor alleles of rs7097028 and rs11000205 have increased BMI values and the risk of osteoporosis. However, the obesity paradox between osteoporosis and obesity remains controversial. Taes et al. reported that increased fat mass was related to having small bone sizes, in contrast to the entrenched view that a low BMI is a destructive factor for osteoporosis.[19]. Zhao et al. and Janicka et al. demonstrated that increased body fat rates may negatively affect bone mass in each group [20, 21]. Chang et al. also suggested that obesity was negatively associated with osteoporosis in older women [22].
Tzanavari et al. showed that TNF-α is present in and secreted by fat tissue and its levels correlated with the degree of adiposity [23]. Adipose tissue, taking a classical role as an energy repository, is also a main endocrine organ whose circulating levels are affected by the degree of fat mass. Obesity leads to the permeation of the expanded fat tissue by macrophages and increased levels of proinflammatory cytokines. The evidence for increased cytokine discharge in obesity was reported for the first time by the findings of increased TNF-α expression, a proinflammatory cytokine, in the fat tissue of obese rodents [24]. We could relate this study to the study by Silvia et al. [9]. They showed that ASCC1 blocked NF-kB activation and that a truncated and inactive variant of ASCC1 was related to the progression and prognosis of rheumatoid arthritis. They performed massive sequencing of the NF-kB pathway in rheumatoid arthritis patients, focusing on the ASCC1 gene, and indicated that it severely inhibited the expression of NF-kB target genes (TNF-α, IL8, and others) and interrupted the activation of an NF-kB-luciferase reporter construct in human cell lines. Moreover, ASCC1 consistently reduced the expression of NF-kB target genes, including TNF-α, and the activation of NF-kB by TNF-α was drastically reduced in the presence of wild-type ASCC1. However, no inhibition was observed when the cells were transfected with the truncated variant. In summary, adipose tissue enhanced the expression of TNF-α, TNF-α activated NF-kB, and the presence of wild-type ASCC1 was reduced. Finally, we concluded that ASCC1 mutation downregulated the genes associated with neurogenetic disease and bone development.
While some authors have reported low body mass index as a recognized risk factor for bone density [25], Cui et al. reported that the body fat rates in Korean may positively contribute to BMD only in postmenopausal females and old males. Lekamwasam et al. also found similar results in premenopausal women in Sri Lanka [26, 27]. Mohammad et al. provided additional evidence for the obesity paradox in individuals with osteoporosis by indicating that there was a negative association between fat mass and a positive association of lean mass with BMD [28]. The contradictions between these results could be due to the specific study populations (specific gender or age), ethnicity, sampling methods, participant numbers, and genetic differences.
A recent ethnic study found that the clinical vertebral-to-hip fracture ratios in Hong Kong Chinese and Japanese populations were higher than those in a Caucasian population [29]. Bow et al. demonstrated that Asian women had a much higher vertebral fracture risk than Caucasian women. Although our population had a low hip fracture rate, Hong Kong females had a higher frequency of osteoporosis (bone mineral density T score ≤ − 2.5) than Caucasian females (35.8% vs. 20%, respectively) [30, 31]. In ethnic differences studies, it is important to obtain precise and varied fracture risk information on each population to characterize the complete fracture risk of individual subjects. In addition, we found that rs11000205 in the ASCC1 gene were rarely found in Caucasians, whereas they were quite frequent in East Asians or Africans (Fig. 2), similar to previous studies.
In summary, we found that ASCC1 SNPs were highly associated with both osteoporosis and obesity in Asians, especially Korean postmenopausal women, suggesting that people with gene variants are at an increased risk for osteoporosis. In the future study, more SNPs in ASCC1 gene and FAM50B should be taken into account including various gene–environment interaction analyses. There has not been much research on SNPs related to the ASCC1 gene, possibly because they are not frequently expressed in Caucasians.
In conclusion, the results of this study indicated that genetic variants in ASCC1 were associated with both osteoporosis and obesity. Further study is needed to focus on ASCC1 genes in African or other Asian populations.
