Abstract
A history of childhood trauma is associated with increased risk for psychopathology and interpersonal difficulties in adulthood and, for those who have children, impairments in parenting and increased risk of negative outcomes in offspring. Physiological and behavioral mechanisms are poorly understood. In the current study, maternal history of childhood trauma was hypothesized to predict differences in maternal affect and HPA axis functioning. Mother-infant dyads (N = 255) were assessed at 6 months postpartum. Mothers were videotaped during a 3-min naturalistic interaction, and their behavior was coded for positive, neutral, and negative affect. Maternal salivary cortisol was measured six times across the study visit, which also included an infant stressor paradigm. Results showed that childhood trauma history predicted increased neutral affect and decreased mean cortisol in the mothers and that cortisol mediated the association between trauma history and maternal affect. Maternal depression was not associated with affective measures or cortisol. Results suggest that early childhood trauma may disrupt the development of the HPA axis, which in turn impairs affective expression during mother-infant interactions in postpartum women. Interventions aimed at treating psychiatric illness in postpartum women may benefit from specific components to assess and treat trauma-related symptoms and prevent secondary effects on parenting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While several investigators have described the impact of maternal trauma on infant and child well-being, less attention has been paid to the enduring effect of prior trauma on subsequent maternal parenting behavior and the mother’s affective experience of interacting with her child. Existing empirical research suggests that mothers with a history of early life trauma are more intrusive in interactions with their infants compared with controls (Moehler et al. 2007) and that mothers with a history of childhood sexual abuse are more likely to engage in negative parenting strategies, including physical punishment (Banyard 1997). Traumatic experiences are also known to have lasting effects on an individual’s affect regulation, and many patients with early life trauma develop avoidant coping strategies that include numbing, intolerance of affect, and dissociation. Consistent with these strategies, women with a history of sexual abuse are more likely to display flatness of affect and appear disengaged on measures of maternal involvement with their infant (Lyons-Ruth and Block 1996).
A recent review suggests that maternal early-life trauma may result in poor quality of mothering in part due to the impact of early trauma on the mother’s hypothalamic-pituitary-adrenal (HPA) axis functioning (Barrett and Fleming 2011). For example, data from a community sample of mothers and infants revealed an indirect path from maternal early-life trauma to lower maternal sensitivity through higher diurnal cortisol (Gonzalez et al. 2012). The primary goal of the present study is to extend these findings using an alternative statistical method, examining a clinical sample and maternal data collected in the context of a laboratory study that included an infant stressor paradigm.
Of note, the direction of the associations between early-life trauma and cortisol and cortisol and parenting quality has not been consistent across studies. For example, our group found that mothers with a history of childhood abuse showed steeper declines in cortisol after infant stressor tasks (Brand et al. 2010). However, alternate findings link early-life stress to HPA axis hyperactivity (as opposed to hypoactivity) (Heim et al. 2000). In the case of cortisol and parenting, Mills-Koonce et al. (2009) found that mothers with higher cortisol levels displayed more negative intrusiveness during parent-child interactions than those with lower cortisol levels. Conversely, lower maternal cortisol levels have also been linked to insensitive and negative-intrusive parenting practices (Schechter et al. 2004). The direction of the cortisol effects may be less important than the overall finding that maternal HPA axis dysregulation is associated with both early-life trauma and parenting quality, and therefore may act as a biological mechanism linking the two.
In this study, we investigated the relationship between maternal early-life trauma and measures of parenting behaviors, focusing on maternal cortisol as a mediator. We tested our hypotheses in the context of a clinical sample in which the majority of mothers were receiving psychiatric treatment during pregnancy or the postpartum. First, we examined mean maternal cortisol levels across a laboratory visit that included a mother-infant stressor task, expecting to see evidence of a dysregulated cortisol response among mothers with a history of childhood trauma. Second, we coded mothers’ affect as positive, neutral, or negative during a videotaped post-stressor reunion. We hypothesized that affective responses during the reunion might be attenuated among mothers with a history of early-life trauma, irrespective of maternal psychopathology. Third, we examined whether maternal cortisol levels acted as a mediator between childhood trauma history and maternal affective quality in response to her infant.
