Abstract
We examined the efficacy and safety of denosumab as treatment for glucocorticoid-induced osteoporosis (GIOP) patients complicated with rheumatic diseases, by measuring patients’ lumber bone mineral density (BMD) and bone turnover markers. A total of 66 consecutive patients for whom denosumab was initiated between July 2013 and August 2016 were enrolled and evaluated for 12 months. All of the patients were treated with glucocorticoids for underlying rheumatic diseases. The clinical assessment included measurements of the BMD of the lumbar spine (L2–L4) by a dual-energy X-ray absorptiometry technique and the bone turnover markers N-terminal telopeptide of type 1 collagen (NTX) in urine, serum intact procollagen type 1 N-terminal propeptide (P1NP), and bone-specific alkaline phosphatase (BAP) at baseline, 6 months and 12 months after the start of denosumab treatment. Adverse events (AEs) until 12 months were also analyzed. The mean percentage changes in BMD from baseline to 6 and 12 months were significant (2.85% increase, p < 0.0001 and 4.40% increase, p < 0.0001, respectively) regardless of the prior anti-osteoporotic drugs treatment (16 no transition from anti-osteoporotic drugs, 27 transition from bisphosphonate, 23 transition from teriparatide). The decreases in NTX, P1NP and BAP at 6 and 12 months were also significant. No serious AEs were noted. A multivariable logistic analysis showed that the prednisolone dose at baseline was associated with the clinical response to denosumab. In a real-world setting, denosumab was effective and safe for treating GIOP patients complicated with rheumatic diseases regardless of prior anti-osteoporotic drug treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the treatment of rheumatic disease, glucocorticoid (GC) is still a main-stay despite its several unfavorable effects. Glucocorticoid-induced osteoporosis (GIOP) is an example of this, as GIOP leads to an individual’s vulnerability to fragility fractures and a potential loss of daily-life functioning. A fragility fracture may occur in 30–50% of patients who are treated with GC over a long term; one report revealed that the vertebral fracture risk increases to 17-fold with treatment using a prednisolone-equivalent dose of 10–12 mg for > 3 months [1]. It was also reported that within the first 6 months after GC treatment begins, a decrease in bone mineral density (BMD) occurs [2]. In addition to BMD loss, GC treatment has a deleterious effect on bone quality: even before the BMD loss occurred, the risk of fracture increased by as much as 75% within the first 3 months [3]. Moreover, compared to postmenopausal osteoporosis, the risk of fragility fractures is much higher at the same BMD values in GIOP [4].
Osteoporosis can be classified into two types: primary osteoporosis and secondary osteoporosis. Most of the osteoporosis patients are classified as having primary osteoporosis, which is observed mainly in postmenopausal women. Secondary osteoporosis is usually caused by the administration of a drug such as a steroid, or it is caused by other diseases such as hyperparathyroidism. The clinical practice guidelines for the treatment of GIOP are different from the guidelines for postmenopausal osteoporosis. Generally, compared to postmenopausal osteoporosis, the clinical evidence of the efficacy of anti-osteoporotic drugs with new mechanism is weak due to the heterogeneity of disease and the use of concomitant medications such as immunosuppressive agents. The limited use of anti-osteoporotic drug also makes it difficult to conduct a large randomized trial.
Denosumab is a monoclonal antibody for receptor activator for nuclear factor kappa-B ligand (RANKL). Denosumab prevents the binding of RANKL and its receptor RANK, resulting in a suppression of the differentiation of precursor cells into mature osteoclasts and decreases in the function and survival of activated osteoclasts. Several randomized controlled trials (RCTs) have been conducted to evaluate denosumab in patients with primary osteoporosis, and these RCTs have confirmed the efficacy of denosumab for increasing bone density and reducing the rate of fractures [5,6,7]. However, regarding the use of denosumab for treating GIOP, there has been few RCT (for the above-mentioned reasons), and little information is available from clinical cases. Considering the frequency of fractures caused by GIOP and the influence of GIOP on patients’ activities of daily living (ADLs) after a fracture, it is necessary to determine the effectiveness and safety of denosumab for treating GIOP. Here, we evaluated the efficacy and safety of denosumab in patients under GC treatment.
