Abstract
The purpose of this study was to examine peripartum depression (PD) screening patterns within and across the prenatal and postpartum periods and assess the incidence of new positive screens during standard screening protocol timepoints to inform practice, particularly when limited screenings can be conducted.
This is a retrospective observational study of women screened for PD through a large, integrated health system using the Edinburgh Postnatal Depression Scale (EPDS) within their obstetrics and pediatric practices. Pregnancies with an EPDS score for at least one obstetric and one pediatric appointment between November 2016 and October 2019 were included (n = 3240). The data were analyzed using chi-squared test, Student’s t-test, and binary logistic regression analyses. An EPDS score of 10 or higher was considered a positive screen.
The positive screening rate for this cohort was 18.5%, with a prenatal positive rate of 9.9% and a postpartum positive rate of 8.6%. Single relationship status showed a higher rate of PD overall. Two thirds of women were not screened until their third trimester, resulting in delayed detection for an estimated 28% of women who ultimately screened positive. Few new positive screens (1.3%) were detected after 9 weeks postpartum in women who had completed all recommended prior screens.
Obstetric providers should screen for PD as early in pregnancy as possible and continue to screen as often as feasible regardless of previous negative EPDS scores. Prioritizing screening more often in pregnancy and before 9 weeks postpartum is optimal to avoid delays in detection and intervention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Depression impacts an estimated 7–15% of pregnant women (Bennett et al. 2004; Gavin et al. 2005) and 9–23.5% of postpartum women in the USA (Bauman et al. 2020), with higher rates found among teen mothers and those in low-income households (Knitzer et al. 2008). Peripartum depression (PD) is characterized by depressed mood, anxiety, irritability, compulsive thoughts, poor sleep and appetite, fatigue, and feelings of guilt and despair (Horowitz & Goodman 2004; Miller 2002). PD may present with suicidal ideation, and suicide is a leading cause of maternal deaths (Oates 2003). Untreated depression during pregnancy has further been associated with low birth weight, increased rates of preterm birth, and increased rates of cesarean section (Jarde et al. 2016; Rejnö et al. 2019). Mothers suffering from PD may experience an altered emotional attachment and interference in mother-infant bonding that can lead to infants having delayed physical and cognitive development, difficult temperament, poor self-regulation, and increased likelihood of hospitalization and mortality (Dubber et al. 2015; Fransson et al. 2020; Moehler et al. 2006; Murray 1992). Identification and treatment of PD have been shown to improve outcomes for both mothers and their children (Halfin 2007; Li et al. 2020; Weissman et al. 2016).
The American Academy of Pediatrics (AAP), the American College of Obstetrics and Gynecologists (ACOG), and the U.S. Preventive Services Task Force (USPSTF) all recommend screening for PD; however, only the AAP provides guidance on the timing of the screening (American College of Obstetricians and Gynecologists 2018; Earls et al. 2019; Felder 2019). This is likely due to the lack of evidence regarding appropriate timing for screening and which timepoint(s) would identify the greatest number of women experiencing symptoms (Bick & Howard 2010). As noted by Bick and Howard (2010), the timing of screening coincides with routine appointment times rather than being based on evidence of optimal screening times for PD.
Further complicating decisions on when and how often to screen is the ability of providers to be reimbursed for the screening from Medicaid and private insurance programs. For those without private insurance, Medicaid provides free health coverage for low-income women during pregnancy and up to 2 months after the birth of the baby. In 2016, the Centers for Medicare & Medicaid Services released an informational bulletin to provide clarity around the agency’s policy for PD screening, stating that “state Medicaid agencies may allow such screenings to be claimed as a service for the child as part of the Early and Periodic Screening, Diagnostic and Treatment (EPSDT) benefit.” (Wachino, 2016). Additionally, state Medicaid agencies have discretion over the reimbursement approaches available to pediatric providers screening mothers within a child’s visit. States have developed reimbursement models for PD screening, which vary from one assessment allowed in Texas and Colorado to up to three screenings in North Dakota (Texas Legislature 2017; Wachino 2016).
