Abstract
Sclerostin and dickkopf-1(DKK1) are Wnt/β-catenin signal antagonists that play an important role in bone formation. Ossification of the posterior longitudinal ligament (OPLL) of the spine is characterized by pathological ectopic ossification of the posterior longitudinal ligament and ankylosing spinal hyperostosis. The aims of this study were to evaluate serum sclerostin and DKK1 levels in persons with OPLL and to identify its relationship with bone metabolism and bone mass in persons with OPLL. This was a case–control study, and 78 patients with OPLL were compared with 39 age- and sex-matched volunteers without OPLL. We analyzed the relationship with calciotropic hormones, bone turnover markers, OPLL localization, number of ossified vertebrae, and bone mineral density of total hip (TH-BMD). Serum sclerostin levels in men with OPLL were significantly higher than in men in the control group (control group: mean = 45.3 pmol/L; OPLL group: mean = 75.7 pmol/L; P = 0.002). Age and sclerostin levels were positively correlated in men with OPLL (r = 0.43; P = 0.002). Serum sclerostin levels in men with OPLL had a positive correlation with TH-BMD Z-score (r = 0.511; P = 0.011, n = 30). There was a strong negative correlation between serum sclerostin levels and serum DKK1 levels in men with OPLL (r = −0.506; P < 0.001). Bone and mineral metabolism in OPLL differs between men and women. In men with OPLL, systemic secretion of sclerostin increases with advancing age and with higher bone mass. These two Wnt/β-catenin signal antagonists have the opposite effect in persons with OPLL, and higher serum sclerostin levels are counterbalanced by underproduction of DKK1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ossification of the posterior longitudinal ligament (OPLL) of the spine is an intractable disease of unknown etiology [1]. Development of pathological ectopic ossification of the posterior longitudinal ligament induces compression myelopathy or radiculopathy by spinal stenosis and the loss of spinal flexibility by ankylosing spinal hyperostosis [2]. The etiology of OPLL has not been fully clarified [3], but OPLL seems to occur and develop as a result of systemic and local factors in combination with a genetic abnormality [4–7].
Several studies have investigated the relationship between OPLL and bone and mineral metabolism [8–13]. Matsui et al. [8] demonstrated the presence of increased serum procollagen type I carboxyl-terminal peptide and intact osteocalcin in patients with OPLL. Sohn and Chung found that bone mineral density (BMD) in the lumbar spine and femur were higher in persons with OPLL than in control-group members and that rates of osteopenia and osteoporosis were lower in persons with OPLL than in control-group members [12]. Yoshimura et al. [13] reported that the presence of cervical OPLL showed a significant association between femoral neck BMD and the presence of diffuse idiopathic spinal hyperostosis (DISH) in 1,562 Japanese from a population-based cohort. Findings from these studies suggest that systemic factors such as constitutional predisposition and abnormal metabolic disorders may cause OPLL and lead to increased BMD. However, bone and mineral metabolism in persons with OPLL has not been fully elucidated.
Sclerosteosis and van Buchem disease are rare bone diseases first described in 1950 [14]. In 2001 the SOST gene, which encodes sclerostin, was identified as the gene responsible for sclerosteosis [15, 16]. SOST mRNA as well as sclerostin protein expression is restricted to osteocytes, and sclerostin (secreted exclusively by mature osteocytes embedded within the mineralized matrix) is a key protein that inhibits bone formation [17, 18]. Both sclerostin and dickkopf-1 (DKK1) are antagonists for canonical Wnt/β-catenin signaling and regulate bone mass by competitive binding to low-density lipoprotein receptor-related protein 5 [19]. Canonical Wnt/β-catenin signaling plays an important role in bone formation, and activation of this signaling pathway results in propagation of osteoprogenitor cells as well as reduced apoptosis of osteoblasts, leading to anabolic effects on bone [19, 20]. The role of sclerostin and DKK1 has been investigated in various diseases related to bone and mineral metabolism [21–24]. However, no studies have investigated serum sclerostin and DKK1 levels in OPLL in either animals or humans.
The objectives of our study were (1) to compare serum sclerostin and DKK1 levels between persons with OPLL and those without the disease and (2) to identify the relationship between Wnt/β-catenin signaling antagonists and bone turnover markers in persons with OPLL.