Materials and methods
Patients
We retrospectively analyzed all patients with rheumatic disease who were registered and followed by the Department of Immunology and Rheumatology, Nagasaki University Graduate School of Biomedical Sciences, Sasebo Chuo Hospital treated with denosumab for GIOP. A total of 66 consecutive patients for whom denosumab was initiated between July 2013 and August 2016 were enrolled and evaluated for ≥ 1 y year. None of the patients had dropped out from denosumab treatment at 1 year. All patients were receiving prednisolone (PSL) (2–20 mg). All patients were classified as being in an anti-osteoporotic treatment-required category based on the Japanese Society for Bone and Mineral Research (JSBMR) guidelines for GIOP [8].
The patients gave their informed consent to be subjected to the protocol, which was approved by the Institutional Review Board of Nagasaki University (IRB Approval No.: 17041715) and Sasebo Chuo Hospital (IRB Approval No.: 2018-01). The demographic data recorded at the initiation of denosumab treatment included age, sex, duration of steroid therapy, and previous treatment for osteoporosis. All patients received 60 mg of denosumab subcutaneously every 6 months. All patients received daily supplemental vitamin D and calcium (n = 15) or active vitamin D (n = 51).
Clinical assessment including bone mineral density measurement
Effectiveness of denosumab was assessed by measure of BMD. The BMD of the lumbar spine (L2–L4) of all patients was measured by a dual-energy X-ray absorptiometry (DXA) technique using a Discovery™ Wi QDR densitometer (Hologic, Bedford, MA, USA) or a Lunar PRODIGY densitometer (GE Healthcare, Buckinghamshire, UK) at baseline, 6 months, and 12 months. Radiographs of the thoracic and lumbar spine were taken for the detection of pre-existing vertebral fractures and new fractures at the same time as a BMD measurement. Vertebral fractures were evaluated using semiquantitative method [9]. Adverse events (AEs) until 12 months were analyzed.
Assessment of bone turnover markers
We also evaluated bone turnover markers in almost half of the enrolled patients. The N-terminal telopeptide of type 1 collagen (NTX) in urine was determined as a marker of bone resorption. The NTX in urine is expressed as a ratio to urinary creatinine concentration. As bone formation markers, we determined the intact procollagen type 1 N-terminal propeptide (P1NP) and bone-specific alkaline phosphatase (BAP) levels in serum, at baseline, 6 months and 12 months.
Statistical analysis
GraphPad prism software (GraphPad Software, San Diego, CA) and JMP Statistical Software (SAS Institute, Cary, NC) were used for the statistical analysis. Normal distribution of the data was confirmed using the Kolmogorov–Smirnov test. The distribution of baseline variables between patient subgroups was examined by analysis of variance (ANOVA) with Tukey’s multiple-comparisons test (parametric data), Steel–Dwass test with Dunn’s multiple-comparisons test (non-parametric data) and Chi-square test (non-parametric data). The Wilcoxon signed rank test was used to detect significant differences in changes of BMD and bone turnover markers. For the cases of missing data, we used the complete case analysis method. An ANOVA with Tukey’s multiple-comparisons test was used to estimate changes of BMD between patient subgroups. Univariate and multivariable ordinal logistic regression analyses were used to determine the predictive factor of clinical responses. Variables with p values < 0.2 in the univariate logistic regression analyses were entered in the multivariate logistic regression analysis. All data are expressed as the mean and standard deviation (SD). A p value < 0.05 was considered significant.
Results
Baseline characteristics
The study population was 66 patients. Their baseline demographic characteristics are illustrated in Table 1. The mean age of the patients was 63.4 ± 12.8 years, and the majority of the patients were female (84.9%). The mean dose of prednisolone was 5.92 ± 3.79 mg/day with a mean duration of 11.6 ± 8.5 years. Radiographs of the thoracic and lumbar spine at baseline showed the presence of vertebral fractures in 21 patients (31.8%). The underlying diseases of the patients leading to prednisolone use were: rheumatoid arthritis (37.9%), systemic lupus erythematosus (19.7%), dermatomyositis/polymyositis (3.0%), Behçet disease (4.6%), polymyalgia rheumatica (4.6%), overlap syndrome (12.1%) and others (18.1%, e.g., vasculitis). Twenty-three patients (34.9%) had been treated with daily teriparatide prior to denosumab, and 27 patients (40.9%) had been treated with bisphosphonate (BP) prior to denosumab. In the other 16 patients, there was no transition from an anti-osteoporotic drug to denosumab, but two of these 16 patients had a history of BP intake (for 1 month and 6 months). The decision to change each treatment was made based on a consensus between the patients and physicians. The most common reason for transition from daily teriparatide treatment was the duration of teriparatide treatment (all patients were treated with daily teriparatide, not weekly teriparatide, and the duration of daily teriparatide treatment approved in Japan is up to 2 years). Regarding the transition from BP treatment, one patient changed treatment because of nausea and one patient changed for preventive effect against the bone destruction of rheumatoid arthritis. The reason for the transition from BP in the other 25 patients was the diminished efficacy of the BP treatment, e.g., a decrease in BMD.