Methods
Participants
Mother-infant dyads (N = 255) participated in a laboratory stressor study measuring maternal psychiatric status, cortisol, and affect during a mother-infant interaction. Mothers in the current study were recruited from the Emory Women’s Mental Health Program (WMHP), a tertiary care center for women with psychiatric disorders. While women were seen during pregnancy as part of a longitudinal study on perinatal mood disorders, the current study focuses on data collected at the 6-month postnatal visit and from birth records. Women meeting criteria for substance abuse or dependence during pregnancy or within 6 months of becoming pregnant were excluded from the current study. Infants with major congenital malformations (e.g., spina bifida) were excluded. The sample contained two sets of twins. Equipment malfunction resulted in missing behavioral coding data for 63 dyads. Mothers with and without these data did not differ with respect to maternal age, education history, marital status, race/ethnicity, history of depression or history of childhood trauma, and current level of depressive symptoms, as well as infant age or gender (ps > 0.05). Infants (n = 192) were 6 months of age (M = 183 days, SD = 16 days) and 51 % were male. Mothers’ age ranged from 22 to 44 years (M = 34.2, SD = 4.06) and 92.7 % of mothers were married or cohabitating. Mothers were primarily Caucasian (92.6 %) with a median education level of college graduate (Table 1).
Procedure
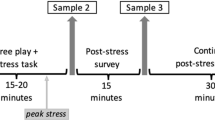
Study procedures were approved by the Emory University Institutional Review Board and conducted at the Early Child Development Lab at Emory University. All procedures and samples were collected between 2000 and 2008 in a single lab visit that began between 1:00 and 1:30 PM and was completed by 4:00 PM. The study involved additional infant procedures that have been described elsewhere (Brennan et al. 2008; Johnson et al. 2014) and are not included below. Following maternal informed consent, five stages of data collection were completed as follows.
First, baseline maternal cortisol was obtained (T0—baseline) in a quiet room with the mother and infant seated together. To obtain maternal cortisol, the mother provided a saliva sample by swabbing a dental cotton roll inside her mouth. Saliva was then transferred from the cotton roll to a 15-cc polypropylene tube via syringe. All cortisol samples were collected using the same methodology and frozen at −20 °C within 15 min of collection. Second, the mother completed questionnaires while her infant was held by a research assistant (RA) within view of the mother. After 20 min of separation, the second saliva sample was obtained (T1—post-separation stressor). Third, the infant was exposed to two laboratory stressor tasks, including a brief arm restraint and noise burst, while the mother monitored her infant’s behavior on a computer screen. The third maternal cortisol sample was obtained immediately following the completion of the stressor tasks (T2—post laboratory stressor I). Fourth, a semi-structured 3-min interaction between the mother and infant was videotaped (details below). The fourth cortisol sample was collected from the mother after this interaction, approximately 20 min after the conclusion of the arm restraint task (T3—post laboratory stressor II). Fifth, the mother and infant were reunited and escorted to a private room where the infant sat on the mother’s lap for the clinical interview and psychosocial assessment. The fifth and sixth cortisol samples were obtained during or after the interview portion of the study, approximately 80 min (T4—post clinical interview) and 100 min after the arm restraint task (T5—pre study exit). Following the final cortisol sample collection, participants were compensated and given a t-shirt for completing the study.
Measures
Maternal cortisol
Samples were assayed using a commercially available radioimmunoassay kit (DiaSorin GammaCoat, Stillwater, Minnesota) with a cortisol sensitivity of 0.05 μg/dL. Inter- and intraassay coefficients of variation for this kit are 6.0 and 3.5 %, respectively. An RA blind to maternal trauma history and the time point at which each sample was collected assayed all standards and samples in duplicate.
Maternal trauma exposure
The Structured Clinical Interview for DSM-IV (SCID) (First et al. 2002) was administered by Masters-level RAs. A reliability analysis performed by an independent judge on 10 % of the sample yielded kappas over 0.75 for all major diagnoses. History of trauma was evaluated according to DSM-IV criteria using the posttraumatic stress disorder (PTSD) module of the SCID. The DSM-IV defines trauma as direct personal experience with an event involving actual or threatened death, serious injury, a threat to one’s physical integrity, or witnessing someone else be killed or seriously harmed. For each trauma reported, the mother’s age was recorded. Any trauma that took place prior to age 18 was considered early-life trauma. Using the SCID PTSD module to assess trauma history has been shown to be a cost-effective assessment of previous trauma in mental health patients (Franklin et al. 2002) as well as in college students and primary care patients (Elhai et al. 2008). Forty mothers in the sample (20.8 %) reported experiencing a trauma during childhood. Although restricted power precluded our ability to examine specific types of trauma, it should be noted that sexual trauma was reported in over half of the cases (n = 24).
Maternal psychiatric status
The current sample was recruited from a psychiatric clinic. The SCID was administered at the 6-month visit to assess current and lifetime psychiatric status. A majority of women (n = 165, 85.9 %) experienced at least one psychiatric illness across their lifetime, with the most common diagnosis being depression (n = 125, 65.1 %). In addition, most of the women received psychopharmacologic treatment during pregnancy. Prenatal exposures for the major classes of drugs were as follows (with some women receiving drugs from more than one class): antidepressants n = 130, antiepileptics n = 21, antipsychotics n = 16, anxiolytics, n = 29, hypnotics n = 17). The Beck Depression Inventory (BDI) (Beck et al. 1988) was administered to assess current levels of depressive symptoms (M = 9.72, SD = 8.86, range = 0–51); 22 % of the sample reported clinically elevated scores (>15) on the BDI.