The purpose of this study was to examine PD screening results in pregnancy and postpartum to better understand screening patterns within and across the prenatal and postpartum periods and assess the incidence of new positive screens during standard screening protocol timepoints to inform practice, particularly if providers are limited by clinical or financial constraints.
Methods
Setting
Texas Children’s Hospital (TCH) is an integrated healthcare system composed of three children’s hospitals, four obstetric practices, and over 50 pediatric practices located across greater Houston, Texas. In 2014, the system began a quality improvement initiative to increase access to maternal mental health services through universal screening for PD at obstetric and pediatric practices and to facilitate referrals for evaluation and treatment (Puryear et al, 2019). All obstetric and pediatric practices were trained to administer the Edinburgh Postnatal Depression Scale (EPDS). Obstetric practices administered EPDS screenings at 11–13 weeks and 35–37 weeks during pregnancy and at 6 weeks postpartum. Pediatric practices screened women at the 2-week and 2-, 4-, and 6-month well-baby visits.
Study population
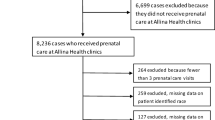
Data collected between November 2016 and October 2019 from obstetric and pediatric practice screening for PD in the TCH health system were analyzed. Pediatric and obstetrics appointment data were linked using unique patient identifiers for mother and infant. Pregnancies with an EPDS score for at least one obstetrics and one pediatric appointment were included in the study (n = 3240). In total, 3235 women were represented. Obstetrics appointments that took place during pregnancy were coded as prenatal visits; those that took place up to a year after delivery and could not be explained by a subsequent pregnancy were coded as postpartum visits. Gestational/infant age was calculated as weeks relative to the date of birth.
Measures
The EPDS is a validated, self-reported instrument that consists of 10 items to assess maternal mood and anxiety symptoms experienced within the past 7 days (Cox, Holden, & Sagovsky 1987). Except in rare instances of low literacy or language barriers, women completed the EPDS on their own during the clinic visit. The questions were available in both English and Spanish, and translation services were available for families speaking other languages. The instrument demonstrated adequate internal consistency for our dataset (α = 0.817). An EPDS score ≥ 10 was considered a positive screen. For pregnancies with at least one positive EPDS score recorded, the first positive screen was used for downstream analysis. For pregnancies with no positive EPDS score recorded, the screen with the highest EPDS score was used for downstream analysis.
Demographic variables collected from the electronic medical record (EMR) included maternal age, race, relationship status, and primary insurance. Race was coded as “White,” “Black,” or “Asian”; relationship status was coded as “Single,” “Married or in a Relationship,” or “Divorced, Separated, or Widowed”; and insurance status was coded as “Medicaid” or “non-Medicaid.” In Texas, where this study was conducted, pretax annual income eligibility for Medicaid for Pregnant Women is $25,512 for a single woman and $34,500 for a couple experiencing their first pregnancy (Texas Health and Human Services N.D).
Analysis
Data were analyzed in SPSS version 24 using descriptive and inferential statistical methods including Chi-squared test, Student’s t-test, and binary logistic regression. For binary logistic regression analysis, pregnancies with complete data on all variables of interest were included.
Ethics
This retrospective study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Baylor College of Medicine Institutional Review Board (H-46478).
Results
In this sample, the mean maternal age was 31.84 (SD = 5.04), and most women were white (73.8%), married (78.6%), and using non-Medicaid insurance (86.6%) (Table 1). The vast majority of women represented had one pregnancy (99.85%, n = 3230) during the study period, while 0.15% (n = 5) had two pregnancies meeting criteria for inclusion. Of 3240 total pregnancies represented in the study, 601 screened positive (EPDS ≥ 10) at least one time, indicating an 18.5% positive screen rate. Of the pregnancies that screened positive, 53.4% were first detected prenatally and 46.6% postpartum, resulting in a 9.9% prenatal positive screen rate and an 8.6% postpartum positive screen rate. The mean EPDS score of a first positive screen was 12.43 (SD = 2.83).
