Abstract
Several cellular and molecular processes participate in the pathologic changes of osteoarthritis (OA). However, the core molecular regulators of these processes are unclear, and no effective treatment for OA disease has been developed so far. ANGPTL2 is well known for its tissue remolding and pro-inflammation properties. However, the role of ANGPTL2 in osteoarthritis (OA) still remains unclear. To explore the expression level of ANGPTL2 in human OA cartilage and investigate the function of ANGPTL2 in human chondrocytes injury, qRT-PCR, western blot and immunohistochemistry were employed to investigate the expression of ANGPTL2 between human OA and normal cartilage samples. Next, human primary chondrocytes were treated with IL-1β to mimic OA progress in vitro, and the expression of ANGPTL2 were tested by qRT-PCR and western blot. Furthermore, the effect of ANGPTL2 in the expression of pro-inflammation cytokines (IL-1β, IL-6), proteolytic enzymes (MMP-1, MMP-13) and component of the cartilage matrix (COL2A1 and aggrecan) in human primary chondrocyte were explored by gain-of-function and loss-of-function methods. Finally, the nuclear factor kappa B (NF-κB) and p38/MAPK signaling pathways were also tested by western blot analysis. In this study, firstly, the expression level of ANGPTL2 was elevated both in human OA cartilage samples and IL-1β stimulated human chondrocytes. Secondly, ANGPTL2 upregulation promotes extracellular matrix (ECM) degradation and inflammation mediator production in human chondrocytes. Finally, ANGPTL2 activated the NF-κB and p38/MAPK signaling pathways via integrin α5β1. This study, for the first time, highlights that ANGPTL2 secreted by human chondrocytes plays a negative role in the pathogenesis of osteoarthritis, and it may be a potential therapeutic target in OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is the most prevalent joint disease that causes joint pain and loss of function, which is viewed as a whole organ disease of the knee joint [1]. The pathologic changes occurring in OA joints including degradation of the articular cartilage, formation of osteophytes, thickening of the subchondral bone, inflammation of the synovium, etc. [2]. Several cellular and molecular processes participate in these pathologic changes such as imbalance in catabolism and anabolism in cartilage, hypertrophy and death of chondrocytes and activation of immune cells [2]. However, the core molecular regulators of these processes are unclear. Among the pathologic changes occurred in OA joints, the imbalance in cartilage catabolism and anabolism is one of the crucial factors in degradation of the articular cartilage [2,3,4,5,6]. Several studies have demonstrated that the matrix metalloproteinases (MMPs) family, such as MMP1 and MMP13 promote the excessive cartilage degradation via destroying the cartilage collagen type II network [7,8,9]. Meanwhile, low-grade, chronic inflammation is also associated with OA progression, and the overexpression of inflammatory cytokines, such as IL-1β, IL-6, TNF-α, have been identified in OA joint fluids and cartilage [1, 10, 11]. Current therapies for OA usually target the symptoms of the disease including pain control, viscosupplementation [12,13,14,15,16,17]. However, no effective treatment for OA disease has been developed so far, and as the disease continues to advance, joint replacement is often ultimately required to reduce pain and disability [18]. Novel therapeutics are needed to slow or stop the progression that drive OA pathology [19]. Therefore, exploration of the precise molecular mechanisms in the progression of OA to effectively treat OA is needed urgently.
Angiopoietin-like 2 (ANGPTL2) is a circulating protein, belongs to the angiopoietin-like family and contains an N-terminal coiled domain and a C-terminal fibrinogen-like domain (FLD) [20]. ANGPTL2 was first identified as its pro-angiogenic capacity, but it is better acknowledged that it contributes to promote low-grade chronic inflammation, and tissue remodeling [21]. Accumulating data indicated that ANGPTL2 can not only lead to extracellular matrix degradation, also cause inflammatory gene expression (such as IL-1β, IL-6, TNF-α) in chronic inflammation [21,22,23]. OA was also associated with aging and obesity as previous reported, and abnormal high expression of ANGPTL2 is often associated with aging, obesity, chronic hypoxia and so on [24, 25]. Therefore, these observations elicited our hypothesis that ANGPTL2 might contribute to the pathologic progression of OA.
To validate this, we set out to investigate differences in the expression of ANGPTLT2 in normal and OA human cartilage and found that ANGPTLT2 is highly expressed in human OA cartilage and IL-1β stimulated human primary chondrocytes. Further study suggested that ANGPTLT2 contributed to extracellular matrix (ECM) degradation and inflammation factors production via integrin α5β1/p38 MAPK and NF-κB signaling, and in doing so, defined the function of ANGPTLT2 in OA progression.
Materials and methods
Collection of human cartilage samples
Normal human cartilage samples were collected from 12 donors undergoing total hip replacement operation because of the femoral neck fracture (12 for total RNA and protein extraction, and 4 of them for immunohistochemical analysis). Osteoarthritic human cartilage samples were obtained from knee joints of 22 OA patients after total knee arthroplasty (4 for immunohistochemical analysis, 12 for total RNA and protein extraction and 6 for primary chondrocyte isolation). Patients’ information is shown in Supplementary Table 1. For total RNA and protein extraction, the cartilage samples were cut into 1-mm3 pieces, repeated grinding after adding liquid nitrogen three times and kept frozen at − 80 °C until used.
All cartilage samples were obtained from the First Affiliated Hospital of Anhui Medical University. A written informed consent was obtained from all the patients about the study. This research was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University.
Human primary chondrocyte extraction and culture
Cartilage samples were shaved off from intact, non-fibrillated areas of the articular surface and cut with scissors as smaller as possible. After digested in 0.25% trypsin–EDTA solution (Beyotime, China) for 30 min at 37 °C, the cartilage pieces were digested again in 0.4% collagenase II (Sigma, USA) for 24 h at 37 °C in a humidified atmosphere under 5% CO2 in air. Cells were cultured in growth media (DMEM/F12 1:1, Hyclone, USA) containing 10% fetal bovine serum (CLARK, USA) and 1% penicillin/streptomycin (penicillin–streptomycin solution, MRC, American). All cells were maintained at 37 °C in a humidified atmosphere under 5% CO2 in air and used at the end of second passage.