Maternal affect during a mother-infant interaction
The mother-child interaction occurred after the infant stressors and was videotaped for later scoring. Mothers were instructed to interact normally with their infants for 3 min, but were asked not to physically touch the infant. Maternal affect (positive, negative, and neutral) was rated by trained coders (kappas > 0.70) and the percentage of time mothers exhibited each type of affect was calculated using MANgold INTERACT software. Mother positive affect was defined as a broad smile with cheeks raised, making funny or positive faces at the infant, half smiling, and when whole eyebrows were raised and eyes were wide open while talking with infant. Mother negative affect was identified as anger/disgust/contempt/sadness/fear/pain or a turned down mouth, furrowed brow, raised inner corners of brow, and wincing/scrunching up face with mouth turned down. Mother neutral affect was defined as the absence of observable positive or negative affect as defined above.
Data analytic plan
Descriptive statistics were calculated including mean values and measures of normality and variability. Because almost 95 % of mothers (n = 182) displayed no negative affect during the mother-infant interaction, limited variability precluded our ability to include it in the data analyses. Thus, only neutral affect and positive affect were used as outcome variables.
Mean values of cortisol were calculated using all measures available for each mother. No cortisol was collected from 9 mothers; all 6 samples were collected from 151 mothers (78.6 %) and at least 3 samples were collected from 182 mothers (95 %). Outliers greater than 3 standard deviations, all of which reflected biologically implausible values, were removed. Cortisol levels at each time point were highly correlated (Pearson correlations ranged from 0.57 to 0.87, ps < 0.01); thus, the mean cortisol level over the course of the study was used. The mean cortisol value was log transformed to correct for skew.
Potential covariates were analyzed using independent t tests and Pearson correlations. Results are presented in Table 2. Only covariates that were significantly associated with the dependent variable were included in the models. Linear regression was used to test the direct paths of the study hypotheses: associations between maternal trauma history and positive affect, neutral affect, and mean cortisol, and associations between mean cortisol and positive and neutral affect. Indirect effects were tested using the bootstrapping method put forth by Preacher and Hayes (2008). SPSS Version 20 was used for all analyses.
Results
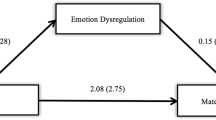
Linear regression revealed that after controlling for mothers’ smoking status and infant age, mothers’ early-life trauma failed to predict mothers’ positive affect during the interaction (β = 0.003, t = 0.045, p = 0.964). After controlling for time since the mother ingested caffeine, mothers’ early-life trauma significantly predicted increased neutral affect during the mother-infant interaction (β = .228, t = −2.761, p = .007). Mothers’ early-life trauma also significantly predicated mothers’ mean cortisol response during the visit (β = −.232, t = −3.209, p = 0.002). A history of early-life trauma was associated with lower mean cortisol. Mothers’ mean cortisol, in turn, significantly predicted neutral affect during the mother-infant interaction, after controlling for infant gender and prematurity at birth (β = −.252, t = −2.947, p = 0.004). Examination of the specific indirect effects indicated that maternal mean cortisol mediated the association between maternal trauma history and maternal neutral affect during the mother-infant interaction (point estimate = 3.494, SE = 1.483, 95 % CI = 1.149–7.128). The model summary suggested that the overall model is significant (F(2, 180) = 8.898, p < 0.001) and accounted for approximately 9.00 % of the variance in mothers’ neutral affect.
Given the sample was recruited from a psychiatric clinic and demonstrated a high incidence of depression, we examined whether current or previous depression status predicted affect or cortisol and thus might explain the results reported on childhood trauma. After controlling for mothers’ smoking status and infant age, mothers’ lifetime history of depression or dysthymia failed to predict mothers’ positive affect during the interaction (β = −0.016, t = −0.225, p = 0.823). Results were similar using current maternal depressive symptoms (β = −0.039, t = −0.512, p = 0.609). After controlling for maternal caffeine consumption, mother’s lifetime history of depression or dysthymia failed to predict mothers’ neutral affect during the interaction (β = −0.081, t = −0.945, p = 0.346). Results were similar using current maternal depressive symptoms (β = −0.040, t = −0.455, p = 0.650). Mother’s lifetime history of depression or dysthymia failed to predict mothers’ mean cortisol (β = 0.067, t = −0.902, p = 0.368). Results were similar using current maternal depressive symptoms, though the standardized beta value was larger (β = −0.123, t = −1.626, p = 0.106).
