Abstract
Vitamin D deficiency is observed worldwide and represents a health hazard for mothers, infants and elderly persons. We know that many young Japanese women experience vitamin D insufficiency; however, there is a lack of knowledge regarding the serum 25-hydroxyvitamin D [25(OH)D] profile of pregnant Japanese women and of the association between maternal 25(OH)D level and maternal bone mass during pregnancy and lactation. In this longitudinal study, 160 pregnant Japanese women were enrolled; of them, 68 have been followed-up from the first trimester through at least 1 year of breast-feeding. We estimated serum 25(OH)D levels, intact PTH levels, calcaneus quantitative ultrasound (QUS: T score) scores, bone mineral density at the distal one-third of the radius, dietary intakes according to the Food Frequency Questionnaire, and sunlight exposure times. We found that Vitamin D deficiency is prevalent in Japanese women, irrespective of pregnancy or lactation, and our analysis suggested that 25(OH)D levels and BMI in the first trimester were related to the lactating women’s bone mass from after delivery to 1 year after delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D deficiency is a well-known cause of rickets in neonates and infants [1]. Recently, vitamin D deficiency during pregnancy and lactation has been associated with maternal preeclampsia [2, 3] and gestational diabetes mellitus [4, 5]. A study reported that children delivered by a mother with vitamin D deficiency may have a significantly lower bone mass at the age of 20 years [6] and that exposure to vitamin D deficiency in utero may have long-lasting effects on the infants. Moreover, it places the neonate at a higher risk for craniotabes [7], low birth-weight [8], preterm birth [9], and infections [10]. Many reports have been published on maternal vitamin D supplementation in an attempt to improve maternal and fetal outcomes [11,12,13,14,15,16]. However, how vitamin D deficiency during pregnancy and breastfeeding affects maternal bone mass [17] has not yet been determined. To clarify the relationship between 25-hydroxyvitamin D [25(OH)D] concentration profiles and maternal bone mass, we carried out a prospective cohort study by following participants from pregnancy and during their first year of breastfeeding.
Materials and methods
A total of 160 healthy Japanese women, who attended the Yoshikata Obstetrics and Gynecology Clinic (Yokohama, Japan) between November 2010 to February 2011 and who were up to 12 weeks pregnant and free from any underlying diseases and drug intake, which might affect bone metabolism, were enrolled in this study. The measurement was conducted within the limitation of recruiting early pregnant women from November to January (winter). We accommodated each pregnancy period, because the level of 25(OH)D varies seasonally [18] The participants were asked to visit us for testing in the first trimester (weeks 8–12), second trimester (weeks 22–24), third trimester (weeks 32–34), delivery (3 days postpartum), 1 month postpartum, 6 months postpartum, and 1 year postpartum. A condition was that mothers breastfed their babies for at least 1 year, and any participants who weaned their babies before their first birthday were excluded from the analysis. We also measured 25(OH)D and intact-parathyroid hormone (PTH) levels of 30 women who were neither pregnant nor lactating as the controls.
This joint research project was called the SKY (named after the three cities where joint research facilities were located: Sakado, Kobe, and Yokohama, Japan) pregnant cohort study. The Institutional Review Board at the Yokohama City University School of Medicine (Yokohama, Japan) approved this clinical study, and all participants provided informed consent.
Measurements
Sunlight exposure
At each hospital visit, participants were asked to record sunlight exposure for the past 2 weeks, including details such as where they applied sunscreen and the extent of sunscreen use. We calculated the ratio of the part of the skin on which sunscreen was used; partially applied sunscreen was defined as not exposed under sunlight. Sunlight exposure times were calculated from this information.
Dietary intake
Nutritional states were evaluated according to the Food Frequency Questionnaire (FFQ) [19, 20]. Participants filled out these forms during their clinic visits, and the Laboratory of Physiological Nutrition, Kagawa Nutrition University (Sakado, Japan) analyzed the results. Participants were also questioned about using supplements. Vitamin D, calcium, vitamin K, and the total calorie intake of meals they had were calculated. Participants were not provided with any nutritional guidance.
Anthropometry
Height and body weight were measured at the initial visit. Body weights were recorded during every subsequent visit.
Serum 25(OH)D, intact parathyroid hormone (intact-PTH)
Serum was kept frozen at − 30 °C until analysis. Serum 25(OH)D was assayed with LIAISON® (LIAISON® N-TACT® PTH II Assay: DiaSorin, Stillwater, MN, USA) [21]. Intact-PTH was measured at the Department of Hygienic Sciences, Kobe Pharmaceutical University (Kobe, Japan).