ANGPTL2 knockdown by siRNA
The cells were inoculated into a six-well plate the day before transfection (18–24 h) to allow the cell density to reach about 50–60% on the second day. Before performing the transfection procedure, each well was replaced with 2 ml of fresh medium (serum and antibiotic free). For cells in each well to be transfected, Opti-MEM (Sigma, USA) was added into two clean sterile centrifuge tubes (125 μl/tube), then 100 pmol siRNA or siNC was added to one of the tubes and 5 μl of Lipo6000TM transfection reagent (Beyotime, China) was mixed in another tube gently. After standing at room temperature for 5 min, the culture medium containing the siRNA was gently added into the culture medium containing Lipo6000TM transfection reagent. After incubating at room temperature for 20 min, the Lipo6000–siRNA mixture was added dropwise to the well. To achieve the highest transfection efficiency, cells were replaced with fresh complete cultures after 5 h of transfection. Cultured for 24 h after transfection, cells were stimulated or not with 10 ng/ml IL-1β and cultured for 24 h to isolate mRNA or proteins (siANGPTL2: sense 5′-gcaaggguuugggaacauutt-3′, antisense 5-aauguucccaaacccuugctt-3′; negative control: sense 5′- uucuccgaacgugucacgutt-3′, antisense 5′-acgugacacguucggagaatt-3′).
RNA isolation, reverse transcription and real-time PCR
For total RNAs isolated from cartilage samples (repeated grinding after adding liquid nitrogen as described), Column Cartilage RNAout (TIANDZ, CHINA) was used according to the manufacturer’s instructions. For chondrocytes (cultured in a six-well plate) RNA extraction, TRIzol Reagent (Invitrogen, USA) was used according to the introductions. RNA concentration was measured by spectrophotometric analysis with a NANODROP 2000c (Thermo, USA). Reverse transcription was performed using a PrimeScript RT reagent Kit (TaKaRa, Japan) on a Mastercycler nexus gradient (Eppendorf, German) and real-time PCR was performed using a SYBR Premix Ex Taq II (TaKaRa, Japan) on an Agilent Technologies Stratagene Mx3000P (USA). GAPDH was used as a housekeeping control. Results were calculated using the relative quantitative method (2−ΔΔCT). Primer sequences are shown in Supplementary Table 2.
Western blotting analysis
Cartilage samples (repeated grinding after adding liquid nitrogen as described) and chondrocyte were lysed in RIPA Lysis Buffer (Beyotime, China) supplemented with PMSF (Beyotime, China) and phosphatase inhibitor (Beyotime, China). Proteins were separated on 10% or 12% SDS-PAGE gels and transferred to PVDF membranes (Immobilon-P Transfer Membrane 0.45 μm, Millipore, USA). After blocked with 5% skimmed milk powder in TBS-T, membranes were incubated with primary antibodies at 4 °C overnight, followed by incubation with HRP-conjugated secondary antibodies and imaged using the BeyoECL Moon (Beyotime, China) on Tanon 4500SF imaging system (China). All antibodies are shown in Supplementary Table 3.
Immunohistochemistry
Cartilage samples were fixed in 4% paraformaldehyde (Biosharp, China) for 24 h at 4 °C, decalcified in EDTA for 8 weeks at room temperature. After dehydration, the samples were embedded in paraffin and cut into sections. Then the paraffin sections were deparaffinized, rehydrated and heated for antigen retrieval. For inhibiting endogenous peroxidases, the sections were treated in 3% H2O2 for 10 min. After blocking with 5% normal goat serum for 2 h, the sections were incubated in the primary antibody overnight at 4 °C, followed by incubation with HRP-conjugated secondary antibodies for 2 h. The signal was developed with DAB (ZSGB-BIO, China) and nuclei were counterstained with hematoxylin. The sections were photographed by confocal laser scanning microscope (LSM880 + airyscan, Germany).
Statistical analysis
All the results of qRT-PCR reported were harvested from at least three independent experiments and each experimental data is the mean of three technical repeats. The Shapiro–Wilk test was performed to evaluate normal distribution of date and the Levene’s test was performed for homogeneity of variance. To compare two groups, the independent-samples t test was performed. To compare four groups, the data were analyzed using either one-way analysis of variance (ANOVA) followed by Bonferroni test (equal variances) or Welch test followed by Games-Howell (equal variances not assumed). SPSS software (IBM Corporation) was used for date analyses.
Results
ANGPTL2 is upregulated in human OA cartilage and IL-1β-stimulated human primary chondrocytes
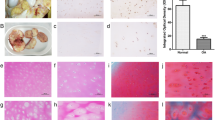
To explore the association of ANGPTL2 with OA pathogenesis, the expression levels of ANGPTL2 were first examined in human OA cartilages and IL-1β treated human primary chondrocytes. Identification of human primary chondrocytes was shown in SF.1A–D. As shown in Fig. 1A–C, western blot and Q-PCR assays revealed that the expression level of ANGPTL2 were markedly elevated in human OA cartilages (n = 12) compared with normal cartilage (n = 12). Consistent with these results, immunohistochemical staining (Fig. 1D) revealed that ANGPTL2 protein levels were markedly elevated in OA cartilages. IL-1β has been widely used to stimulate chondrocytes to induce osteoarthritis-like phenotype in vitro [26,27,28]. Therefore, we investigated the expression of ANGPTL2 in IL-1β treated human primary chondrocytes and found that the expression level of ANGPTL2 was significantly upregulated in human primary chondrocytes under stimulated with IL-1β stimulation (Fig. 1E–G). Together, these results suggested that ANGPTL2 was associated with OA pathogenesis.