References
Braithwaite RS, Col NF, Wong JB (2003) Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc 51:364–370
Kelly PJ, Morrison NA, Sambrook PN, Nguyen TV, Eisman JA (1995) Genetic influences on bone turnover, bone density and fracture. Eur J Endocrinol 133:265–271
Zwart M, Azagra R, Encabo G, Aguye A, Roca G, Guell S, Puchol N, Gene E, Lopez-Exposito F, Sola S, Ortiz S, Sancho P, Abado L, Iglesias M, Pujol-Salud J, Diez-Perez A (2011) Measuring health-related quality of life in men with osteoporosis or osteoporotic fracture. BMC Public Health 11:775
Rosen CJ, Klibanski A (2009) Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 122:409–414
Yamaguchi T, Kanazawa I, Yamamoto M, Kurioka S, Yamauchi M, Yano S, Sugimoto T (2009) Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone 45:174–179
Kveiborg M, Flyvbjerg A, Rattan SI, Kassem M (2000) Changes in the insulin-like growth factor-system may contribute to in vitro age-related impaired osteoblast functions. Exp Gerontol 35:1061–1074
Jung DJ, Sung HS, Goo YW, Lee HM, Park OK, Jung SY, Lim J, Kim HJ, Lee SK, Kim TS, Lee JW, Lee YC (2002) Novel transcription coactivator complex containing activating signal cointegrator 1 (in English). Mol Cell Biol 22:5203–5211
Dango S, Mosammaparast N, Sowa ME, Xiong LJ, Wu FZ, Park K, Rubin M, Gygi S, Harper JW, Shi Y (2011) DNA unwinding by ASCC3 helicase is coupled to ALKBH3-dependent DNA alkylation repair and cancer cell proliferation (in English). Mol Cell 44:373–384
Torices S, Alvarez-Rodriguez L, Grande L, Varela I, Munoz P, Pascual D, Balsa A, Lopez-Hoyos M, Martinez-Taboada V, Fernandez-Luna JL (2015) A truncated variant of ASCC1, a novel inhibitor of NF-kappaB, is associated with disease severity in patients with rheumatoid arthritis. J Immunol 195:5415–5420
Orloff M, Peterson C, He X, Ganapathi S, Heald B, Yang YR, Bebek G, Romigh T, Song JH, Wu W, David S, Cheng Y, Meltzer SJ, Eng C (2011) Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 306:410–419
Knierim E, Hirata H, Wolf NI, Morales-Gonzalez S, Schottmann G et al (2016) Mutations in subunits of the activating signal cointegrator 1 complex are associated with prenatal spinal muscular atrophy and congenital bone fractures. Am J Hum Genet 98:473–489
Compston JE, McConachie C, Stott C, Hannon RA, Kaptoge S, Debiram I, Love S, Jaffa A (2006) Changes in bone mineral density, body composition and biochemical markers of bone turnover during weight gain in adolescents with severe anorexia nervosa: a 1 year prospective study. Osteoporos Int 17:77–84
Hu FB (2003) Overweight and obesity in women: health risks and consequences. J Womens Health (Larchmt) 12:163–172
Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH et al (2009) A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet 41:527–534
Weiss M, Ben-Shlomo AB, Hagag P, Rapoport M (2000) Reference database for bone speed of sound measurement by a novel quantitative multi-site ultrasound device. Osteoporos Int 11:688–696
Jin HS, Kim BY, Kim J, Hong KW, Jung SY, Lee YS, Huh D, Oh B, Chung YS, Jeong SY (2013) Association between the SPRY1 gene polymorphism and obesity-related traits and osteoporosis in Korean women. Mol Genet Metab 108:95–101
Knapp KM, Blake GM, Spector TD, Fogelman I (2004) Can the WHO definition of osteoporosis be applied to multi-site axial transmission quantitative ultrasound? Osteoporos Int 15:367–374
Oliveira J, Martins M, Pinto Leite R, Sousa M, Santos R (2017) The new neuromuscular disease related with defects in the ASC-1 complex: report of a second case confirms ASCC1 involvement. Clin Genet 92:434–439
Taes YE, Lapauw B, Vanbillemont G, Bogaert V, De Bacquer D, Zmierczak H, Goemaere S, Kaufman JM (2009) Fat mass is negatively associated with cortical bone size in young healthy male siblings. J Clin Endocrinol Metab 94:2325–2331
Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW (2007) Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 92:1640–1646
Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V (2007) Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab 92:143–147
Chang CS, Chang YF, Wang MW, Chen CY, Chao YJ, Chang HJ, Kuo PH, Yang YC, Wu CH (2013) Inverse relationship between central obesity and osteoporosis in osteoporotic drug naive elderly females: The Tianliao Old People (TOP) Study. J Clin Densitom 16:204–211
Tzanavari T, Giannogonas P, Karalis KP (2010) TNF-alpha and obesity. Curr Dir Autoimmun 11:145–156
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259:87–91
Moayyeri A, Luben RN, Wareham NJ, Khaw KT (2012) Body fat mass is a predictor of risk of osteoporotic fractures in women but not in men: a prospective population study. J Intern Med 271:472–480
Cui LH, Shin MH, Kweon SS, Park KS, Lee YH, Chung EK, Nam HS, Choi JS (2007) Relative contribution of body composition to bone mineral density at different sites in men and women of South Korea. J Bone Miner Metab 25:165–171
Lekamwasam S, Weerarathna T, Rodrigo M, Arachchi WK, Munidasa D (2009) Association between bone mineral density, lean mass, and fat mass among healthy middle-aged premenopausal women: a cross-sectional study in southern Sri Lanka. J Bone Miner Metab 27:83–88
Salamat MR, Salamat AH, Janghorbani M (2016) Association between obesity and bone mineral density by gender and menopausal status. Endocrinol Metab (Seoul) 31:547–558
Bow CH, Cheung E, Cheung CL, Xiao SM, Loong C, Soong C, Tan KC, Luckey MM, Cauley JA, Fujiwara S, Kung AW (2012) Ethnic difference of clinical vertebral fracture risk. Osteoporos Int 23:879–885
Kung AW, Lee KK, Ho AY, Tang G, Luk KD (2007) Ten-year risk of osteoporotic fractures in postmenopausal Chinese women according to clinical risk factors and BMD T-scores: a prospective study. J Bone Miner Res 22:1080–1087
Melton LJ 3rd (1995) How many women have osteoporosis now? J Bone Miner Res 10:175–177
Acknowledgements
This work was supported by the Soonchunhyang University research fund, and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [NRF-2020R1F1A1071977].
Author information
Authors and Affiliations
Contributions
YBE and HSJ participated in the design of the study, contributed to data reduction/analysis and interpretation of results; HWC contributed to data analysis and interpretation of results. All authors contributed to the manuscript writing. All authors reviewed and approved the final version of the manuscript and agree with the order of presentation of the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board of the Korean National Institute of Health (KNIH, KBN-2017-046) and Hoeso University (1041231170418-HR-056-02). Written informed consent was obtained from all subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Cho, HW., Jin, HS. & Eom, YB. Association between non-Caucasian-specific ASCC1 gene polymorphism and osteoporosis and obesity in Korean postmenopausal women. J Bone Miner Metab 38, 868–877 (2020). https://doi.org/10.1007/s00774-020-01120-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01120-2