Overall, more screenings occurred in later pregnancy and within the first 9 weeks post-delivery. During the first 9 weeks postpartum, the rate of first positive screens was 7.16%, with an overall positivity rate of 11.4% when including cases of PD detected during pregnancy that continued into the postnatal period. Screening occurring at or around the 12-week prenatal visit had the highest rate of first positive screens and second highest overall positivity rate (8.5%), but the fewest screens of all timepoints (Table 2). For women with prenatal positive EPDS screens, the majority were first screened and detected at the 36-week gestational age (GA) obstetrics appointment. Only 26% of prenatal screening occurred at the 12-week appointment, and we estimate that a lack of screening at this timepoint resulted in delayed detection for approximately 28% of those who ultimately screened positive. After accounting for this screening disparity, first positive screen detection rates were not significantly different between the two timepoints (8.5% at ~ 12-week GA vs 7.3% at ~ 36-week GA, Chi-Squared test p = 0.22). To further disentangle screening frequency from newly emergent depressive symptomatology in the prenatal period, we also assessed the subset of the data encompassing women who were assessed at both the 12- and 36-week appointments (n = 913) and found that in this subset the percentage of prenatal positive EPDS screens was similar to the cohort as a whole at 11.2%, but the rate of first positive screen detection was 8.2% at 12 weeks compared to 3.3% at 36 weeks.
The postpartum period accounted for 46.6% of newly detected PD. An overwhelming 77% of pregnancies that screened EPDS positive postpartum did so in the first 9 weeks after delivery. Less than 3% of pregnancies first screened positive after the infant was 9 weeks old. For those in the full cohort who were positive for the first time postpartum (n = 280), pediatric surveillance was responsible for the detection of 22.9% of positive screens.
Finally, for women who had a negative screen prior to screening positive, the mean EPDS score of the negative screen was 4.93 (SD = 2.80) compared to a mean highest score of 4.33 (SD = 2.70) for women who remained negative throughout pregnancy and postpartum (Student’s t-test, p < 0.001). While 13.3% of women with an EPDS score of 6–9 were positive at the next screening compared to 9.2% of women with a score of 0–5, 12.6% of women who screened positive had an EPDS score of 0 at their previous screen.
Positive EPDS screens were more commonly detected in women who were Black, single, and insured by Medicaid. However, maternal age, race, Medicaid status, and total number of EPDS screens were not significantly associated with EPDS positive status (Table 3). Among women who were single, the odds of a positive screen were 44% higher than for those in a relationship (OR 1.437, 95% CI 1.122–1.841; p < 0.01).
Discussion
The objective of this study was to examine patterns of PD screening and positive screens detected in the prenatal and postpartum periods within the standard obstetric and pediatric screening timepoints. We examined 3240 pregnancies that occurred within a large, multisite, integrated healthcare system in the USA. The integration of appointment data from multiple specialty providers allowed for a longitudinal view of PD screening and detection throughout the prenatal and postpartum periods. Given the lack of an existing consensus regarding the recommended number and optimal time(s) for PD screening, our findings can help inform when PD screening provides the most clinical value and, if necessary, when discontinuing screening would be reasonable.
The positive screen rate for this cohort was 18.5%, with a total prenatal rate of 9.9% and a postpartum rate of 13.0%. These values are consistent with what has been reported by other large studies, in which prenatal depression rates have typically ranged from 7 to 15% (Bennett et al. 2004; Gavin et al. 2005) and postpartum depression rates from 9 to 23.5% (Bauman et al. 2020). Women who screened EPDS positive at any point during pregnancy or postpartum were significantly more likely to have a relationship status of single, but other demographic features such as age, race, and Medicaid status were not associated with a positive screen. This is consistent with other research indicating higher rates of PD in single and unpartnered women and the value of emotional closeness and relationship satisfaction in protecting against PD (Bilszta et al. 2008; Pilkington et al. 2015).