ANGPTL2 is upregulated in human OA cartilage and IL-1β stimulated human primary chondrocytes. A Western blots analysis for the protein expression of ANGPTL2 isolated from human normal (n = 4) and OA (n = 5) cartilage samples, and B quantification of protein bands measured by Image J software. C qRT-PCR analysis for the mRNA expression levels of ANGPTL2 isolated from human normal (n = 12) and OA (n = 12) cartilages samples normalized to GAPDH expression as a control. D Immunohistochemical staining for ANGPTL2 on human normal and OA cartilage sections. E Western blots analysis for the protein expression of ANGPTL2 isolated from human chondrocytes stimulated with IL-1β (10 ng/ml) or not after 24 (n = 3) and F quantification of protein bands, measured by Image J software. G qRT-PCR analysis for the mRNA expression levels of ANGPTL2 isolated from human primary chondrocytes stimulated with IL-1β (10 ng/ml) or not after 24 (n = 4) and normalized to GAPDH expression as a control. F Results are expressed as mean ± SD, independent-samples t test. Scale bars (D, overview) = 500 μm. Scale bars (D, superficial, middle, deep zone) = 50 μm
Effects of ANGPTL2 on MMPs and COL2A1 production in human primary chondrocytes
MMP-1 and MMP-13 are important catabolic factor for extracellular matrix degradation in OA. Interestingly, MMPs expression has been reported to be increased by ANGPTL2 in osteosarcoma [23]. However, it remains unknown whether ANGPTL2 influence MMPs expression in OA pathogenesis. In this study, ANGPTL2 was silenced by siRNA (SF. 2AB). Our results indicated that treatment of chondrocytes with IL-1β could markedly enhance the expression of MMP-1 and MMP-13, whereas their expression level in mRNA and protein were significantly inhibited when ANGPTL2 was silenced in human primary chondrocytes (Fig. 2. A–C). On the contrary, rhANGPTL2 (PROSPEC, Israel) could also promote MMP-1 and MMP-13 production in a dose dependent manner (Fig. 2D–F). COL2A1 and aggrecan, secreted by chondrocytes, are important components of the cartilage matrix. Our results indicated that treatment of chondrocytes with IL-1β could markedly inhibited the expression of COL2A1 and aggrecan, and only COL2A1 expression level in mRNA and protein were significantly enhanced when ANGPTL2 was silenced in human primary chondrocytes (SF.3A–C). On the contrary, rhANGPTL2 (PROSPEC, Israel) could also suppressed COL2A1 production in a dose dependent manner and there was no significant change in aggrecan expression (SF.3D–F). In addition, as shown in SF 4, there was no significant change in the expression of caspase-3, a key enzyme related to cell apoptosis, when knocking down ANGPTL2 in human primary chondrocytes, knocking down of ANGPTL2 may not affect the apoptosis of human primary chondrocytes. All these suggested that ANGPTL2 upregulation contributed to extracellular matrix degradation by increasing the expression of MMP-1 and MMP-13 and decreasing COL2A1 in human chondrocytes.
Effects of ANGPTL2 on MMP-1 and MMP-13 production in human primary chondrocytes. A–C Silencing of ANGPTL2 reduced the expression of MMP-1 and MMP-13. Human chondrocytes were divided into four groups: Control, IL-1β (10 ng/mL), IL-1β (10 ng/ml) + siNC, IL-1β (10 ng/mL) + siANGPTL2. Chondrocytes were pretreated with siNC or siANGPTL2 for 24 h followed by stimulation with IL-1β (10 ng/ml) for 24 h, and then, RNA or protein was extracted from chondrocytes. A, B qRT-PCR analysis for the mRNA expression levels of MMP-1 and MMP-13. C Western blots analysis for the protein expression of MMP-1 and MMP-13. D–F rhANGPTL2 increased the expression of MMP-1 and MMP-13. Human primary chondrocytes were treated with a serial dosage (0,0.1,0.2,0.4 μg/ml) of rhANGPTL2 for 24 h. D, E qRT-PCR analysis for the mRNA expression levels of MMP-1 and MMP-13. F Western blots analysis for the protein expression of MMP-1 and MMP-13. Results are expressed as mean ± SD. E Analysis of variance (ANOVA) followed by Bonferroni test (equal variances). A, B, D Welch test followed by Games-Howell test (equal variances not assumed)
Effects of ANGPTL2 on IL-1β and IL-6 production in human primary chondrocytes
Low-grade, chronic inflammation has a central role in the pathogenesis of OA. Interestingly, ANGPTL2 is also acknowledged for its pro-inflammatory properties. However, the pro-inflammatory properties of ANGPLT2 remain unknown in OA pathogenesis. IL-1β-stimulation of human chondrocytes resulted in a marked up-regulation of the mRNA and protein levels of IL-1β and IL-6, but knockdown of ANGPLT2 resulted in significant inhibition of expression of IL-1β and IL-6 (Fig. 3A–C). Additionally, treatment of chondrocytes with rhANGPTL2 can markedly promote IL-1β and IL-6 production (Fig. 3D–F). These results suggested that ANGPTL2 might exert potent pro-inflammatory effects through increasing the expression of IL-1β and IL-6 in chondrocytes.