At 52 weeks from baseline, all of the patients were continuing their denosumab treatment. The results of our comparison of the baseline characteristics of the patient subgroups divided by prior anti-osteoporotic drug are summarized in Table 1. Reflecting their prior treatment, the BMD values were significantly high at the transition to denosumab in the teriparatide-treated group compared to the other groups. However, there were no significant differences among the subgroups in other baseline variables including the mean dose of PSL.
Overall efficacy of denosumab treatment
The changes in the BMD of lumbar spine are illustrated in Fig. 1a. The mean percentage changes in the BMD of the lumbar spine from baseline to 6 and 12 months were significant (2.85% increase, p < 0.0001 and 4.40% increase, p < 0.0001, respectively). The proportions of BMD gains at 12 months are shown in Fig. 1b. Gains higher than 3% (putative least significant change [10,11,12]) were observed in 68.2% of the patients. Few patients showed decreased BMD at 12 months after the initiation of denosumab (16.67%).
a Percent changes in BMD at the lumbar spine for the 12-month study period. Points and bars represent means and standard deviations, respectively. *p < 0.0001 versus baseline by the Wilcoxon signed rank test. b The proportion of subjects by BMD response category based on the BMD percent change from baseline to 12 months at the lumbar spine. BMD bone mineral density
Bone turnover markers
In approx. one-half of the enrolled patients, we analyzed bone turnover markers during the denosumab treatment. As shown in Fig. 2, all bone turnover markers determined in this study were rapidly decreased at 6 months from baseline, followed by a slow decrease or increase at 12 months compared to the 6-month values. The median value of serum BAP in 35 patients was 12.5 µ/L at baseline, 8.5 µ/L at 6 months and 8.6 µ/L at 12 months. The median value of urinary NTX in 31 patients was 23.9 nmolBCE/mmolCr at baseline, 17.95 nmolBCE/mmolCr at 6 months and 15.9 nmolBCE/mmolCr at 12 months. The median value of serum P1NP in 32 patients was 26.0 µg/L at baseline, 14.0 µg/L at 6 months and 12.7 µg/L at 12 months.
Value changes of serum BAP (a), serum P1NP (b), and urinary NTX (c) for the 12-month study period. Points and bars represent median and IQR, respectively. *p < 0.05 versus baseline by the Wilcoxon signed rank test. BAP bone-specific alkaline phosphatase, P1NP procollagen type 1 N-terminal propeptide, NTX N-terminal telopeptide of type 1 collagen, IQR interquartile range
Safety and adverse events
Ten patients (15.2%) experienced one or two AEs within 12 months of the start of denosumab treatment. All AEs are reported in Table 2. As expected, the most common AE was infection (29.2%), especially upper respiratory infection and herpes zoster. Four of the five patients who experienced infections were being treated concomitantly with an immunosuppressive agent. No patients discontinued denosumab due to AEs. No hypocalcemia or osteonecrosis of the jaw was observed during the study period.
Fracture incidence
No new vertebral fracture detected by radiographs occurred during the study period. However, two fragility fractures of the pelvic ring occurred. In the two patients who experienced this type of fragility fracture, there were great increases in BMD at 12 months (7.57% and 9.88% increases, respectively), but these patients’ baseline BMD values were significantly low (0.555 g/cm2 and 0.567 g/cm2, respectively).
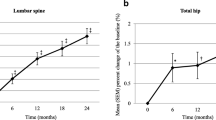
A comparison of the efficacy of denosumab based on prior treatment/no treatment for osteoporosis
Previous phase 3 study showed that the order in which denosumab is used affected the efficacy of denosumab [13]. We, therefore, next analyzed the relation between the efficacy of denosumab and prior treatment for osteoporosis. In the present study, the patients who transitioned from BPs to denosumab (n = 27, 40.9%) showed a further increase in BMD (4.71% increase, 0.73 ± 0.18 g/cm2 to 0.76 ± 0.19 g/cm2, p < 0.001) compared to the patients who transitioned from teriparatide to denosumab (n = 23, 34.9%) (3.71% increase, 0.89 ± 0.20 g/cm2 to 0.92 ± 0.21 g/cm2, p < 0.001) at 12 months. However, the difference between the two groups was not significant. The changes in BMD in the patients who did not transit from an anti-osteoporotic drug to denosumab (n = 16, 24.2%) also showed no significant difference compared to the other two groups (Fig. 3).