Bone ultrasound measurement
To avoid any radiation exposure during pregnancy, we measured the bone density by calcaneus quantitative ultrasound (QUS) using the Lunar A-1000 EXPRESS, GE Healthcare. Measurements were carried out in a temperature and humidity-controlled room by one of three designated technicians. Results were derived in the form of broadband ultrasound attenuation (BUA), speed of sound (SOS), and stiffness. In this study, participants were evaluated with T scores of Stiffness [22].
Bone mineral density (BMD)
After birth, in addition to QUS measurements, BMD of the distal third of the radius in the non-dominant arm was determined by dual-energy X-ray absorptiometry: DXA (DTX-200, Osteometer MediTech Inc). A single technician recorded all the measurements for BMD.
Statistical analysis
Data were analyzed at the Department of Hygienic Sciences, Kobe Pharmaceutical University (Kobe, Japan) and Yoshikata Obstetrics and Gynecology Clinic using the JMP® (SAS Institute, USA) statistical software program.
Results
Subjects’ characteristics
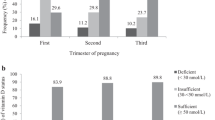
The characteristics of the participants at the point of enrollments are shown in Table 1. Of the 160 pregnant women entering the study, the following number of women were available for follow-up: 158 in the first trimester of their pregnancy (2 participants dropped out due to early-stage miscarriage); 147 in the second trimester (11 dropped out due to early-stage miscarriage or a change of address); 144 in the third trimester (2 dropped out due to premature abruption, and 1 neonate had congenital malformations and was transferred to an advanced medical facility). Of the 142 participants who gave birth, 1 suffered placental abruption and 1 had a severely small-for-dates neonate who was required to transfer to an advanced medical institution. A total of 142 participants were available for follow-up at 1 month postpartum. Obstetric care is usually terminated at 1 month postpartum in Japan. We provided accounts of further examination to 142 participants and obtained consent from 76 of them. At 6 months postpartum, 76 participants were available for follow-up and 68 were available at 1 year postpartum (8 dropped out due to weaning). In this study, we analyzed the results of participants who could be followed until 1 year postpartum (Fig. 1.)
25(OH)D
All participant’s blood levels of 25(OH)D are showed in Table 2. The levels were 9.2 ± 3.6 ng/mL in the first trimester, 8.3 ± 2.9 ng/mL in the second trimester, and 10.6 ± 4.3 ng/mL in the third trimester. After delivery, they were 10.1 ± 4.5 ng/mL at 3 days postpartum, 13.1 ± 5.0 ng/mL at 1 month postpartum, 16.0 ± 4.8 ng/mL at 6 months postpartum, and 21.4 ± 5.7 ng/mL at 1 year postpartum. Throughout the entire observation period, 25(OH)D levels were extremely low, and participants were estimated as either vitamin D deficient or insufficient. The group of non-pregnant and non-lactating women had levels of 13.1 ± 4.6 ng/mL, which are as low as pregnant women.
68 women’s blood levels of 25(OH)D which could be followed until 1 year after delivery, were 9.4 ± 3.8 ng/mL in the first trimester, 8.3 ± 2.8 ng/mL in the second trimester, 10.9 ± 4.7 ng/mL in the third trimester, and 10.2 ± 4.4 ng/mL at delivery. Levels of 25(OH)D were 12.9 ± 4.8 ng/mL at 1 month postpartum, 15.8 ± 4.8 ng/mL at 6 months postpartum, and 21.4 ± 5.7 ng/mL at 1 year postpartum. Analyzing the results with ANOVA revealed a statistically significant difference. We conducted the analysis using the HSD test of the Tukey–Kramer method; 25(OH)D levels at 6 months postpartum and 1 year postpartum were higher than those in the first trimester (p < 0.0001), and those at 1 month postpartum were higher than those in the first trimester (p = 0.0002). The 25(OH)D levels at 6 months postpartum and 1 year postpartum were higher than those at delivery (p < 0.0001), and so were those at 1 month postpartum (p = 0.0121) (Fig. 2a).