Just over half of pregnancies that screened positive were detected through obstetric practices prenatally (as noted in Table 1). While most women who screened positive prenatally were detected at the 36-week GA appointment, many of those women would likely have screened positive sooner had they been screened earlier in their pregnancy, indicating a missed opportunity for early intervention. Although there were two recommended screening timepoints during pregnancy, the majority of women were screened only once. Women were nearly three times more likely to be screened at the 36-week appointment despite existing protocol to screen at the 12-week obstetrics visit, perhaps due to time constraints at earlier appointments or late presentations to prenatal care (Osterman & Martin 2018). After adjusting for screening frequency, there was no significant difference between the new positive EPDS screen rates between the two timepoints, indicating that screening earlier does not produce inferior results with respect to sensitivity if a clinician can screen only once. Given that approximately two out of every three women screened at the 36-week checkup were not assessed prior, positive screens at 36 weeks could be detecting symptoms that have been ongoing throughout pregnancy. Support for this possibility was further strengthened by findings from the subset of the cohort that did have data from both timepoints, which indicated that the rate of a newly positive screen at the 36-week GA appointment was considerably lower (3.3% at 36 weeks compared to 8.2% at 12 weeks). These results suggest that what appears to be a particularly high yield of EPDS positive screens at the 36-week appointment is artificially inflated by a relative dearth of screening earlier in pregnancy, and that just over half of positives at the 36-week appointment may fall into the category of “missed” early prenatal depression.
The clinical implications of these results are that obstetric practices should be incorporating PD screening into their workflow as early in pregnancy as possible because depressive symptoms in pregnancy are linked to poorer maternal–fetal and postpartum bonding, as well as adverse neonatal outcomes including preterm birth and long-term consequences for neurodevelopmental outcomes in children such as attentional, emotional, and behavioral disturbances (Dubber et al. 2015; Fransson et al. 2020; Moehler et al. 2006; Murray 1992; Straub et al. 2012). The need for and potential benefit of additional screenings throughout pregnancy is also supported by our findings, as there was a nontrivial proportion of women who indicated symptoms later in pregnancy. Furthermore, while women who screened positive after an initial negative screen had significantly higher average total EPDS scores on the preceding screen when compared to the highest EPDS scores of women who remained negative throughout, the absolute difference was small and this finding did not suggest that a low initial score was an indication to defer or discontinue further screening. In fact, of women who did screen positive during pregnancy, one in eight reported a complete absence of symptoms on the EPDS assessment preceding their positive screen, underscoring the challenges of predicting who will develop symptoms and the need for regular screening in all women. Future studies would be useful to determine if there are identifiable score trajectories when considering maternal demographic factors and all timepoints. Recent work has addressed the possibility and potential value of such trajectories in the postpartum period but did not assess symptoms prior to 4 months postpartum (Putnick et al. 2020).
Nearly half of EPDS positive pregnancies in this dataset were detected for the first time postpartum despite receiving prenatal screening, highlighting the value of both prenatal and postpartum depression screening. Screening in pediatric practices plays a critical role because a sizable proportion of women (one in five) who screened newly positive postpartum was not screened by obstetrics postpartum. Few new positive screens were detected after 5 months postpartum despite over half the cohort being assessed past this timepoint. Given that this study required both a prenatal and postpartum screen for inclusion, women with preexisting affective disorder may have been detected earlier in our study, a possibility further supported by the low rate (1.4%) of new positive screens after even 9 weeks postpartum in women who had been maximally screened in pregnancy and early postpartum. Despite the low rate of new positive screens after 9 weeks postpartum in this study, more extensive or longer-term postpartum screening may still be indicated for groups at known high risk of postpartum mood disorders, including mothers of preterm babies in the neonatal intensive care unit (Pace et al. 2016), and clarifying screening practices in such subpopulations would be a valuable topic for future research.
Importantly, affective disorders in women that begin or recur independently of a specific peripartum process can still have a large impact on parenting efficacy and child well-being, and extending maternal depression screening in a pediatric context in a way that does not overburden clinicians could certainly have benefits beyond the scope of PD screening (Olson et al. 2002; Pelaez et al. 2008).