Materials and methods
This study is a case–control study. From October 2011 to September 2012, we consecutively recruited patients with OPLL who visited our hospital for routine follow-up care. The OPLL group included 78 patients with a diagnosis of cervical OPLL and/or thoracic OPLL. Cervical OPLL was diagnosed using plain lateral radiographs and/or whole-spine multiplanar reconstruction computed tomography (CT-MPR). The diagnosis of cervical OPLL was based on the OPLL criteria described by Tsuyama [1]. Thoracic OPLL was diagnosed using whole-spine CT-MPR. The control group included 39 age- and sex-matched patients who were admitted to our hospital to undergo CT myelogram during the same period, and whole-spine CT myelogram showed that they did not have OPLL. Our study protocol was approved by the ethics review board of our institution and was in compliance with the Helsinki Declaration. All participants in the study provided written informed consent.
Inclusion and exclusion criteria
All participants were enrolled in our study according to the following criteria—were self-reliant in activities of daily living, were aged between 30 and 85 years, and had normal levels for renal creatinine and hepatic function. No patients enrolled in our study had diseases affecting bone metabolism such as osteomalacia, Paget disease, rheumatoid arthritis, hyperparathyroidism, hypercortisolism, chronic kidney disease with bone and mineral disorder, or malignant tumors. Patients who had sustained a fracture within the preceding 6 months were also excluded.
Clinical examination and assessment of comorbidities
At recruitment, height, weight, and body mass index (BMI) were recorded for all study participants, and BMI was calculated as weight in kilograms divided by the square of height in meters. We evaluated each participant for the presence of comorbidities such as hypertension, hyperlipidemia, and diabetes mellitus and for previous or current treatment with drugs affecting bone metabolism (calcium supplements, vitamin D preparations, selective estrogen-receptor modulators, estrogen therapy, antiresorptive therapy, teriparatide, thiazides, steroids, glucocorticoids, and anticoagulants).
Serum measurements
Blood samples of venous blood were collected from fasting patients and sera were stored at −70 °C until examination. Serum concentrations of calcium (Ca), phosphorus, total protein, albumin (Alb), creatinine, alkaline phosphatase, and glycated hemoglobin were measured using standard automated laboratory techniques. Estimated glomerular filtration rate was calculated according to a Japanese standard formula based on inulin clearance:
For females, the value subtracted from age is 0.739.
Serum Ca levels were corrected for Alb using the following formula:
where Alb <4.0 g/dL.
Serum intact parathyroid hormone (iPTH) was measured by electrochemiluminescence immunoassay (Roche Diagnostics, Tokyo, Japan). Serum 1.25-hydroxyvitamin D was measured using a Toray-Fuji Bionics 1.25-dihydroxyvitamin D RIA kit (Immunodiagnostic Systems Ltd., Boldon, UK). Bone turnover markers were measured as follows—total osteocalcin by immunoradiometric assay (BGP-IRMA; Mitsubishi Chemical Medience Corporation, Tokyo, Japan), bone-specific alkaline phosphatase by enzyme immunoassay (Osteolinks BAP; Quidel Corporation, San Diego, CA, USA), intact N-terminal propeptide of type I procollagen (P1NP) by radioimmunoassay (Orion Diagnostica, Espoo, Finland), and tartrate-resistant acid phosphatase 5b (TRACP-5b) by enzyme immunoassay (Osteolinks TRAP-5b; Nittobo Medical Co. Ltd., Tokyo, Japan).
Serum sclerostin (standard range 0−240 pmol/L) was measured using a commercially available enzyme-linked immunosorbent assay (Biomedica Gruppe, Vienna, Austria) and quantified with detection limits of 8.9 pmol/L with intra-assay and inter-assay CV of 5 % and 3–6 %, respectively. Serum DKK1 (standard range 1357–5240 pmol/L) was measured using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) and quantified with detection limits of 4.23 pg/mL with intra-assay and inter-assay CV of 3.3–4.2 % and 4.6–7.6 %, respectively.