a The blood levels of 25(OH)D which could be followed until 1 year after delivery (n = 68): no significant change was found during pregnancy. The 25(OH)D levels were significantly higher at 1 month, 6 months, and 1 year postpartum than the first trimester and delivery. The data are represented as mean ± SD. (++p value< 0.0001, +p value< 0.001 vs. first trimester, **p < 0.0001, *p < 0.05 vs. delivery; with ANOVA, by HSD test of Tukey–Kramer). b Intact-PTH levels during pregnancy and lactation. The intact-PTH level decreased at delivery, gradually increased during lactation, and reached approximately the same level as in the control group at 1 year postpartum. Compared with the first trimester, levels were significantly lower at delivery and 1 month postpartum, and significantly higher at 1 year postpartum (++p value < 0.0001 vs. first trimester). Levels were significantly higher at 6 months, and 1 year postpartum than at delivery (**p value < 0.0001 vs. delivery; with ANOVA, by HSD test of Tukey–Kramer)
Intact-PTH
Blood levels of intact-PTH, which were analyzed in all participants including non-pregnant and non-lactating women, are shown in Table 2. A total of 68 women’s blood levels of intact-PTH which could be followed until 1 year after delivery, were at 35.9 ± 16.6 pg/mL in the first trimester, 33.9 ± 13.1 pg/mL in the second trimester and 36.6 ± 15.6 pg/mL in the third trimester. Intact-PTH did not change significantly during pregnancy. A significant decrease from baseline was noted at delivery, dropping to 19.1 ± 11.6 pg/mL. However, the levels significantly went up to 21.8 ± 13.5 pg/mL after at 1 month postpartum, 38.8 ± 16.7 pg/mL at 6 months postpartum, and 55.3 ± 21.4 pg/mL at 1 year postpartum. The levels remained within the normal limits throughout the entire study period. Non-pregnant and non-lactating women had levels of 56.5 ± 11.5 pg/mL. ANOVA analysis revealed a statistically significant difference (p < 0.0001). Moreover, intact-PTH levels at delivery and 1 month postpartum were significantly lower than those in the first trimester (p < 0.0001: Turkey–Kramer). Levels measured at 1 year were significantly higher than those during the first trimester. The levels during pregnancy, at 6 months, and at 1 year postpartum were higher than the level at delivery (p < 0.0001: Turkey–Kramer) (Fig. 2b).
Participant’s dietary intake
Table 3 shows the information collected from the FFQ regarding the dietary intake of participants. The total energy intake and total calcium intake did not reach the Dietary Reference Intakes for Japanese individuals recommended by the Ministry of Health, Labor and Welfare.
Sunlight exposure times
Sunlight exposure in a time span of 2 weeks was of 6.5 ± 10.5 h in the first trimester, 7.3 ± 11.8 h in the second trimester, 7.3 ± 12.0 h in the third trimester, 4.8 ± 9.1 h at the time of delivery, 2.0 ± 4.0 h at 1 month postpartum, 6.6 ± 10.2 h at 3 months postpartum, 7.2 ± 10.7 h at 6 months postpartum, and 8.9 ± 16.6 h at 1 year postpartum. Sunlight exposure time at 1 month postpartum was significantly lower than during the first trimester (p < 0.01: ANOVA, Tukey–Kramer HSD test). The rate of sunscreen use was of 56.5% in the first trimester, 63.9% in the second trimester, 64.3% in the third trimester, 72.0% at the time of delivery, 66.7% at 1 month postpartum, 63.5% at 3 months postpartum, 59.3% at 6 months postpartum, and 66.3% at 1 year postpartum. The reduced duration of sunlight exposure was attributed to the fewer opportunities to leave the house from the time before delivery to 1 month postpartum. Regarding the lack of exposure to sunlight, 47% of participants were exposed to less than 3.5 h for every 2 weeks during pregnancy.
Calcaneus QUS: stiffness derived T scores
The average T scores of stiffness were noted in the first trimester (101.3 ± 15.4%), second trimester (99.9 ± 17.8%), and third trimester (98.6 ± 16.7%), at delivery (94.3 ± 16.2%), at 1 month postpartum (98.3 ± 16.6%), at 3 months postpartum (100.8 ± 16.8%), at 6 months postpartum (102.3 ± 19.1%), and 1 year postpartum (96.0 ± 17.5%). The results obtained with ANOVA did not show any significant change (Fig. 3a).