Limitations in this study include minimal maternal demographic information. Notably, this study was not able to distinguish between Hispanic and non-Hispanic White patients and thus was unable to leverage the inherent diversity of the study setting, in which 43% of the city population identifies as Hispanic or Latinx, for more detailed analysis. Social support, socioeconomic status, life stress, and past psychiatric history have all been shown to be associated with PD but were not assessed here (Beck 2001). However, the choice of study variables reflects the maternal factors that are most commonly and consistently recorded in clinical practice and easily available for consideration by healthcare providers. Moreover, while nearly two-thirds of women with positive prenatal screens did not screen positive again postpartum, we do not have data informing who received pharmacologic and therapeutic interventions in the interim. Because of protocols in place within the electronic medical record (EMR) facilitating electronic referrals to psychiatric care for those who screened positive, a high rate of intervention in this sample is likely.
This study has a number of important clinical implications. First, obstetrics providers should screen for PD as early in pregnancy as possible and continue to screen as often as is feasible, even if women reported low or no symptoms on previous screens. Automatic alerts for providers within the EMR to identify women who need screening, regardless of when they enter into care, may prove beneficial for increasing early detection. Second, although awareness of demographic variables may be useful to providers in conceptualizing patient priority groups, increased screening would be advantageous to and recommended for all women. Across multiple decades of research, survey studies (e.g., Leddy et al. 2011; LaRocco-Cockburn et al. 2003) have indicated that obstetricians view appointment time constraints, insufficient reimbursement for screening, and discomfort treating depression as obstacles to screening. While creative online training modules (e.g., Byatt et al. 2021) have recently been developed to address obstetrician comfort with screening for and treating perinatal depression, institutional and structural support for such training, longer patient appointments, more specific guidance from ACOG regarding timing of perinatal screening, and systemic changes to insurance valuations of mental health care are also needed to effect meaningful change. Augmentation and standardization of Medicaid reimbursement rates nationally may present a target for policy initiatives, as existing research on this topic indicates that increased Medicaid reimbursement for prenatal visits is associated with a higher number of prenatal visits (Sonshak 2015). Third, since a substantial proportion of women did not have a postpartum EPDS screen with their obstetrician, pediatric screening proves crucial to detect cases of PD that might otherwise be missed. However, while unresolved depressive symptoms after a positive prenatal or postpartum screen were not uncommon, few new positive screens were detected after 9 weeks postpartum for our cohort in women with all recommended prior screens. Prioritizing screening more often in pregnancy and early postpartum is ideal to avoid delays in detection and intervention.
Availability of data and materials
This is proprietary data and not available for public use.
Code availability
None.
References
American College of Obstetricians and Gynecologists. (2018). ACOG Committee opinion no. 757 summary: screening for perinatal depression. Obstetrics and gynecology, 132(5), 1314–1316. https://doi.org/10.1097/AOG.0000000000002928
Bauman, B. L., Ko, J. Y., Cox, S., D’Angelo, MPH, D. V., Warner, L., Folger, S., Barfield, W. D. (2020). Vital signs: postpartum depressive symptoms and provider discussions about perinatal depression — United States, 2018. MMWR morbidity and mortality weekly report, 69, 575–581. https://doi.org/10.15585/mmwr.mm6919a2
Beck CT (2001) Predictors of postpartum depression: an update. Nurs Res 50(5):275–285. https://doi.org/10.1097/00006199-200109000-00004
Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR (2004) Prevalence of depression during pregnancy: systematic review. Obstet Gynecol 103(4):698–709. https://doi.org/10.1097/01.AOG.0000116689.75396.5f
Bick D, Howard L (2010) When should women be screened for postnatal depression? Expert Rev Neurother 10(2):151–154. https://doi.org/10.1586/ern.09.156
Bilszta J, Tang M, Meyer D, Milgrom J, Ericksen J, Buist A (2008) Single motherhood versus poor partner relationship: outcomes for antenatal mental health. Aust N Z J Psychiatry 42(1):56–65. https://doi.org/10.1080/00048670701732731
Byatt N. Master, G. A. Twyman J. Hunt A. Hamad C. Maslin M. & Moore Simas T. A. (2021). Building obstetric provider capacity to address perinatal depression through online training. Journal of women's health (2002), 30(10), 1386–1394. https://doi.org/10.1089/jwh.2020.8843
Centers for Disease Control and Prevention (2010) Current depression among adults–-United States, 2006 and 2008. MMWR Morb Mortal Wkly Rep 59(38):1229–1235
Cox JL Holden JM Sagovsky R (1987) Detection of postnatal depression. Development of the 10- item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150, 782–786.