Radiological examinations
Plain radiographs of cervical, thoracic, and lumbar spines were obtained for all participants with OPLL. OPLL localization and numbers of ossified vertebra were investigated using plain lateral radiographs and/or CT-MPR. OPLL localization was categorized into 3 types—(A) localized cervical type, in which OPLL is localized to 1 or 2 cervical spine levels; (B) extensive cervical type, in which OPLL spreads across a wide area (over 3 spine levels) from the upper cervical to the lower cervical or upper thoracic spine; and (C) thoracic type, in which OPLL is widespread only in the thoracic spine (Fig. 1). Plain radiographs of thoracic and lumbar spines and/or whole spine CT-MPR were obtained for members of the OPLL group for use in examining for DISH, which was diagnosed according to the classification system of Resnick et al., which requires involvement of at least 4 contiguous vertebrae of the thoracic spine [2].
Three categories of ossification of the posterior longitudinal ligament (OPLL) of the spine—a localized cervical type, in which OPLL is localized to only 2 cervical spine levels (C4–C5); b extensive cervical type, in which OPLL spreads across a wide area from the upper cervical to lower cervical or upper thoracic spine (C2–T1); and c thoracic type, in which OPLL is widespread only in the thoracic spine (T1–L1)
BMD measurement
BMD values in total hip (TH-BMD) were measured by dual-energy X-ray absorptiometry (Discovery A; Hologic, Inc., Waltham, MA, USA). TH-BMD was expressed relative to the standard deviation of age- and sex-matched normal Japanese BMD values provided by the manufacturer (Z score).
Statistical analysis
Baseline characteristics were summarized using descriptive statistics. All data are expressed here as the mean or the mean ± SD. Probability values of <0.05 were considered to indicate statistical significance. Shapiro–Wilk test was used to check the normality of distribution of continuous variables. Parameters not showing Gaussian distribution were log transformed. Associations are expressed as results of the Pearson correlation coefficient test. Comparisons of continuous variables between the 2 groups were performed using the unpaired Student t test or Mann–Whitney U test depending on the distribution. To determine the influence of independent variables to explain serum sclerostin levels, liner regression models were used. ANCOVA was performed to compare individual regression line and to show differences adjusting age between the 2 groups. All statistical analyses were performed using JMP software (version 11.0.0; SAS Institute, Cary, NC, USA).
Results
Characteristics of the 2 groups
Table 1 shows the characteristics of members of the OPLL and control groups. The groups were comparable in most baseline characteristics. It is well known that persons with OPLL often have impaired glucose tolerance, and disorders of glucose metabolism play a role in the pathogenesis of OPLL [3, 4, 6]. As expected, we found that OPLL was associated with a much higher incidence of diabetes mellitus than those without OPLL (control group, 15 %; OPLL group, 35 %), and glycated hemoglobin levels were significantly higher in the OPLL group than in the control group (P = 0.02).
Serum iPTH levels were significantly higher in men with OPLL than in men in the control group (P = 0.01). Serum P1NP and TRACP-5b levels were significantly lower in men with OPLL than in men in the control group (P1NP: P = 0.01; TRACP-5b: P = 0.01). Bone turnover markers were lower in men with OPLL than in men in the control group.
Serum sclerostin and dickkopf-1 levels in the 2 groups
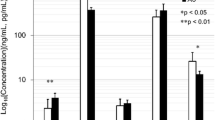
Serum sclerostin levels as well as log (sclerostin) in men with OPLL were significantly higher than in men in the control group (control group: mean 45.3, SD 16.0 pmol/L; OPLL group: mean 75.7, SD 42.9 pmol/L; P = 0.002). Serum sclerostin levels were significantly higher in men than in women in the OPLL group (men: mean 75.7, SD 40.9 pmol/L; women: mean 45.3, SD 16.1 pmol/L; P < 0.0001), but there were no significant differences between men and women in the control group. There was no significant difference in DKK1 levels between men and women in either group.
Serum sclerostin levels were positively correlated with age only in men with OPLL (r = 0.430; P = 0.002), but there were no correlations in women with OPLL or women in the control group (r = 0.157; P = 0.42) (Fig. 2). Results from ANCOVA analysis indicated that the regression slopes were not different in men in either group (P = 0.14), but serum sclerostin levels were significantly higher in men in the OPLL group after adjusting age. Linear regression analysis was performed to determine the independent variables that would explain serum sclerostin levels—sex (β = 0.308; P = 0.001), age (β = 0.262; P = 0.004), and study group (β = 0.260; P = 0.006).