a Calcaneus quantitative ultrasound (QUS) T score from first trimester to 1 year postpartum. No significant change was found during pregnancy and lactation. The data are represented as mean ± SD. b Bone mineral density (BMD) at the distal third of the radius, the percent changes in BMD was − 4.1 ± 21.3% from delivery to 6 months postpartum. It declined − 3.1 ± 17.7% at 1 year postpartum. There is not any significant change
BMD at the distal third of the radius
The average value of the young adult mean (YAM) at each stage was 96 ± 11.2% at delivery (n = 140), 93 ± 11.6% at 6 months postpartum (n = 76), 92 ± 11.0% at 1 year postpartum (n = 68). The percentage changes in the BMD of participants who could be followed until 1 year postpartum, were − 4.1 ± 21.3% from delivery to 6 months postpartum and declined to − 3.1 ± 17.7% at 1 year postpartum (n = 68). ANOVA analysis did not show a significant change (Fig. 3b).
Factors affecting maternal bone mass extracted
Using the calcaneus QUS (T score) and BMD (YAM) as determinants, single regression analyses were performed for each of the following prognostic factors: 25(OH)D, intact-PTH, BMI, age, calcium intake, vitamin D intake, vitamin K intake, and sunlight exposure times. Both 25(OH)D level and BMI were significant factors for bone mass at each stage. (25(OH)D: 0.003 < r2 ≤ 0.042, p < 0.05; BMI: 0.017 < r2 ≤ 0.308, p < 0.001) (data not shown). Furthermore, as a result of multiple regression analyses using 25(OH)D level and BMI as predictors of calcaneus QUS (T score) and BMD (YAM), BMI was a stronger factor (BMI: 1.51 < t ≤ 4.31, p < 0.05) (data not shown).
BMI and 25(OH)D correlation with radius BMD (YAM)
We examined the longitudinal effects of 25(OH)D levels and BMI on BMD during lactation. Single regression analyses of the 25(OH)D levels of each period and BMD in lactation revealed that first trimester 25(OH)D levels were associated with BMD in lactation (at delivery: r2 = 0.068, p < 0.05; 6 months after delivery: r2 = 0.088, p < 0.05; 1 year after delivery: r2 = 0.095, p < 0.05). No significant association with 25(OH)D levels in second or third trimester was evident (data not shown).
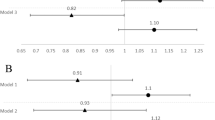
In this study, BMI during the first trimester was positively correlated with BMD in all after delivery stages (r = 0.49 at delivery, r = 0.54 at 6 months postpartum, and r = 0.47 at 1 year postpartum). Based on this correlation, we performed a subgroup analysis of the low-BMI participants with a BMI lower than the median value (20 kg/m2) in the first trimester. Participants were also divided into two groups according to the median 25(OH)D level (8.9 ng/mL) of the first trimester. As a result, in this low-BMI group, BMD in the high-25(OH)D group was significantly higher than that of the low-25(OH)D group over an entire period after delivery (p < 0.01 at delivery, p < 0.05 at 6 months postpartum and 1 year postpartum: unpaired t test, Welch), whereas in the high-BMI group, we found no difference in BMD regarding 25(OH)D levels (Fig. 4).
Comparisons of BMD (YAM) between high-25(OH)D groups: 25(OH)D at the first trimester > 8.9 ng/mL, and low-25(OH)D groups: 25(OH)D at the first trimester ≤ 8.9 ng/mL. a Subgroup analysis of the low-BMI participants: BMI in the first trimester ≤ 20 kg/m2. BMD in the high-25(OH)D groups were significantly higher than that in the low-25(OH)D groups at all stages (*p < 0.05, **p < 0.01: unpaired t test, Welch). b Subgroup analysis of the high-BMI participants: BMI in the first trimester > 20 kg/m2. It’s showed no significant differences in BMD regarding 25(OH)D levels
Discussion
We conducted a prospective cohort study to examine the 25(OH)D profiles of Japanese pregnant and lactating women, and the association between 25(OH)D levels and maternal bone mass. Accordingly, two findings were revealed. First, 25(OH)D remained extremely low throughout pregnancy and lactation in Japanese women. Levels in participants consistently remained below 20 ng/mL from the first trimester of pregnancy to 6 months postpartum, which is defined as being vitamin D deficient [17, 18, 23]. At 1 year postpartum, levels in participants were still below 30 ng/mL, which is considered to be vitamin D insufficient. Second, maternal bone mass during pregnancy and lactation was found to be related to 25(OH)D levels and BMI in the first trimester. If women with low levels of both BMI and 25(OH)D become pregnant it seems impossible that their bone mass can be sustained during lactation.