Factors influencing the BMD of patients treated with denosumab
To assess factors that might influence BMD changes when denosumab is administered, we conducted univariate and multivariate analyses. The factors that were shown to influence BMD changes throughout the study period by the univariate logistic analysis are shown in Table 3. The univariate analysis indicated that the dose/duration of PSL and body weight were associated with a BMD increase of > 3% at 12 months. Among these candidate factors, the multivariable logistic analysis showed that the dose of PSL and body weight were associated with the clinical response to denosumab (Table 3).
Discussion
We evaluated the efficacy of denosumab for GIOP in a “real-world” setting, and our analyses revealed that the patients’ BMD was increased at 12 months of denosumab treatment regardless of prior treatment. The percent change of lumbar spine BMD in our study was 4.4%, an improvement rate was similar to the other trials. In a large phase 3 trial called FREEDOM which evaluated the efficacy of denosumab in postmenopausal women, the percent change of BMD of the lumbar spine at 36 months was > 3% in over 90% of the denosumab-treated patients [10]. Two other phase 3 trials demonstrated a 5.7% increase in the lumbar spine BMD at 12 months and a 5.6% increase in the lumbar spine BMD at 24 months, respectively [14, 15]. Regarding GIOP, one large RCT which compared the efficacy of denosumab and risedronate in 795 GIOP patients reported that denosumab was superior to risedronate at 12 months for the effect on BMD at lumbar spine, and 4.4% increase was shown in BMD [16]. Unlike primary osteoporosis, there is only one large RCT for the evaluation of the efficacy of denosumab treatment of GIOP, but several observational studies and small RCTs are available. In one of those investigations, the 42 patients who were receiving long-term prednisolone and oral BPs were randomized to either continue oral BPs or switch to denosumab and the efficacy of each treatment was determined [17]. In the denosumab group (n = 21), the lumbar spine BMD had increased by 3.4% at 12 months. An observational study evaluating the efficacy of denosumab for GIOP showed that the BMD of the lumbar spine had increased by 3.2% at 12 months [18]. Taken together, the past and present findings indicate that the effect of denosumab on the BMD of patients with GIOP seems almost equal to that observed in primary osteoporosis patients, at least in the initial months after initiation of denosumab. Regarding long-term efficacy, several studies investigating the long-term efficacy of denosumab in primary osteoporosis patients revealed continuous increases in BMD [19, 20]. In contrast, the long-term efficacy of denosumab for treating GIOP remains unclear, and further studies are, therefore, necessary to determine whether denosumab’s long-term efficacy in GIOP cases shows the same tendency as that observed in primary osteoporosis.
Bone turnover markers reflect the status of bone remodeling, the bone resorption phase, or the bone formation phase. In this study, we measured P1NP and BAP in serum as bone formation markers and NTX in urine as a bone resorption marker. GIOP is regarded as low bone turnover caused by multiple mechanisms such as an inhibition of sex steroid hormones, an inhibition of the gastrointestinal absorption as well as renal reabsorption of calcium, and an inhibition of bone formation by a suppression of osteoblasts and enhancement of osteoclastogenesis through an osteoprotegerin(OPG)-RANKL production imbalance. In contrast, postmenopausal osteoporosis is regarded as high bone turnover. In our present study, denosumab treatment resulted in reduced bone turnover markers after 6 months in GIOP, and this result is similar to that reported in postmenopausal osteoporosis [21, 22]. Moreover, regardless of prior treatment (in all three subgroups in this study), the bone turnover markers showed a tendency to be decreased (e.g., the median serum P1NP values at baseline, 6 months, and 12 months were 24.8 µg/L, 12.3 µg/L, and 9.2 µg/L in no transition group (n = 6); 23.6 µg/L, 19.8 µg/L, and 16.1 µg/L in the transition from BPs group (n = 10); and 44.4 µg/L, 13.6 µg/L, and 12.9 µg/L in the transition from teriparatide group (n = 16). Other data are available upon request from the corresponding author). In the DEFEND [23], FREEDOM [20], and DIRECT [7] randomized controlled trials, markers of bone resorption were rapidly reduced by denosumab in the early phase (1 month), whereas the reduction of bone formation markers was more gradual with an increase in BMD, even at 1 month. Although we did not examine the changes of bone turnover markers in the early phase after the introduction of denosumab, it can be speculated that denosumab could modify the bone metabolism to bone formation in the early phase regardless of the prior status of bone metabolism or the prior therapeutic regime, and thus denosumab might be effective for treating patients with various states of bone metabolism, as we observed in the present study.