Effects of ANGPTL2 on IL-1β and IL-6 production in human primary chondrocytes. A–C Silencing of ANGPTL2 reduced the expression of MMP-1 and MMP-13. Human primary chondrocytes were divided into four groups: Control, IL-1β (10 ng/mL), IL-1β (10 ng/mL) + siNC, IL-1β (10 ng/ml) + siANGPTL2. Chondrocytes were pretreated with siNC or siANGPTL2 for 24 h followed by stimulated with IL-1β (10 ng/ml) for 24 h, and then, RNA or protein was extracted from chondrocytes. A, B qRT-PCR analysis for the mRNA expression levels of IL-1β and IL-6. C Western blots analysis for the protein expression of IL-1β and IL-6. D–F rhANGPTL2 increased the expression of IL-1β and IL-6. Human primary chondrocytes were treated with a serial dosage (0,0.1,0.2,0.4 μg/ml) of rhANGPTL2 for 24 h. D, E qRT-PCR analysis for the mRNA expression levels of IL-1β and IL-6. F Western blot analysis for the protein expression of IL-1β and IL-6. Results are expressed as mean ± SD. D Analysis of variance (ANOVA) followed by Bonferroni test (equal variances). A, B, E Welch test followed by Games-Howell (equal variances not assumed)
ANGPTL2 activates NF-κB and p38/MAPK signaling via integrin α5β1 in human primary chondrocytes
Much research in recent years has indicated that ANGPTL2 can influence the p38 MAPK and NF-κB signaling pathways, and both pathways play an important role in mediating the expression of MMPs and inflammatory mediators in OA progression [23, 24, 29, 30]. To investigate the molecular mechanisms responsible for the observed chondrocyte injury of ANGPTL2, we determined whether ANGPTL2 promotes chondrocyte injury through the activation of p38 MAPK and NF-κB signaling. Results in Fig. 4A–C illustrate that stimulation of chondrocytes with IL-1β activated the phosphorylation of p38 and p65. Surprisingly, and silencing ANGPTL2 in chondrocytes can suppress the p38 and p65 phosphorylation levels. In addition, we found that rhANGPTL2 (0.1, 0.2, 0.4 μg/ml) could also enhance the p38 MAPK and p65 phosphorylation levels (Fig. 4.D–F). It was reported that integrin α5β1 functions as ANGPTL2 receptors in ATDC and other cells [23, 25, 30]. To investigate the relationship between integrin α5β1 and ANGPTL2, we first examined the expression of ITGA5 (integrin α5) and ITGB (integrin β1) in normal and OA human cartilage and chondrocyte (treated with IL-1β) [30]. Our results indicated that ITGA5 (integrin α5) and ITGB (integrin β1) are highly expressed in human OA cartilage and IL-1β stimulated human primary chondrocytes (Fig. 5.A–D). ATN-161 is an antagonist of integrin α5β1 and has been widely used in integrin α5β1 blocking studies [31,32,33]. Our results indicated inhibition of integrin α5β1 with ATN-161(100 μg/ml) significantly antagonizes the phosphorylation levels of p38 and p65 in primary chondrocyte treated with rhANGPTL2 (0.4 μg/ml) (Fig. 5. E–G).
Effects of ANGPTL2 on the NF-κB and p38/MAPK signaling pathways. A–C Silencing of ANGPTL2 reduced the activation of NF-κB and p38/MAPK signaling pathways. Human primary chondrocytes were divided into four groups: Control, IL-1β (10 ng/mL), IL-1β (10 ng/mL) + siNC, IL-1β (10 ng/mL) + siANGPTL2. Chondrocytes were pretreated with siNC or siANGPTL2 for 24 h followed by stimulated with IL-1β (10 ng/ml) for 24 h, and then, protein was extracted from chondrocytes. A Western blots analysis for the protein expression of p65, phosphorylated p65, p38 and phosphorylated p38. B The ratios between phosphorylated p38 and p38. C The ratios between phosphorylated p65 and p65. Quantification of protein bands measured by Image J software. Results are expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Bonferroni test (equal variances). D–F rhANGPTL2 increased the activation of NF-κB and p38/MAPK signaling pathways. Human primary chondrocytes were treated with a serial dosages (0,0.1,0.2,0.4 μg/ml) of rhANGPTL2 for 24 h. D Western blots analysis for the protein expression of p65, phosphorylated p65, p38 and phosphorylated p38 extracted from chondrocytes. E The ratios between phosphorylated p38 and p38. F The ratios between phosphorylated p65 and p65. Quantification of protein bands measured by Image J software. Results are expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Bonferroni test (equal variances)
ANGPTL2 activates NF-κB and p38/MAPK signaling via integrin α5β1. A, B qRT-PCR analysis for the mRNA expression levels of ITGA5 and ITGB1 isolated from human normal (n = 12) and OA (n = 12) cartilages samples normalized to GAPDH expression as a control. C, D qRT-PCR analysis for the mRNA expression levels of ITGA5 and ITGB1 isolated from human primary chondrocytes stimulated with IL-1β (10 ng/ml) or not after 24 (n = 4) and normalized to GAPDH expression as a control. E, F, G Human chondrocytes were divided into three groups: control, rhANGPTL2 (0.4 μg/ml), rhANGPTL2 (0.4 μg/ml) +ATN-161 (100 μg/ml). Chondrocytes were pretreated with ATN-161 (100 μg/ml) for 24 h followed by stimulated with rhANGPTl2 for 24 h, and then, protein was extracted from chondrocytes. Western blots analysis was used to evaluate the expression of p65, phosphorylated p65, p38 and phosphorylated p38. F The ratios between phosphorylated p38 and p38. G The ratios between phosphorylated p65 and p65. Quantification of protein bands measured by Image J software. Results are expressed as mean ± SD. A, B, C, D Independent-samples t test. F, G One-way analysis of variance (ANOVA) followed by Bonferroni test (equal variances)
These data suggested that high expression of ANGPTL2 in OA progression exerts its function by regulating p38 MAPK and NF-κB signaling via integrin α5β1.