Levels of serum sclerostin, calcium-regulating hormone, and bone turnover markers
Table 2 details relationships among serum sclerostin, calcium-regulating hormone, and bone turnover marker levels in men in the 2 groups.
There was no association between serum sclerostin levels and iPTH levels in men in the control group, but there was a positive correlation between serum sclerostin levels and iPTH levels in men in the OPLL group (r = 0.280; P = 0.048). There was no association between serum DKK1 levels and iPTH levels in men in either group.
In men in the control group, serum sclerostin levels had a strong positive correlation with P1NP, osteocalcin, and TRACP-5b. In the women in the control group, serum sclerostin levels also had a strong positive correlation with TRACP-5b. In contrast, there was no correlation with bone turnover markers in either the men or the women in the OPLL group. Regarding DKK1, there was no correlation with bone turnover markers in either men or women in the control group, but serum DKK1 levels had a positive correlation with P1NP in the men of the OPLL group.
Serum sclerostin levels had a strong negative correlation with serum DKK1 levels in men with OPLL (r = −0.506; P < 0.001). In the control group, there was no correlation between serum sclerostin levels and DKK1 levels. These 2 Wnt/β-catenin signaling antagonists had the opposite effect in the OPLL group.
Serum sclerostin levels, localization of ossification of the posterior longitudinal ligament, number of ossified vertebrae and DISH
OPLL localization was categorized into 3 types—localized cervical type (8); extensive cervical type (56); and thoracic type (14). There was no association with serum sclerostin levels among OPLL localization types (localized cervical type: mean 75.0, SD 41.9 pmol/L; extensive cervical type: mean 66.4, SD 41.0 pmol/L; thoracic type: mean 48.0, SD 20.0 pmol/L). Furthermore, there was no correlation between serum sclerostin levels and the number of ossified vertebrae with OPLL (r = 0.07; P = 0.84). There was no correlation between serum sclerostin levels and OPLL localization or the number of ossified vertebrae with OPLL between men and women in either group. The percentage of persons with OPLL and DISH was 26 % (men 31 %, female 16 %), and there was no correlation between serum sclerostin levels and the presence of DISH.
Serum sclerostin levels and TH-BMD
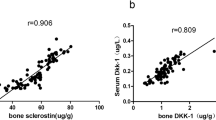
Almost half of the OPLL group had a symptom of cervical or thoracic myelopathy and had some kind of muscular weakness of lower limbs or gait disturbance postoperatively. TH-BMD was measured in 38 OPLL patients with no neurological deficits (30 men and 8 women). Patients with OPLL exhibited a large increase in TH-BMD Z-score (men: mean 1.38, SD 1.16, women: mean 0.60, SD 0.65). There were no tendency for age-related TH-BMD increase in men in the OPLL group (P = 0.22). In men in the OPLL group, serum sclerostin levels had a strong positive correlation with the TH-BMD Z score (r = 0.511, P = 0.011) (Fig. 3).
Discussion
The mechanism behind the development of spinal ligament ossification has not been fully elucidated, but we have clarified the relationship between OPLL and a systemic negative regulator of bone formation. Bone and mineral metabolism in OPLL differs between men and women, and we found higher serum sclerostin levels in men with OPLL compared with age- and sex-matched members of our control group. Systemic secretion of sclerostin significantly increased with advancing age and with higher bone mass in men with OPLL, and higher serum sclerostin levels are counterbalanced by underproduction of DKK1.
Serum sclerostin levels in patients with OPLL
Serum sclerostin levels were significantly higher in men with OPLL than in age-matched men of our control group. Serum sclerostin levels were positively correlated with age only in men with OPLL (r = 0.43; P = 0.002). Some large-sample studies have documented that men have higher serum sclerostin levels than women using a population which is highly characteristic of a Caucasian healthy population, but blacks and Asians are underrepresented, and that these levels increase with age in this population [25–27]. Szulc et al. [25] speculated that the age-related changes in serum sclerostin reflect changes in bone mass and turnover rate. Bone tissue enters a new remodeling cycle in younger men compared with older men, and the activation of bone remodeling in younger men results in a lower mass of mature osteocytes producing sclerostin despite high total bone mass [25]. Mödder et al. [26] speculated that there is increased sclerostin production by individual osteocytes with aging. They noted that circulating sclerostin levels might reflect total-body skeletal mass, and thus because men generally have larger skeletons than women, their bodies simply may produce and release more sclerostin into circulation than women [26]. In contrast with the studies [25–27] which used healthy men, serum sclerostin levels in men with OPLL were extremely high, and we found no tendency for age-related changes in serum sclerostin to slow down in older men with OPLL.