We compared the effect of denosumab by performing a sub-analysis of the patients’ prior treatment, and we observed that after the transition from teriparatide, the lumbar BMD showed a continuous increase. This result was consistent with the result from previous large study “DATA-SWITCH” trial [13]. Postmenopausal women with osteoporosis in which subjects were randomized to receive teriparatide, denosumab, or both drugs for 24 months, and then the subjects originally randomized to teriparatide received 24-months of denosumab, whereas subjects originally randomized to denosumab received 24-months of teriparatide. In the teriparatide–denosumab group, the lumbar BMD showed a continuous increase by 8.6 ± 5.0% after the transition, and this post-transition BMD increase was greater compared to that in the denosumab–teriparatide group. Our present study is the first to show that the clinical significance of transitioning from teriparatide to denosumab in GIOP, and we plan to investigate which strategy (denosumab–teriparatide or teriparatide–denosumab) is more effective in GIOP in a future study.
Regarding the transition from BPs, although the difference was not significant, we observed that the increase in BMD after transition was better compared with teriparatide–denosumab group. Although there is still controversial, long-term treatment with BPs might slightly increase the risk of osteonecrosis of the jaw and atypical fractures [24, 25]. Considering this and the reduction in efficacy over 3–5 years of BP treatment, a transition to denosumab should be considered in patients who have been under long-term BP treatment [26]. To investigate the optimal use of denosumab in GIOP, we analyzed various factors associated with the response to denosumab, and the multivariate analysis revealed that the dose of prednisolone was associated with the clinical response to denosumab. This response might be associated with RANKL expression, as glucocorticoids enhance RANKL expression [27], and thus the inhibition of RANKL might be more effective when there is an excess of RANKL induced by excess glucocorticoid. This suggests that denosumab should be used for the treatment of GIOP when the patient is being treated with a high dose of glucocorticoid. However, the patient series in the present study was small and this was not an RCT; this hypothesis must be tested in a larger study. New guidelines for the prevention and treatment of GIOP were recently published by the American College of Rheumatology (ACR) [28]. The guidelines still describe oral BPs as a preferred treatment for GIOP, and they note that denosumab can be used as an alternative treatment if oral BPs are not appropriate. The reason for this positioning of denosumab in the guidelines is based on the lack of safety data in people treated with immunosuppressive agents. In the present study, most of the patients were treated with additional immunosuppressive agent besides PSL; however, no serious AEs occurred. For a determination of optimal use of denosumab in GIOP, more clinical observational studies like this study and RCTs are needed.
AEs occurred in 15.2% of our patients. In the RCT of postmenopausal women, the incidence of skin infection (cellulitis and erysipelas of the skin) was higher in the treated group compared to the placebo group [6]. No skin infections developed in our present patients during study period, but several other infections occurred. It is difficult to assess whether these infections were caused by denosumab, because the concomitant use of PSL or immunosuppressive agent could attribute to these AEs. Nevertheless, these infections were not serious, and all patients recovered without hospitalization; our findings, thus, indicate that the treatment of GIOP with denosumab was relatively safe. A recent meta-analysis of denosumab treatment also showed that the relative risk of infectious serious AEs was not significantly different from that of placebo treatment [29]. Moreover, atypical fracture, osteonecrosis of the jaw, and severe symptomatic hypocalcemia did not develop in the present patient series.
Several limitations of this study must be mentioned. The number of patients was small and the long-term efficacy and safety of denosumab were not explored; in particular, the observation period was too short to assess the efficacy of denosumab for reducing fragility fractures. We could not analyze the effect of denosumab on non-vertebral bone because the BMD at the femoral neck was measured in only some of the patients. The effects of anti-osteoporotic drugs against cancellous bone and cortical bone differ. Although several RCTs which examined the effects of denosumab treatment in postmenopausal women showed similar effects on the BMD at the lumbar spine and that at the femoral neck [5, 20], we should investigate the efficacy of denosumab in the BMD at the femoral neck of GIOP patients in the near future. Moreover, the statistical reliability of the multivariate analysis that we performed in this study is not very strong, because of the small patient numbers and the use of data from three different subgroups. Finally, this study was not a randomized controlled trial conducted to compare the effects between denosumab and other conventional anti-osteoporotic agents. However, our findings are valuable because this was the first study to compare the efficacy of denosumab in GIOP by prior anti-osteoporotic treatment.