Previous studies in other countries have found low levels of 25(OH)D during pregnancy and lactation [24,25,26]. When compared to the rest of the world, unfortunately, Japanese women’s 25(OH)D levels were substantially lower than women of the same generation from other countries. In order to confirm data reliability, 30 women were tested who lived in the same areas as the study subjects and who were neither pregnant nor nursing. Their 25(OH)D levels were as deficient as those of the pregnant women.
We investigated the relationship between 25(OH)D levels and sunlight exposure times, and a significant positive correlation was noted at 6 months postpartum (r = 0.36, p < 0.01). Yokohama is located at 35.67° north latitude and 139.65° east longitude. Plugging these numbers into Holick’s rule [27], which calculates daily sunlight exposure times equivalent to 25 µg (1000 IU/day) of vitamin D intake, the minimally perceptible erythema (MED) irradiation equivalent to a quarter of the body surface area would require a minimum of 15 min per day (3.5 h in 2 weeks) of sunlight exposure in Yokohama. The averages at each measured period except 1 month postpartum were above the recommended number, and there is much variation in these values. Investigating the proportion of each participant’s average sunlight exposure time from the sum during pregnancy, we found as many as 47% of them exposed to sunlight for less than 3.5 h in 2 weeks. Young Japanese women generally use a high amount of sunscreen against UV rays to maintain a light complexion. Therefore, the limited exposure to sunlight in this population may be one of the causes of low 25(OH)D levels. Dawodu et al. have reported that pregnant women in the United Arab Emirates (UAE) cannot expose their skin for religious reasons and have extremely low levels of 25(OH)D [14]. Levels in pregnant women from the UAE and Japanese women who participated in this study are quite similar. Also, both are remarkably lower than data from other nations [28].
Calculations to determine vitamin D intake from meals using the FFQ method do satisfy The Ministry of Health, Labor and Welfare’s Dietary Reference Intakes for Japanese (estimated energy requirement) of 8 μg/day during pregnancy, and 9 μg/day during lactation. In this study, a significant correlation was noted between the vitamin D intake and 25(OH)D levels at 1 month postpartum (r = 0.39; p < 0.01) and 6 months postpartum (r = 0.34; p < 0.01).
Regarding 25(OH)D levels, there were no changes during pregnancy; however, during lactation, they increased up to 1 year postpartum gradually. They were significantly higher than in the first trimester at 1 year postpartum. It may be because daily sunlight exposure times gradually increased since delivery to 1 year postpartum. Although the average vitamin D intake of the subjects in the present study was more than adequate (5.5 μg/day) according to the Dietary Reference Intake for Japanese 2015, formulated by Ministry of Health, Labor and Welfare, their 25(OH)D levels remained low.
These results suggest that current adequate vitamin D intake but with limited sun exposure may not be enough to attain sufficient vitamin D levels.
In addition, no significant differences in the 25(OH)D values were noted when women who resumed menstruation within 6 months postpartum were compared to those who did not resume menstruation within 6 months (data not shown). A recent study showed that race/ethnicity and BMI play an important role in pregnancy bone health and that a dose effect of vitamin D supplementation does not support bone health [16]. The effect of BMI was coincident with our findings. In another study, vitamin D supplementation during pregnancy was associated with a reduced risk of SGA and improved infant growth without risk of fetal or neonatal mortality or congenital abnormality [29], increased circulating 25(OH)D level, birth weight, and birth length, but not with other maternal and neonatal outcomes [30]. Maternal vitamin D supplementation from mid-pregnancy until birth or 6 months postpartum did not improve fetal or infant growth [31]. Pre-pregnancy vitamin D supplementation may prevent the disorder, considering the result that the 25(OH)D level in the first trimester was associated with maternal bone mass in our study. Four quadrants by the median values of 25(OH)D and BMI in the second or third trimester were formed. Higher levels of second trimester 25(OH)D were associated with higher BMD during lactation, in the low BMI group, but the difference was not significant. Furthermore, no effect was observed in the third trimester. Although the reason why only first trimester levels of 25(OH)D affected maternal BMD during lactation in the low BMI group is unclear from the findings of our study, the fact that BMI increasing as pregnancy progresses, greatly affecting bone mass, maybe one reason.