Dubber S, Reck C, Müller M, Gawlik S (2015) Postpartum bonding: the role of perinatal depression, anxiety and maternal–fetal bonding during pregnancy. Archives of Women’s Mental Health 18(2):187–195. https://doi.org/10.1007/s00737-014-0445-4
Earls MF, Yogman MW, Mattson G, Rafferty J (2019) Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics 143(1):20183259. https://doi.org/10.1542/peds.2018-3259
Felder JN (2019) April 1). Implementing the USPSTF recommendations on prevention of perinatal depression - opportunities and challenges. JAMA Intern Med 179:467–468. https://doi.org/10.1001/jamainternmed.2018.7729
Fransson E, Sörensen F, KunovacKallak T, Ramklint M, Eckerdal P, Heimgärtner M, Skalkidou A (2020) Maternal perinatal depressive symptoms trajectories and impact on toddler behavior – the importance of symptom duration and maternal bonding. J Affect Disord 273:542–551. https://doi.org/10.1016/j.jad.2020.04.003
Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T (2005) Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106(5 Pt 1):1071–1083. https://doi.org/10.1097/01.AOG.0000183597.31630.db
Halfin A (2007) Depression: the benefits of early and appropriate treatment. American Journal of Managed Care 13(4 Suppl):S92-97
Horowitz JA, Goodman J (2004) A longitudinal study of maternal postpartum depression symptoms. Res Theory Nurs Pract 18(2/3):149. https://doi.org/10.1891/rtnp.18.2.149.61285
Jarde A, Morais M, Kingston D, Giallo R, MacQueen GM, Giglia L, McDonald SD (2016) Neonatal outcomes in women with untreated antenatal depression compared with women without depression: a systematic review and meta-analysis. JAMA Psychiat 73(8):826–837. https://doi.org/10.1001/jamapsychiatry.2016.0934
Knitzer J. Johnson K. & Theberge S. (2008). Reducing maternal depression and its impact on young children: towards a responsive early childhood policy framework. In National Center for Children in Poverty. Retrieved from http://www.nccp.org/publications/pdf/text_791.pdf
LaRocco-Cockburn A, Melville J, Bell M, Katon W (2003) Depression screening attitudes and practices among obstetrician-gynecologists. Obstet Gynecol 101(5 Pt 1):892–898. https://doi.org/10.1016/s0029-7844(03)00171-6
Leddy M, Haaga D, Gray J, Schulkin J (2011) Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol 32(1):27–34. https://doi.org/10.3109/0167482X.2010.547639
Li C, Sun X, Li Q, Sun Q, Wu B, Duan D (2020) Role of psychotherapy on antenatal depression, anxiety, and maternal quality of life: a meta-analysis. Medicine 99(27):e20947. https://doi.org/10.1097/MD.0000000000020947
Miller LJ (2002) Postpartum depression. JAMA 287(6):762–765. https://doi.org/10.1001/jama.287.6.762
Moehler E, Brunner R, Wiebel A, Reck C, Resch F (2006) Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Archives of Women’s Mental Health 9(5):273–278. https://doi.org/10.1007/s00737-006-0149-5
Murray L (1992) The Impact of postnatal depression on infant development. J Child Psychol Psychiatry 33(3):543–561. https://doi.org/10.1111/j.1469-7610.1992.tb00890.x
Oates, M. (2003). Perinatal psychiatric disorders: a leading cause of maternal morbidity and mortality. British Medical Bulletin, 67, 219–229. http:// doi: https://doi.org/10.1093/bmb/ldg011
Olson A. L. Kemper K. J. Kelleher K. J. Hammond C. S. Zuckerman B. S. & Dietrich A. J. (2002). Primary care pediatricians’ roles and perceived responsibilities in the identification and management of maternal depression. Pediatrics, 110(6 I), 1169–1176. https://doi.org/10.1542/peds.110.6.1169
Osterman MJK, Martin JA (2018) Timing and adequacy of prenatal care in the United States, 2016. Natl Vital Stat Rep 67(3):1–14
Pace CC, Spittle AJ, Molesworth CM, Lee KJ, Northam EA, Cheong JL, Davis PG, Doyle LW, Treyvaud K, Anderson PJ (2016) Evolution of depression and anxiety symptoms in parents of very preterm infants during the newborn period. JAMA Pediatr 170(9):863–870. https://doi.org/10.1001/jamapediatrics.2016.0810
Pelaez M, Field T, Pickens JN, Hart S (2008) Disengaged and authoritarian parenting behavior of depressed mothers with their toddlers. Infant Behav Dev 31(1):145–148. https://doi.org/10.1016/j.infbeh.2007.06.002
Pilkington PD, Milne LC, Cairns KE, Lewis J, Whelan TA (2015) June 1). Modifiable partner factors associated with perinatal depression and anxiety: systematic review and meta-analysis. J Affect Disord 178:165–180. https://doi.org/10.1016/j.jad.2015.02.023
Puryear LJ, Nong YH, Correa NP, Cox K, Greeley CS (2019) Outcomes of implementing routine screening and referrals for perinatal mood disorders in an integrated multi-site pediatric and obstetric setting. Maternal and child health journal, 23(10), 1292-1298.