Conclusion
In conclusion, our results demonstrate that denosumab increased the BMD of GIOP patients in a real-world setting. Even in the patients with previous BP or teriparatide treatment, denosumab treatment resulted in a continuous increase of BMD after the transition from those anti-osteoporotic drugs. Despite the concomitant use of prednisolone and immunosuppressive agents, no serious AEs occurred for 12 months. Although the long-term effect on the prevention of vertebral fractures was not elucidated in this study, we should consider denosumab as one of the therapeutic options for GIOP patients, especially when the efficacy of BP treatment is diminished or when teriparatide treatment is being discontinued.
References
Steinbuch M, Youket TE, Cohen S (2004) Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int 15:323–328
LoCascio V, Bonucci E, Imbimbo B et al (1990) Bone loss in response to long-term glucocorticoid therapy. Bone Miner 8:39–51
Weinstein RS (2011) Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med 365:62–70
Kaji H, Yamauchi M, Chihara K, Sugimoto T (2006) The threshold of bone mineral density for vertebral fracture in female patients with glucocorticoid-induced osteoporosis. Endocr J 53:27–34
Brown JP, Prince RL, Deal C et al (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24:153–161
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Sugimoto T, Matsumoto T, Hosoi T et al (2015) Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos Int 26:765–774
Suzuki Y, Nawata H, Soen S et al (2014) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 32:337–350
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Bolognese MA, Teglbjaerg CS, Zanchetta JR et al (2013) Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 16:147–153
Fogelman I, Blake GM (2000) Different approaches to bone densitometry. J Nucl Med 41:2015–2025
Genant HK, Engelke K, Fuerst T et al (1996) Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res 11:707–730
Leder BZ, Tsai JN, Uihlein AV et al (2015) Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 386:1147–1155
Orwoll E, Teglbjaerg CS, Langdahl BL et al (2012) A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 97:3161–3169
Smith MR, Egerdie B, Hernandez Toriz N et al (2009) Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361:745–755
Saag KG, Wagman RB, Geusens P et al (2018) Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. https://doi.org/10.1016/S2213-8587(18)30075-5
Mok CC, Ho LY, Ma KM (2015) Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone 75:222–228
Ishiguro S, Ito K, Nakagawa S, Hataji O, Sudo A (2017) The clinical benefits of denosumab for prophylaxis of steroid-induced osteoporosis in patients with pulmonary disease. Arch Osteoporos 12:44
Freemantle N, Satram-Hoang S, Tang ET et al (2012) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23:317–326
Papapoulos S, Lippuner K, Roux C et al (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int 26:2773–2783
Nakamura Y, Suzuki T, Kamimura M et al (2017) Two-year clinical outcome of denosumab treatment alone and in combination with teriparatide in Japanese treatment-naive postmenopausal osteoporotic women. Bone Res 5:16055
Tsai JN, Uihlein AV, Lee H et al (2013) Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 382:50–56
Bone HG, Bolognese MA, Yuen CK et al (2008) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 93:2149–2157
Bejhed RS, Kharazmi M, Hallberg P (2016) Identification of risk factors for bisphosphonate-associated atypical femoral fractures and osteonecrosis of the jaw in a pharmacovigilance database. Ann Pharmacother 50:616–624
Lloyd AA, Gludovatz B, Riedel C et al (2017) Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 114:8722–8727
Diab DL, Watts NB (2013) Bisphosphonate drug holiday: who, when and how long. Ther Adv Musculoskelet Dis 5:107–111
Komori T (2016) Glucocorticoid Signaling and Bone Biology. Horm Metab Res 48:755–763
Buckley L, Guyatt G, Fink HA et al (2017) 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Care Res (Hoboken) 69:1095–1110
von Keyserlingk C, Hopkins R, Anastasilakis A et al (2011) Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Semin Arthritis Rheum 41:178–186
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
About this article
Cite this article
Iwamoto, N., Okamoto, M., Tsuji, S. et al. Denosumab is effective toward glucocorticoid-induced osteoporosis patients complicated with rheumatic diseases regardless of prior anti-osteoporotic drugs. J Bone Miner Metab 37, 554–562 (2019). https://doi.org/10.1007/s00774-018-0955-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-018-0955-7