Maternal bone mass was measured by calcaneus QUS to avoid exposing the fetuses to X-rays during pregnancy. In our study, no significant change was noted during pregnancy and lactation. Several papers in the literature have reported decreases in the QUS value at delivery [32,33,34,35,36]. Instead, our results agree with the suggestions of literature reports, so there is no difference in QUS values during lactation based on how long a woman breastfeeds her baby [32, 36]. QUS values could be maintained regardless of whether the study participant engaged in long-term lactation. Although, results of QUS were affected by the change in body fluid volume and edema during pregnancy bone quality may reflect them. The use of QUS remains to be further studied.
Multiple papers have reported that the radius BMD decreases during postnatal breastfeeding [37,38,39,40,41,42]. We observed a similar finding in our study: BMD tended to decrease at 1 year postpartum. In a cohort study of participants for up to 9 months postpartum, BMD decreased [43]. However, if breastfeeding continued for less than 4 months, BMD recovered to pre-pregnancy levels, and the duration of breastfeeding was deemed to affect this recovery process. Since our participants were lactating, radius BMD also showed a declining tendency at 1 year postpartum (Fig. 3b). Kurabayashi et al. indicated that osteoporotic or osteopenic women identified with the puerperal scans were still osteopenic after 5–10 years, as assessed by puerperal scans recorded 5–10 years later, and they were associated with a significantly lower BMI compared to normal women [44]. In the long term, appropriate BMD and BMI signify a good condition in reproductive life. Compared with 25(OH)D level, BMI had a stronger association with maternal bone mass in this cohort. This may be reason why 25(OH)D level was not relevant in the high BMI group (Fig. 4b).
Obstetric outcomes revealed an infant birth weight of 3028 ± 339 g (mean ± SD) including 7 low birth weight neonates (4.93%), but there was no correlation with 25(OH)D levels. Craniotabes was noted in two cases (0.6%), and there were no cases of preeclampsia in this cohort.
In our prospective cohort study where 68 participants were followed from early pregnancy throughout 1 year postpartum, changes in 25(OH)D levels and bone mass over time were shown during long-term breastfeeding. Maternal bone mass was found to be associated with 25(OH)D levels and BMI during this period. An acknowledged low BMI is risky in maintaining bone mineral density [45], furthermore, low levels of 25(OH)D are even more harmful. It is important for maternal bone health that 25(OH)D and BMI levels during the onset of pregnancy are appropriate.
There were several limitations in this study that should be acknowledged. First, maternal baseline data before pregnancy were not known. Also, we did not measure estrogen, 1,25(OH)D, or PTHrP levels, and the mechanism behind the changes in 25(OH)D over time could not be elucidated. These results did not necessarily reflect the condition of all Japanese women, because we were limited to participants living in an urban area.
In conclusion, we found that Vitamin D deficiency is prevalent in Japanese women, irrespective of pregnancy or lactation. Our analysis suggested that the low levels of 25(OH)D and BMI in the first trimester were risk factors for a low BMD during lactation. Therefore, providing direction to expectant mothers to increase blood concentrations of 25(OH)D and achieve an appropriate weight in the early pregnancy phase are vital to maintaining a proper maternal bone mass.