Discussion
OA has been recognized as a degenerative disease of cartilage in elderly population, which damages the entire joint structure and leads to chronic pain and joint disability [2, 34,35,36,37,38]. In recent decades, accumulating research has identified various risk factors for the incidence of OA. However, to date, there is no curative treatment for OA. Therefore, clarifying the relative molecular regulators in the progression of OA may contribute to improve therapeutic effect, even cure it. In this study, we observed the obvious elevation of ANGPTL2 in human OA cartilage samples. Furthermore, IL-1β, a key contributor to OA development, also promoted the expression of ANGPTL2. Therefore, these results suggested that ANGPTL2 plays a potential crucial role of ANGPTL2 in the progression of OA.
The destruction of articular cartilage plays an important role in OA development. COL2A1 and aggrecan, secreted by chondrocytes, are important components of the cartilage matrix. In normal context, the cartilage matrix synthesis and degradation are maintained in a dynamic balance in articular cartilage. However, the homeostasis will be broken under OA pathologic condition. It is well acknowledged that matrix-degrading genes, such as MMP-1, MMP-3, MMP-13, play an important role in physiological turnover of OA cartilage by degrading the extracellular matrix (ECM) molecules [5, 10, 39, 40]. In addition, ANGPTL2 enhances tumor cell invasion by increasing expression and activity of MMPs in osteosarcoma [23]. Therefore, to examine the function of ANGPTL2 in OA progression, we investigated its role in chondrocyte metabolic dysfunction. Similar to other studies, IL-1β treatment promoted the expression of MMP-1 and MMP-13 production. Intriguingly, ANGPTL2 inhibition could counteract the production of MMP-1 and MMP-13 upon IL-1β stimulation. In addition, MMP-1, MMP-13 were also increased in chondrocyte treated with rhANGPTL2. And besides, we also found that knockdown of ANGPTL2 increased COL2A1 expression and chondrocyte treated with rhANGPTL2 inhibited COL2A1 expression. Accordingly, these results suggest that ANGPTL2 suppression may ameliorate OA pathologic progression by inhibiting the extracellular matrix degradation.
Accumulating studies indicate that low-grade chronic inflammation plays also a pivotal role in OA pathogenesis by releasing various inflammatory cytokines and mediators [1, 11, 41]. Among these cytokines, IL-1β, IL-6 and TNF-α, have been extensively studied in OA, and exerts a significant role in OA development. IL-1β stimulation in chondrocytes can increase the expression of MMPs and inflammatory cytokines that further exacerbate inflammatory response and promote OA progression. An increasing body of evidence has shown that inhibition of chondrocyte inflammation was a promising therapeutic strategy in OA. Previews studies have shown that ANGPTL2 can lead to inflammatory response in the context of obesity and diabetes. Here, our data revealed that blocking ANGPTL2 expression could significantly antagonize the IL-1β-triggered inflammatory genes expression including IL-1β and IL-6. Intriguingly, rhANGPTL2 treatment promoted inflammatory response by elevating the releasing of IL-1β and IL-6.
The mechanism of response to OA progression is complicated, and several signal pathways are involved, such as NF-κB pathway and p38 MAPK pathway [23, 24, 29, 30]. The inhibitors of NF-κB proteins (IκB) phosphorylation is induced by IL-1β stimulation, results in the phosphorylation of p65 and activated p65 is translocated from the cytoplasm to the nucleus. Consequently, the expression of catabolic enzymes and inflammatory mediators is triggered in the nucleus [42, 43]. In addition, p38 MAPK pathway is implicated in multiple pathologic activities, like tumor metastasis and some inflammatory diseases, but mass evidence has shown that p38 MAPK pathway plays an important role in OA pathogenesis and progression. Moreover, IL-1β treatment in chondrocytes can enhance the phosphorylation of p38. It was also reported this pathway can trigger the expression of inflammation-related genes and catabolic enzymes, and consequent cartilage destruction, including IL-6, MMP-1 and MMP-13. Integrin α5β1, composed of one α5- and one β1-subunit, reportedly functions as ANGPTL2 receptor in ATDC and other cells. In this study, our data indicated that IL-1β-stimulation of human chondrocytes resulted in a marked up-regulation of the expression levels of p-p65, p-p38, ITGA5 and ITGB1, however, knockdown of ANGPLT2 resulted in significant inhibition of expression of p-p65, p-p38. Additionally, treatment of chondrocytes with rhANGPTL2 can markedly promote p-p65 and p-p38 production and inhibition of integrin α5β1 with ATN-161 significantly antagonize the phosphorylation levels of p38 and p65 in primary chondrocyte treated with rhANGPTL2. These results suggested that ANGPTL2 might promote chondrocyte injury through the NF-κB and p38 MAPK signaling pathways via integrin α5β1.
Both articular cartilage and epiphyseal cartilage belong to hyaline cartilage, but they are different in structure and function. In epiphyseal cartilage, endochondral osteogenesis is a progressive process involved in bone formation and growth. Undifferentiated mesenchymal stem cells initially condensate and differentiate into immature chondrocytes (early-phase differentiation), which then undergo successive maturation steps to differentiation become hypertrophic chondrocytes (late-phase differentiation) [44]. In a previous study, ATDC cells (chondrogenic cell line, not chondrocyte) were treated with insulin to mimic progress chondrogenic differentiation in vitro. Knockdown of ANGPLT2 resulted in significant inhibition of expression of chondrocyte marker (COL2A1 and aggrecan) in early-phase differentiation and the hypertrophic chondrocyte marker (Col10a1, MMP-13) in late-phase differentiation (in our study, we also observed that knockdown of ANGPLT2 decreased the expression of MMP-13 in vitro OA model). In ANGPTL2 knockout mice, they observed a decrease both in size and quantity of hypertrophic chondrocytes. And the expression level of the hypertrophic chondrocyte marker (Col10a1, MMP-13) was also inhibited. By contrast, apoptosis chondrocyte in the hypertrophic zone increased in ANGPTL2 knockout mice [30]. All these reveled that ANGPTL2 promote chondrogenic differentiation (mesenchymal stem cells into chondrocytes and chondrocytes into hypertrophic chondrocytes) and ultimately cartilage ossification in epiphyseal cartilage during bone growth. Different from epiphyseal cartilage, it is widely accepted in the textbooks that articular cartilage in adult consists of only terminal differentiated chondrocyte and lacks mesenchymal stem cells. In addition, both hypertrophy of articular chondrocytes and ossification of cartilage are important pathogenesis of OA [45, 46]. On the whole, ANGPTL2 is not a protective protein of cartilage which eventually converts epiphyseal cartilage into bone, promoting chronic inflammation of articular cartilage and degradation of cartilage matrix.