Many previous studies showed that persons with OPLL, who often also have DISH, have a significantly higher BMD than control-group members in the lumbar spine, radius, lower limbs and ribs [8–13]. In this study, men with OPLL also exhibited a large increase in TH-BMD Z score although we did not measure TH-BMD in any members of the control group. In men in the OPLL group, serum sclerostin levels had a strong positive correlation with TH-BMD Z score. In men with OPLL, a larger skeletal mass compared with that of women, along with systemic hyperostosis and the development of poorly remodeled bone tissue such as OPLL and DISH, dramatically increases systemic secretion of sclerostin. We found no relationships between serum sclerostin levels and OPLL localization or number of ossified vertebrae or the presence or absence of DISH. Therefore, these observations suggest that higher bone mass in men with OPLL is related to higher serum sclerostin levels rather than development of OPLL and DISH. Conceptually, higher bone mass increases sclerostin production by osteocytes, and inactivation of bone remodeling in older men with OPLL also increases sclerostin production by mature osteocytes.
Sex difference in patients with ossification of the posterior longitudinal ligament
This study show that the mechanism of bone and mineral metabolism, which focuses on Wnt/β signaling, differs between men and women with OPLL, and this fact suggested that the underlying mechanism of OPLL development is different between the sexes. Serum sclerostin levels were positively correlated with age only in men with OPLL, but there were no correlations in women with OPLL or women in the control group. Ikeda et al. [28] found that serum leptin concentration is associated with extension of OPLL in women. It is possible that hyperleptinemia in combination with hyperinsulinemia contributes to the development of OPLL, because a study by Wada et al. [29] showed that serum estrogen levels in women with OPLL are related to the extent of heterotopic ligament ossification. Ardawai et al. reported increases in serum sclerostin levels in women with increasing levels of serum follicle-stimulating hormone and luteinizing hormone, and that follicle-stimulating hormone had a stronger association with serum sclerostin than luteinizing hormone, particularly in postmenopausal women [30]. In women with OPLL, various complicated pathological factors such as levels of serum leptin, estradiol, follicle-stimulating hormone and luteinizing hormone may influence bone metabolism and may contribute to the development of OPLL. Sclerostin has little impact on bone metabolism in women with OPLL.
Relationships between ossification of the posterior longitudinal ligament and bone turnover markers
Several studies report the relationships between OPLL and bone turnover markers [31, 32]. Sugimori et al. [31] reported that the concentrations of intact osteocalcin, osteocalcin, and carboxy-terminal propeptide of type I procollagen (P1CP) were significantly higher in patients with cervical OPLL alone than in patients with OPLL at multiple spine levels. However, Ishihara et al. reported that there were no significant differences in serum P1CP, osteocalcin, urinary levels of pyridinoline, and deoxypyridinoline between persons with OPLL and age-matched members of a control group [32]. Until now, the relationship between OPLL and bone turnover markers is controversial. In our study, serum P1NP and TRACP-5b levels were significantly lower in men with OPLL than in control-group members. Compared with previous studies, our study focused on a large sample of OPLL patients who were matched by age, sex, and renal function with a control group. Furthermore, we used bone turnover markers with less error of measurement such as TRACP-5b.