Putnick D. L. Sundaram R. Bell E. M. Ghassabian A. Goldstein R. B. Robinson S. L. Yeung, E. (2020). Trajectories of maternal postpartum depressive symptoms. Pediatrics, 146(5). https://doi.org/10.1542/peds.2020-0857
Rejnö G. Lundholm C. Öberg S. Lichtenstein P. Larsson H. D’Onofrio B. Almqvist C. (2019). Maternal anxiety, depression and asthma and adverse pregnancy outcomes – a population based study. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-49508-z
Sonchak L (2015) Medicaid reimbursement, prenatal care and infant health. J Health Econ 44:10–24. https://doi.org/10.1016/j.jhealeco.2015.08.008
Straub H, Adams M, Kim JJ, Silver RK (2012) Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol 207(4):329.e1-329.e4. https://doi.org/10.1016/j.ajog.2012.06.033
Texas Health and Human Services Commission (N.D.). Medicaid for pregnant women and CHIP perinatal. https://www.hhs.texas.gov/services/health/medicaid-chip/medicaid-chip-programs-services/programs-women/medicaid-pregnant-women-chip-perinatal
Texas Legislature. (2017). Texas House Bill 2466. Retrieved December 4, 2020, from https://capitol.texas.gov/billlookup/BillSummary.aspx?LegSess=85R&Bill=HB2466
Wachino V (2016) Maternal depression screening and treatment: a critical role for medicaid in the care of mothers and children introduction. Retrieved from https://www.medicaid.gov/federal-policy-guidance/downloads/cib051116.pdf
Weissman MM, Wickramaratne P, Pilowsky DJ, Poh E, Batten LA, Hernandez M, Stewart JW (2016) Treatment of maternal depression in a medication clinical trial and its effect on children. Focus 14(1):103–112. https://doi.org/10.1176/appi.focus.140103
M Wilcox BA McGee DF Ionescu M Leonte L LaCross J Reps K Wildenhaus (2020) Perinatal depressive symptoms often start in the prenatal rather than postpartum period: results from a longitudinal study Archives of Women’s Mental Health. https://doi.org/10.1007/s00737-020-01017-z
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data abstraction were performed by Lucy Puryear, Yen Nong, and Cary Cain. Analysis was performed by Amanda Koire. The first draft of the manuscript was written by Amanda Koire, Bethanie Van Horne, Yen Nong, and Cary Cain. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was reviewed and approved by the Institutional Review Board at Baylor College of Medicine.
Consent to participate
Consent was not required because this was an observational study using data routinely collected as part of standard of care.
Conflict of interest
The authors declare that they have conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koire, A., Van Horne, B.S., Nong, Y.H. et al. Patterns of peripartum depression screening and detection in a large, multi-site, integrated healthcare system. Arch Womens Ment Health 25, 603–610 (2022). https://doi.org/10.1007/s00737-022-01223-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00737-022-01223-x