In conclusion, this study is the first research showing that ANGPTL2 secreted by human chondrocytes plays a negative role in the pathogenesis of osteoarthritis, and it may be a potential therapeutic target in osteoarthritis.
Abbreviations
- ANGPTL2:
-
Angiopoietin-like 2
- NF-κB:
-
Nuclear factor kB
- MAPK:
-
Mitogen-activated protein kinase
- OA:
-
Osteoarthritis
- MMP:
-
Matrix metalloproteinase
- ECM:
-
Extracellular matrix
- IL:
-
Interleukin
- ITGA5:
-
Integrin α5
- ITGB1:
-
Integrin β1
References
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J (2016) Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis (in eng). Nat Rev Rheumatol 12:580–592. https://doi.org/10.1038/nrrheum.2016.136
Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ (in eng). Arthritis Rheum 64:1697–1707. https://doi.org/10.1002/art.34453
Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C, Theilmeier G, Pap T (2009) Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis (in eng). Nat Med 15:1072–1076. https://doi.org/10.1038/nm.1998
Karsenty G (2005) An aggrecanase and osteoarthritis (in eng). The New England journal of medicine 353:522–523. https://doi.org/10.1056/NEJMcibr051399
Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ (2001) Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice (in eng). J Clin Investig 107:35–44. https://doi.org/10.1172/jci10564
Zhang M, Mani SB, He Y, Hall AM, Xu L, Li Y, Zurakowski D, Jay GD, Warman ML (2016) Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis (in eng). J Clin Investig 126:2893–2902. https://doi.org/10.1172/jci83676
Chen WP, Hu ZN, Jin LB, Wu LD (2017) Licochalcone A Inhibits MMPs and ADAMTSs via the NF-kappaB and Wnt/beta-Catenin Signaling Pathways in Rat Chondrocytes (in eng). Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 43:937–944. https://doi.org/10.1159/000481645
Yuan Y, Tan H, Dai P (2017) Kruppel-Like Factor 2 Regulates Degradation of Type II Collagen by Suppressing the Expression of Matrix Metalloproteinase (MMP)-13 (in eng). Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 42:2159–2168. https://doi.org/10.1159/000479991
Ma CH, Wu CH, Jou IM, Tu YK, Hung CH, Hsieh PL, Tsai KL (2018) PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes (in eng). Redox biology 14:72–81. https://doi.org/10.1016/j.redox.2017.08.011
Son YO, Park S, Kwak JS, Won Y, Choi WS, Rhee J, Chun CH, Ryu JH, Kim DK, Choi HS, Chun JS (2017) Estrogen-related receptor gamma causes osteoarthritis by upregulating extracellular matrix-degrading enzymes (in eng). Nature communications 8:2133. https://doi.org/10.1038/s41467-017-01868-8
Pelletier JP, Martel-Pelletier J, Abramson SB (2001) Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets (in eng). Arthritis Rheum 44:1237–1247. https://doi.org/10.1002/1529-0131(200106)44:6%3c1237:AID-ART214%3e3.0.CO;2-F
Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR et al (2006) Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis (in eng). The New England journal of medicine 354:795–808. https://doi.org/10.1056/NEJMoa052771
Hunter DJ (2015) Viscosupplementation for Osteoarthritis of the Knee (in eng). The New England journal of medicine 372:2570. https://doi.org/10.1056/NEJMc1505801
Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI (1991) Comparison of an antiinflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee (in eng). The New England journal of medicine 325:87–91. https://doi.org/10.1056/nejm199107113250203
da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Juni P, Trelle S (2016) RETRACTED: effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis (in eng). Lancet (London, England) 387:2093–2105. https://doi.org/10.1016/s0140-6736(16)30002-2
Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT (2010) Tanezumab for the treatment of pain from osteoarthritis of the knee (in eng). The New England journal of medicine 363:1521–1531. https://doi.org/10.1056/NEJMoa0901510
Puljak L, Marin A, Vrdoljak D, Markotic F, Utrobicic A, Tugwell P (2017) Celecoxib for osteoarthritis (in eng). The Cochrane database of systematic reviews 5:CD009865. https://doi.org/10.1002/14651858.CD009865.pub2
Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, Rasmussen S (2015) A Randomized, Controlled Trial of Total Knee Replacement (in eng). The New England journal of medicine 373:1597–1606. https://doi.org/10.1056/NEJMoa1505467
Loeser RF, Collins JA, Diekman BO (2016) Ageing and the pathogenesis of osteoarthritis (in eng). Nat Rev Rheumatol 12:412–420. https://doi.org/10.1038/nrrheum.2016.65
Kadomatsu T, Endo M, Miyata K, Oike Y (2014) Diverse roles of ANGPTL2 in physiology and pathophysiology (in eng). Trends in endocrinology and metabolism: TEM 25:245–254. https://doi.org/10.1016/j.tem.2014.03.012
Thorin-Trescases N, Thorin E (2014) Angiopoietin-like-2: a multifaceted protein with physiological and pathophysiological properties (in eng). Expert Rev Mol Med 16:e17. https://doi.org/10.1017/erm.2014.19
Aoi J, Endo M, Kadomatsu T, Miyata K, Nakano M, Horiguchi H, Ogata A, Odagiri H, Yano M, Araki K, Jinnin M, Ito T, Hirakawa S, Ihn H, Oike Y (2011) Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis (in eng). Can Res 71:7502–7512. https://doi.org/10.1158/0008-5472.can-11-1758
Odagiri H, Kadomatsu T, Endo M, Masuda T, Morioka MS, Fukuhara S, Miyamoto T, Kobayashi E, Miyata K, Aoi J, Horiguchi H, Nishimura N, Terada K, Yakushiji T, Manabe I, Mochizuki N, Mizuta H, Oike Y (2014) The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin alpha5beta1, p38 MAPK, and matrix metalloproteinases (in eng). Science Signaling 7:7. https://doi.org/10.1126/scisignal.2004612
Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K et al (2014) Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression (in eng). Arterioscler Thromb Vasc Biol 34:790–800. https://doi.org/10.1161/atvbaha.113.303116
Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y et al (2009) Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance (in eng). Cell Metab 10:178–188. https://doi.org/10.1016/j.cmet.2009.08.003
Corciulo C, Lendhey M, Wilder T, Schoen H, Cornelissen AS, Chang G, Kennedy OD, Cronstein BN (2017) Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression (in eng). Nature communications 8:15019. https://doi.org/10.1038/ncomms15019
Jacques C, Bereziat G, Humbert L, Olivier JL, Corvol MT, Masliah J, Berenbaum F (1997) Posttranscriptional effect of insulin-like growth factor-I on interleukin-1beta-induced type II-secreted phospholipase A2 gene expression in rabbit articular chondrocytes (in eng). J Clin Investig 99:1864–1872. https://doi.org/10.1172/jci119353
Meng F, Li Z, Zhang Z, Yang Z, Kang Y, Zhao X, Long D, Hu S, Gu M, He S, Wu P, Chang Z, He A, Liao W (2018) MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3 (in eng). Theranostics 8:2862–2883. https://doi.org/10.7150/thno.23547
Hirasawa M, Takubo K, Osada H, Miyake S, Toda E, Endo M, Umezawa K, Tsubota K, Oike Y, Ozawa Y (2016) Angiopoietin-like Protein 2 Is a Multistep Regulator of Inflammatory Neovascularization in a Murine Model of Age-related Macular Degeneration (in eng). The Journal of biological chemistry 291:7373–7385. https://doi.org/10.1074/jbc.M115.710186
Tanoue H, Morinaga J, Yoshizawa T, Yugami M, Itoh H et al (2018) Angiopoietin-like protein 2 promotes chondrogenic differentiation during bone growth as a cartilage matrix factor (in eng). Osteoarthritis and cartilage 26:108–117. https://doi.org/10.1016/j.joca.2017.10.011
Bose S, Li B, He J, Lv H, Liu Y, Lv X, Zhang C, Zhu Y, Ai D (2019) c-Abl regulates YAPY357 phosphorylation to activate endothelial atherogenic responses to disturbed flow (in eng). Nature communications 129:1167–1179. https://doi.org/10.1038/s41467-019-09453-x10.1172/jci122440
Lee SJ, Lee CK, Kang S, Park I, Kim YH, Kim SK, Hong SP, Bae H, He Y, Kubota Y, Koh GY (2018) Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction (in eng). J Clin Investig 128:5018–5033. https://doi.org/10.1016/j.ccell.2018.11.01610.1172/jci99659
Duchet BJ, Hansel CS, Maynard SA, Chow LW, Stevens MM, Sundaram A, Chen C, Khalifeh-Soltani A, Atakilit A, Ren X, Qiu W, Jo H, DeGrado W, Huang X, Sheppard D (2017) Targeting integrin alpha5beta1 ameliorates severe airway hyperresponsiveness in experimental asthma (in eng). ACS Nano 127:365–374. https://doi.org/10.1021/acsnano.6b0597510.1172/jci88555
Goldring MB, Goldring SR (2010) Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis (in eng). Ann N Y Acad Sci 1192:230–237. https://doi.org/10.1111/j.1749-6632.2009.05240.x
Sellam J, Berenbaum F (2010) The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis (in eng). Nat Rev Rheumatol 6:625–635. https://doi.org/10.1038/nrrheum.2010.159
Karsdal MA, Bay-Jensen AC, Lories RJ, Abramson S, Spector T, Pastoureau P, Christiansen C, Attur M, Henriksen K, Goldring SR, Kraus V (2014) The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments? (in eng). Ann Rheum Dis 73:336–348. https://doi.org/10.1136/annrheumdis-2013-204111
Liu-Bryan R, Terkeltaub R (2015) Emerging regulators of the inflammatory process in osteoarthritis (in eng). Nat Rev Rheumatol 11:35–44. https://doi.org/10.1038/nrrheum.2014.162
Felson DT (2006) Clinical practice. Osteoarthritis of the knee (in eng). The New England journal of medicine 354:841–848. https://doi.org/10.1056/NEJMcp051726
Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR (1997) Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage (in eng). J Clin Investig 99:1534–1545. https://doi.org/10.1172/jci119316
Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR, Mumford RA, Lohmander LS (1997) Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints (in eng). J Clin Investig 100:93–106. https://doi.org/10.1172/jci119526
de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, Zuurmond AM, Schoones J, Toes RE, Huizinga TW, Kloppenburg M (2012) Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review (in eng). Osteoarthritis and cartilage 20:1484–1499. https://doi.org/10.1016/j.joca.2012.08.027
Chen LF, Greene WC (2004) Shaping the nuclear action of NF-kappaB (in eng). Nat Rev Mol Cell Biol 5:392–401. https://doi.org/10.1038/nrm1368
Kobayashi H, Chang SH, Mori D, Itoh S, Hirata M, Hosaka Y, Taniguchi Y, Okada K, Mori Y, Yano F, Chung UI, Akiyama H, Kawaguchi H, Tanaka S, Saito T (2016) Biphasic regulation of chondrocytes by Rela through induction of anti-apoptotic and catabolic target genes (in eng). Nature Communications 7:13336. https://doi.org/10.1038/ncomms13336
Kronenberg HM (2003) Developmental regulation of the growth plate (in eng). Nature 423:332–336. https://doi.org/10.1038/nature01657
van der Kraan PM, van den Berg WB (2012) Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? (in eng). Osteoarthritis and cartilage 20:223–232. https://doi.org/10.1016/j.joca.2011.12.003
Pesesse L, Sanchez C, Delcour JP, Bellahcene A, Baudouin C, Msika P, Henrotin Y (2013) Consequences of chondrocyte hypertrophy on osteoarthritic cartilage: potential effect on angiogenesis (in eng). Osteoarthritis and cartilage 21:1913–1923. https://doi.org/10.1016/j.joca.2013.08.018
Acknowledgements
We thank Kai Lin, Yinglei Fang, Penghui Ke for assistance in collecting cartilage samples.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81672161) and National Undergraduate Traning Programs for Innovation and Entrepreneurship-China (No. 201810366025).