There is a possibility that bone turnover markers decrease secondary to elevation of serum sclerostin levels in men with OPLL, but there were no significant differences in other bone formation markers such as bone-specific alkaline phosphatase and osteocalcin between the two groups in this study. Data regarding sclerostin and bone turnover markers were controversial and we could not draw a conclusion. Gracia-Martin et al. [33] reported serum sclerostin levels increased in type 2 diabetes mellitus and showed comparable results of bone turnover markers. In their study, only serum P1NP and TRACP-5b levels were significantly lower in person with type 2 diabetes mellitus. They concluded that data regarding sclerostin and bone turnover markers were controversial, and no clear conclusions could be drawn. In this study, there is a possibility that bone metabolism in men with OPLL is partially controlled independently of sclerostin, but it is easier to understand that bone formation markers decrease secondary to elevation of serum sclerostin level. Szulc et al. [25] speculated the feedback system between serum sclerostin and bone turnover rate, i.e., lower bone turnover results in a higher mass of mature osteocytes producing sclerostin, which further decreases bone turnover. In male patients with OPLL, higher bone mass induces the overproduction of sclerostin which, in turn, may create a loop of decreased bone turnover. Bone turnover markers were lower in men with OPLL than in men in the control group. However, there are fewer correlations between serum sclerostin levels and bone turnover markers in men with OPLL.
Various factors such as type 2 diabetes mellitus and levels of serum DKK1 and PTH may contribute to the difference among various bone formation markers in men with OPLL. The lack of correlation between serum sclerostin levels and bone turnover markers may be mainly explained by the underproduction of serum DKK1. Sclerostin is expressed and secreted by osteocytes [17, 18], whereas DKK1 is widely expressed in embryonic mice and is almost exclusively confined to both osteocytes and osteoblasts in adults [34]. The decreased osteoblast population induced by the increased secretion of sclerostin may lead to an overall decrease in DKK1 production [20]. In support of this theory, we found a negative correlation between serum sclerostin and DKK1 levels (r = −0.356; P < 0.05) and a strong negative correlation between serum sclerostin and DKK1 levels in men with OPLL (r = −0.506; P < 0.001) in our study. Recently, Van Lierop et al. reported that serum DKK1 levels were significantly higher in patients with both sclerosteosis and van Buchem disease (which shows the loss-of-function mutations in the SOST gene) compared to levels in carriers of the two diseases individually and to levels in healthy controls [35]. Their results in the population with sclerosteosis and van Buchem disease are identical to our results in men with OPLL. Decreased serum DKK1 levels observed in men with OPLL represent an adaptive response to decreased bone formation characterizing OPLL, although these decreased levels do not completely compensate for the increased production of sclerostin. Similarly, Viapiana et al. also reported an apparent discrepancy between serum sclerostin and DKK1 levels [22]. They reported that patients with primary hyperparathyroidism have significantly lower sclerostin and higher DKK1 levels compared with healthy women after menopause. Two recent studies investigated the relationship between serum DKK1 and DISH [36, 37]; however, the role of serum DKK1 in patients with DISH remains controversial because of the selection of appropriate control-group members (regarding age and sex) and a small number of objective patients. Fifty percent of patients with DISH also have OPLL [2], and OPLL is thought to be a subtype of DISH. Our study suggests that changes in serum DKK1 levels observed in patients with DISH may represent an adaptive response to changes in serum sclerostin levels.
Limitations
Our study had several potential limitations. First, because this was a case–control study, we could not determine if there is a causal relationship between serum Wnt/β antagonists and bone turnover markers. Further prospective studies are necessary to determine whether this correlation is reflective of a causal relationship. Second, results were for a single measurement instead of serial measurements, so laboratory measurement errors could have affected the accuracy of data. Third, it is unclear whether serum sclerostin and DKK1 levels really reflect the status of these Wnt/β antagonists in local bone lesions. Drake et al. [38] reported that serum sclerostin levels correlate with sclerostin levels in human bone marrow, and that serum sclerostin levels reflect the status of sclerostin in local bone lesions. However, there is evidence to the contrary showing that changes in bone sclerostin levels vary independently of changes in serum sclerostin levels in mice. OPLL is characterized by local pathological ectopic OPLL of the spine, so there is a possibility that changes in bone sclerostin levels differ from changes in serum sclerostin levels. Fourth, the sample size is relatively small in our control group and may affect the statistical power of our study. In this study, there were no significant indifferences in serum sclerostin levels between men and women in the control group. Fifth, TH-BMD in all OPLL subjects was not measured, and could not be measured due to the lack of OPLL subjects with no neurological deficits. Almost half of OPLL subjects in this study had a symptom of cervical or thoracic myelopathy and had some kind of muscular weakness of lower limbs or gait disturbance which affects TH-BMD data. Further studies to assess the relationships between bone mass and serum sclerostin levels are thought to be necessary using OPLL subjects with no neurological deficits.