References
Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW et al (2016) Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab 101:394–415
Kiely ME, Zhang JY, Kinsella M, Khashan AS, Kenny LC (2016) Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am J Clin Nutr 104:354–361
Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM (2007) Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 92:3517–3522
Zhang Q, Cheng Y, He M, Li T, Ma Z, Cheng H (2016) Effect of various doses of vitamin D supplementation on pregnant women with gestational diabetes mellitus: a randomized controlled trial. Exp Ther Med 12:1889–1895
Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, Retnakaran R (2014) Vitamin D and parathyroid hormone status in pregnancy: effect on insulin sensitivity, β-cell function, and gestational diabetes mellitus. J Clin Endocrinol Metab 99:4506–4513
Zhu K, Whitehouse AJ, Hart PH, Kusel M, Mountain J, Stephen L, Pennell C, Walsh JP (2014) Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: a prospective cohort study. J Bone Miner Res 29:1088–1095
Yorifuji J, Yorifuji T, Tachibana K, Nagai S, Kawai M, Momoi T, Nagasaka H, Hatayama H, Nakahata T (2008) Craniotabes in normal newborns: the earliest sign of subclinical vitamin D deficiency. J Clin Endocrinol Metab 93:1784–1788
Gernand AD, Simhan HN, Caritis S, Bodnar LM (2014) Maternal vitamin D status and small-for-gestational-age offspring in women at high risk for preeclampsia. Obstet Gynecol 123:40–48
Qin LL, Lu FG, Yang SH, Xu HL, Luo BA (2016) Does maternal vitamin D deficiency increase the risk of preterm birth: a meta-analysis of observational studies. Nutrients 8:301
Ginde AA, Mansbach JM, Camargo CA Jr (2009) Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 169:384–390
Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, Gupta S, Singh S, Saxena P, Bhatia V (2012) Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr 108:1052–1058
Bodnar LM, Platt RW, Simhan HN (2015) Early-pregnancy vitamin D deficiency and risk of preterm birth subtypes. Obstet Gynecol 125:439–447
Hossain N, Kanani FH, Ramzan S, Kausar R, Ayaz S, Khanani R, Pal L (2014) Obstetric and neonatal outcomes of maternal vitamin D supplementation: results of an open-label, randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J Clin Endocrinol Metab 99:2448–2455
Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW (2013) Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab 98:2337–2346
Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, Fraser R, Gandhi SV, Carr A, D’Angelo S, Crozier SR, Moon RJ, Arden NK, Dennison EM, Godfrey KM, Inskip HM, Prentice A, Mughal MZ, Eastell R, MAVIDOS Study Group et al (2016) Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol 4:393–402
Wei W, Shary JR, Garrett-Mayer E, Anderson B, Forestieri NE, Hollis BW, Wagner CL (2017) Bone mineral density during pregnancy in women participating in a randomized controlled trial of vitamin D supplementation. Am J Clin Nutr 106:1422–1430
Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne ST, Ross AC, Shapses SA, Taylor CL (2012) IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab 97:1146–1152
Moon RJ, Harvey NC, Cooper C, D’Angelo S, Crozier SR, Inskip HM, Schoenmakers I, Prentice A, Arden NK, Bishop NJ, Carr A, Dennison EM, Eastell R, Fraser R, Gandhi SV, Godfrey KM, Kennedy S, Mughal MZ, Papageorghiou AT et al (2016) Determinants of the maternal 25-hydroxyvitamin D response to vitamin D supplementation during pregnancy. J Clin Endocrinol Metab 101:5012–5020
Uenishi K, Ishida H, Nakamura K (2008) Development of a simple food questionnaire to estimate intakes of calcium and other nutrients for the prevention and management of osteoporosis. J Nutr Sci Vitaminol 54:25–29
Egami I, Wakai K, Kato K, Line Y, Kawamura T, Tamakoshi A, Aoki R, Kojima M, Nakayama T, Wada M, Ohno Y (1999) A simple food frequency questionnaire for Japanese diet-Part II. Reproducibility and validity for nutrient intakes. J Epidemiol 9:227–234
Moon HW, Cho JH, Hur M, Song J, Oh GY, Park CM, Yun YM, Kim JQ (2012) Comparison of four current 25-hydroxyvitamin D assays. Clin Biochem 45:326–330
Liu JM, Ma LY, Bi YF, Xu Y, Huang Y, Xu M, Zhao HY, Sun LH, Tao B, Li XY, Wang WQ, Ning G (2012) A population-based study examining calcaneus quantitative ultrasound and its optimal cut-points to discriminate osteoporotic fractures among 9352 Chinese women and men. J Clin Endocrinol Metab 97:800–809
Okazaki R, Ozono K, Fukumoto S, Inoue D, Yamauchi M, Minagawa M, Michigami T, Takeuchi Y, Matsumoto T, Sugimoto T (2017) Assessment criteria for vitamin D deficiency/insufficiency in Japan—proposal by an expert panel supported by Research Program of Intractable Diseases, Ministry of Health, Labour and Welfare, Japan, The Japanese Society for Bone and Mineral Research and The Japan Endocrine Society (opinion). Endocr J 64:1–6