Author information
Authors and Affiliations
Contributions
Zongsheng Yin, Wei He and Jiegou Xu designed the study. Wenshan Shan, Zhenfei Ding, Guanjun Cui performed in vitro experiments. Chao Chen, Wei Huang, Wei Lu and Fuen Liu detected the expression level of ANGPTL2 in OA cartilage samples. Sha Luo participated in the supplementary experiment. Wenshan Shan wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
All cartilage samples were obtained from the First Affiliated Hospital of Anhui Medical University. This research was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University.
Informed consent
All patients obtained a written informed consent about the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
774_2019_1016_MOESM1_ESM.tif
Supplementary Fig. 1. Identification of human primary chondrocytes. SF1. A-C shows that type II collagen was high produced by human primary chondrocytes. Immunofluorescence of human primary chondrocytes with an anti-type II collagen antibody (SF1A). The nuclei of cells were revealed by the DAPI (SF1.B). Staining of human primary chondrocytes with toluidine blue, which reveals high production of proteoglycans in human chondrocytes (SF1.D). Scale bars (ABC) = 200 μm. Scale bars (D) = 100 μm (TIFF 77082 kb)
774_2019_1016_MOESM2_ESM.tif
Supplementary Fig. 2. Silencing of ANGPTL2 in chondrocyte. AB: Silencing of ANGPTL2 in vitro OA model Human chondrocytes were divided into four groups: Control, IL-1β (10 ng/mL), IL-1β (10 ng/ml) + siNC, IL-1β (10 ng/mL) + siANGPTL2. Chondrocytes were pretreated with siNC or siANGPTL2 for 24 h followed by stimulated with IL-1β (10 ng/ml) for 24 h, and then, RNA or protein was extracted from chondrocytes. (A) qRT-PCR and (B) Western Blot were used to evaluate the expression of ANGPTL2. CD: Silencing of ANGPTL2 in human primary chondrocyte without IL-1β. Human chondrocytes were divided into three groups: Control, siNC, siANGPTL2. Chondrocytes were pretreated with siNC or siANGPTL2 for 24 h and then, RNA or protein was extracted from chondrocytes. (A) Western Blot and (B) qRT-PCR were used to evaluate the expression of ANGPTL2. Results are expressed as mean ± SD. Welch Test followed by Games-Howell Test (Equal variances not assumed) (TIFF 9847 kb)
774_2019_1016_MOESM3_ESM.tif
Supplementary Fig. 3. Effects of ANGPTL2 on COL2A1 and aggrecan production in human primary chondrocytes. A-C Human chondrocytes were divided into four groups: Control, IL-1β (10 ng/mL), IL-1β (10 ng/ml) + siNC, IL-1β (10 ng/mL) + siANGPTL2. Chondrocytes were pretreated with siNC or siANGPTL2 for 24 h followed by stimulated with IL-1β (10 ng/ml) for 24 h, and then, RNA or protein was extracted from chondrocytes. (A)(B) qRT-PCR analysis for the mRNA expression levels of COL2A1 and aggrecan. (C)Western blots analysis for the protein expression of COL2A1 and aggrecan. D-F Human primary chondrocytes were treated with a serial dosage (0, 0.1, 0.2, 0.4 μg/ml) of rhANGPTL2 for 24 h. (D)(E) qRT-PCR analysis for the mRNA expression levels of COL2A1 and aggrecan. (F)Western blots analysis for the protein expression of COL2A1 and aggrecan. Results are expressed as mean ± SD. (D)Analysis of variance (ANOVA) followed by Bonferroni Test (equal variances). (A)Welch Test followed by Games-Howell Test (Equal variances not assumed). (BE) ANOVA Test (no significant) (TIFF 9789 kb)
774_2019_1016_MOESM4_ESM.tif
Supplementary Fig. 4. Effects of ANGPTL2 on caspase3 production in human primary chondrocytes. Human chondrocytes were divided into four groups: Control, IL-1β (10 ng/mL), IL-1β (10 ng/ml) + siNC, IL-1β (10 ng/mL) + siANGPTL2. Chondrocytes were pretreated with siNC or siANGPTL2 for 24 h followed by stimulated with IL-1β (10 ng/ml) for 24 h, and then, RNA or protein was extracted from chondrocytes. (A) Western Blot and (B) qRT-PCR were used to evaluate the expression of caspase3. Results are expressed as mean ± SD. ANOVA Test (no significant) (TIFF 4184 kb)
About this article
Cite this article
Shan, W., Cheng, C., Huang, W. et al. Angiopoietin-like 2 upregulation promotes human chondrocyte injury via NF-κB and p38/MAPK signaling pathway. J Bone Miner Metab 37, 976–986 (2019). https://doi.org/10.1007/s00774-019-01016-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-01016-w