Conclusion
Bone and mineral metabolism in OPLL differs between men and women. In men with OPLL, systemic secretion of sclerostin increases with advancing age and with higher bone mass, and sclerostin plays a critical role in bone and mineral metabolism. Both sclerostin and DKK-1 have the opposite effect in patients with OPLL, and higher serum sclerostin levels are counterbalanced by underproduction of DKK1. A clinical study has suggested that the development of OPLL, as documented on radiographic images, slows with advancing age [39]. As this study is cross-sectional, further longitudinal studies are necessary to clarify the relationship between OPLL development and serum sclerostin levels.
References
Tsuyama N (1984) Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res 184:71–84
Resnick D, Guerra J Jr, Robinson CA, Vint VC (1978) Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. Am J Roentgenol 131:1049–1053
Taguchi T (2006) Etiology and Pathogenesis. In: Yonenobu K, Nakamura K, Toyama Y (eds) OPLL; Ossification of the posterior longitudinal ligament, 2nd edn. Springer Japan, Tokyo, pp 33–35
Kawaguchi H, Akune T, Ogata N, Seichi A, Takeshita K, Nakamura K (2006) Contribution of metabolic conditions to ossification of the posterior longitudinal ligament of spine. In: Yonenobu K, Nakamura K, Toyama Y (eds) OPLL; Ossification of the posterior longitudinal ligament, 2nd edn. Springer Japan, Tokyo, pp 37–40
Kawaguchi H, Kurokawa T, Hoshino Y, Kawahara H, Ogata E, Matsumoto T (1992) Immunohistochemical demonstration of bone morphogenetic protein-2 and transforming growth factor-beta in the ossification of the posterior longitudinal ligament of the cervical spine. Spine 17:S33–S36
Akune T, Ogata N, Seichi A, Ohnishi I, Nakamura K, Kawaguchi H (2001) Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J Bone Joint Surg Am 83-A:1537–1544
Nakajima M, Takahashi A, Tsuji T, Karasugi T, Baba H et al (2014) A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat Genet 46:1012–1016
Matsui H, Yudoh K, Tsuji H (1996) Significance of serum levels of type I procollagen peptide and intact osteocalcin and bone mineral density in patients with ossification of the posterior longitudinal ligaments. Calcif Tissue Int 59:397–400
Hirai N, Ikata T, Murase M, Morita T, Katoh S (1995) Bone mineral density of the lumbar spine in patients with ossification of the posterior longitudinal ligament of the cervical spine. J Spinal Disord 8:337–341
Yamauchi T, Taketomi E, Matsunaga S, Sakou T (1999) Bone mineral density in patients with ossification of the posterior longitudinal ligament in the cervical spine. J Bone Miner Metab 17:296–300
Tahara M, Aiba A, Yamazaki M, Ikeda Y, Goto S, Moriya H, Okawa A (2005) The extent of ossification of posterior longitudinal ligament of the spine associated with nucleotide pyrophosphatase gene and leptin receptor gene polymorphisms. Spine 30:877–881
Sohn S, Chung CK (2013) Increased bone mineral density and decreased prevalence of osteoporosis in cervical ossification of the posterior longitudinal ligament: a case-control study. Calcif Tissue Int 92:28–34
Yoshimura N, Nagata K, Muraki S, Oka H, Yoshida M, Enyo Y, Kagotani R, Hashizume H, Yamada H, Ishimoto Y, Teraguchi M, Tanaka S, Kawaguchi H, Toyama Y, Nakamura K, Akune T (2014) Prevalence and progression of radiographic ossification of the posterior longitudinal ligament and associated factors in the Japanese population: a 3-year follow-up of the ROAD study. Osteoporos Int 25:1089–1098
van Buchem FS, Hadders HN, Ubbens R (1955) An uncommon familial systemic disease of the skeleton: hyperostosis corticalis generalisata familiaris. Acta Radiol 44:109–120
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P et al (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543
Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68:577–589
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 15:805–814
Piters E, Boudin E, Van Hul W (2008) Wnt signaling: a win for bone. Arch Biochem Biophys 473:112–116
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Canalis E (2013) Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol 9:575–583