Milman N, Hvas AM, Bergholt T (2011) Vitamin D status during normal pregnancy and postpartum. A longitudinal study in 141 Danish women. J Perinat Med 40:57–61
Gernand AD, Simhan HN, Klebanoff MA, Bodnar LM (2013) Maternal serum 25-hydroxyvitamin D and measures of newborn and placental weight in a US multicenter cohort study. J Clin Endocrinol Metab 98:398–404
Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE (1995) Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr 61:514–523
Dowdy JC, Sayre RM, Holick MF (2010) Holick’s rule and vitamin D from sunlight. J Steroid Biochem Mol Biol 121:328–330
Hossein-nezhad A, Holick MF (2013) Vitamin D for health: a global perspective. Mayo Clin Proc 88:720–755
Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ (2018) Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr 172:635–645
Pérez-López FR, Pasupuleti V, Mezones-Holguin E, Benites-Zapata VA, Thota P, Deshpande A, Hernandez AV (2015) Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril 103:1278–1288
Roth DE, Morris SK, Zlotkin S, Gernand AD, Ahmed T et al (2018) Vitamin D supplementation in pregnancy and lactation and infant growth. N Engl J Med 379:535–546
Hellmeyer L, Hahn B, Fischer C, Hars O, Boekhoff J, Maier J, Hadji P (2015) Quantitative ultrasonometry during pregnancy and lactation: a longitudinal study. Osteoporos Int 26:1147–1154
Kraemer B, Schneider S, Rothmund R, Fehm T, Wallwiener D, Solomayer EF (2012) Influence of pregnancy on bone density: a risk factor for osteoporosis? Measurements of the calcaneus by ultrasonometry. Arch Gynecol Obstet 285:907–912
Della Martina M, Biasioli A, Vascotto L, Rinuncini D, Adorati Menegato A, Liva S, Londero AP, Driul L, Marchesoni D (2010) Bone ultrasonometry measurements during pregnancy. Arch Gynecol Obstet 281:401–407
Javaid MK, Crozier SR, Harvey NC, Taylor P, Inskip HM, Godfrey KM, Cooper C, Southampton Women’s Survey Study Group (2005) Maternal and seasonal predictors of change in calcaneal quantitative ultrasound during pregnancy. J Clin Endocrinol Metab 90:5182–5187
Hadji P, Ziller V, Kalder M, Gottschalk M, Hellmeyer L, Hars O, Schmidt S, Schulz KD (2002) Influence of pregnancy and breast-feeding on quantitative ultrasonometry of bone in postmenopausal women. Climacteric 5:277–285
Sowers M, Eyre DR, Hollis BW, Randolph JF, Shapiro B, Jannausch ML, Crutchfield M (1995) Biochemical markers of bone turnover in lactating and nonlactating postpartum women. J Clin Endocrinol Metab 80:2210–2216
Affinito P, Tommaselli GA, di Carlo C, Guida F, Nappi C (1996) Changes in bone mineral density and calcium metabolism in breastfeeding women: a one year follow-up study. J Clin Endocrinol Metab 81:2314–2318
Kovacs CS, Kronenberg HM (1997) Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18:832–872
Honda A, Kurabayashi T, Yahata T, Tomita M, Takakuwa K, Tanaka K (1998) Lumbar bone mineral density changes during pregnancy and lactation. Int J Gynaecol Obstet 63:253–258
Karlsson MK, Ahlborg HG, Karlsson C (2005) Maternity and bone mineral density. Acta Orthop 76:2–13
Kovacs CS, Ralston SH (2015) Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporosis Int 26:2223–2241
Møller UK, Við Streym S, Mosekilde L, Rejnmark L (2012) Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int 23:1213–1223
Kurabayashi T, Nagata H, Takeyama N, Matsushita H, Tanaka K (2009) Bone mineral density measurement in puerperal women as a predictor of persistent osteopenia. J Bone Miner Metab 27:205–212
Felson DT, Zhang Y, Hannan MT, Anderson JJ (1993) Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 8:567–573
Acknowledgements
We are grateful for support for the Department of Hygienic Sciences, Kobe Pharmaceutical University, Laboratory of Physiological Nutrition, Kagawa Nutrition University, Department of Obstetrics and Gynecology, Yokohama City University School of Medicine. We also thank Dr. Itsuo Gorai, Dr. Ritsuko Kikuchi, Dr. Yoshiyuki Nomura, Dr. Atsuo Yoshizaki, and all of the mothers for their participation in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest to disclose in relation to this manuscript.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
We obtained written informed consent from all participants in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yoshikata, H., Tsugawa, N., Watanabe, Y. et al. 25-Hydroxyvitamin D profiles and maternal bone mass during pregnancy and lactation in Japanese women. J Bone Miner Metab 38, 99–108 (2020). https://doi.org/10.1007/s00774-019-01032-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-01032-w