Viapiana O, Fracassi E, Troplini S, Idolazzi L, Rossini M, Adami S, Gatti D (2013) Sclerostin and DKK1 in primary hyperparathyroidism. Calcif Tissue Int 92:324–329
Ardawi MS, Rouzi AA, Al-Sibiani SA, Al-Senani NS, Qari MH, Mousa SA (2012) High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: the Center of Excellence for Osteoporosis Research Study. J Bone Miner Res 27:2592–2602
Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, Ali AY, Abdulrafee AA, Saeda MY (2013) Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 56:355–362
Szulc P, Boutroy S, Vilayphiou N, Schoppet M, Rauner M, Chapurlat R, Hamann C, Hofbauer LC (2013) Correlates of bone microarchitectural parameters and serum sclerostin levels in men: the STRAMBO study. J Bone Miner Res 28:1760–1770
Mödder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ 3rd, Khosla S (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26:373–379
Bhattoa HP, Wamwaki J, Kalina E, Foldesi R, Balogh A, Antal-Szalmas P (2013) Serum sclerostin levels in healthy men over 50 years of age. J Bone Miner Metab 31:579–584
Ikeda Y, Nakajima A, Aiba A, Koda M, Okawa A, Takahashi K, Yamazaki M (2011) Association between serum leptin and bone metabolic markers, and the development of heterotopic ossification of the spinal ligament in female patients with ossification of the posterior longitudinal ligament. Eur Spine J 20:1450–1458
Wada A (1995) Affinity of estrogen binding in the cultured spinal ligament cells: an in vitro study using cells from spinal ligament ossification patients. Nihon Seikeigeka Gakkai Zasshi 69:440–449 (In Japanese)
Ardawi MS, Al-Kadi HA, Rouzi AA, Qari MH (2011) Determinants of serum sclerostin in healthy pre- and postmenopausal women. J Bone Miner Res 26:2812–2822
Sugimori K, Kawaguchi Y, Ohmori K, Kanamori M, Ishihara H, Kimura T (2003) Significance of bone formation markers in patients with ossification of the posterior longitudinal ligament of the spine. Spine 28:378–379
Ishihara C, Kushida K, Takahashi M, Ohishi T, Murata H, Nagano A, Goto S (2000) The efficacy of biochemical markers in patients with ossification of posterior longitudinal ligament of the spine. Spinal Cord 38:211–213
García-Martín A, Rozas-Moreno P, Reyes-García R, Morales-Santana S, García-Fontana B, García-Salcedo JA, Muñoz-Torres M (2012) Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 97:234–241
Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 2006:934–945
van Lierop AH, Moester MJ, Hamdy NA, Papapoulos SE (2014) Serum Dickkopf 1 levels in sclerostin deficiency. J Clin Endocrinol Metab 99:E252–E256
Senolt L, Hulejova H, Krystufkova O, Forejtova S, Andres Cerezo L, Gatterova J, Pavelka K, Vencovsky J (2012) Low circulating Dickkopf-1 and its link with severity of spinal involvement in diffuse idiopathic skeletal hyperostosis. Ann Rheum Dis 71:71–74
Aeberli D, Schett G, Eser P, Seitz M, Villiger PM (2011) Serum Dkk-1 levels of DISH patients are not different from healthy controls. Joint Bone Spine 78:422–423
Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062
Murakami M, Seichi A, Chikuda H, Takeshita K, Nakamura K, Kimura A (2010) Long-term follow-up of the progression of ossification of the posterior longitudinal ligament. J Neurosurg Spine 12:577–579
Acknowledgments
Medical editor Katharine O’Moore-Klopf, ELS (East Setauket, New York, USA) provided professional English-language editing of this article before its final acceptance for publication.
Conflict of interest
This work was supported by a Grant-in-aid from the Investigation Committee on Ossification of the Spinal Ligaments, Japanese Ministry of Public Health, Labor. None of the authors has any financial interest with any of the commercial entities mentioned in this article.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kashii, M., Matuso, Y., Sugiura, T. et al. Circulating sclerostin and dickkopf-1 levels in ossification of the posterior longitudinal ligament of the spine. J Bone Miner Metab 34, 315–324 (2016). https://doi.org/10.1007/s00774-015-0671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0671-5