Discussion
The current study examined the association between mothers’ early-life trauma history and mothers’ affect during a videotaped interaction with their 6-month-old infants. We predicted that mothers with early-life trauma would show reduced positive affect and increased neutral affect. We further hypothesized that these associations would be mediated by maternal cortisol levels during the study visit.
Results confirmed that while there were no differences in positive affect, mothers with early-life trauma showed increased neutral affect after controlling for relevant covariates. Numerous studies have shown that maternal trauma history predicts negative parenting behaviors (Roberts et al. 2004), including increased intrusiveness (Moehler et al. 2007), impaired bonding (Seng et al. 2013; Muzik et al. 2013), and increased use of physical punishment (Banyard et al. 2003), and at least one study showed that inconsistent caregiving and neglect in childhood was associated with less affectionate behavior at 6 weeks postpartum (Krpan et al. 2005). The current study generally supports this line of research and replicates in a larger sample Lyons-Ruth and Block’s findings of increased neutral affect among mothers with a history of childhood trauma, irrespective of symptomatology. However, our study is the first, to our knowledge, to demonstrate that maternal cortisol mediates the association between maternal early-life trauma and increased neutral maternal affect while interacting with her infant.
A flattened emotional response during mother-infant interaction is not surprising given that flattened affect is a common symptom identified in adults with early-life trauma (Briere 1992; Gil 1988; Marx and Sloan 2002). Studies indicating impaired mother-child bonding (Seng et al. 2013; Muzik et al. 2013) following maternal early-life trauma may be partially explained by the mother’s inability to express appropriate affect or mirror the infant’s affect when interacting with her child. Interestingly, mothers’ early-life trauma was not related to current depressive symptoms or history of depression in our sample. Other researchers have hypothesized that depression may mediate or fully account for the association between maternal trauma and less optimal parenting (Banyard et al. 2003; Lutenbacher and Hall 1998), but results have been mixed (Zuravin and Fontanella 1999). The current sample was recruited from a clinic specializing in peripartum psychiatric illness, and a majority of mothers were being treated with psychotropic medication. These factors may have inhibited our ability to detect depression effects, though BDI scores showed substantial variability. In our sample, the effect on maternal behavior seemed specific to trauma history. This finding is particularly compelling given that targeting depression alone in postpartum women may not be sufficient to improve the mother infant relationship and childhood outcomes (Muzik et al. 2009; Forman et al. 2007). Our findings suggest that maternal trauma history may provide a unique treatment target that deserves clinical attention, particularly in women experiencing or at risk for postpartum depression. Certainly studies examining potential interactions between maternal depression and trauma history would be useful in delineating their potential combined effects.
In our sample, trauma history predicted lower mean cortisol, which, in turn predicted increased neutral affect. As reviewed in the “Introduction,” the precise direction of expected effects with cortisol is mixed, based on the extant literature. For example, our findings differ from Gonzalez et al. (2009) who reported that postpartum mothers with early-life trauma showed higher basal cortisol at awakening and higher levels across the day. The current study assessed laboratory cortisol values in the context of an infant stressor paradigm, rather than examining diurnal cortisol at home, which could partially explain discrepant findings. The lower mean cortisol reported in the current study is compatible with the Brand et al. (2010) findings published from our research group, which showed that maternal trauma history based on self-report questionnaire data was associated with a decline in cortisol over the study visit, while mothers with no trauma history showed no change. The Brand et al. study used a smaller sample, was limited to women with major depression, and did not examine relationships between maternal early-life trauma and parenting styles. Results from the current study add to a growing body of literature that implicates the role of HPA axis dysregulation in women with a trauma history. Our findings are in line with prior studies showing that childhood trauma may exert a lasting, dampening influence on the HPA axis (Heim et al. 2000), which in turn inhibits a woman’s affective expression when interacting with her infant. Neuroimaging studies have demonstrated that early-life trauma predicts dampened neural activation during reward processing (Dillon et al. 2009), thus offering one potential explanation—that mothers with a trauma history are less sensitive to reward cues offered by her infant and are therefore less affectively responsive.
One notable strength of the study is the use of clinician-rated trauma assessment and condition-blind RAs to code maternal affect and examine cortisol levels. This multimodal approach minimized experimenter bias and removed shared-rater variance as a potential confound, which suggests that the present findings are robust. Our protocol included repeated sampling of maternal cortisol, allowing for a more reliable measure of this biological mechanism, and saliva samples were collected at the same time of day for all participants, accounting for the known diurnal variation in cortisol. Maternal behavior and cortisol data were collected in the context of an infant laboratory stressor task. While this may limit generalizability, the infant stressor paradigm is a relevant proxy for measuring maternal response in a stressful parenting context and may reflect her ability to cope with situations that induce parenting stress.
There were several limitations in the present study. First, although the traumatic event screening on the SCID is a valid method of assessing trauma, we could not assess the effects of different types of trauma due to sample size limitations. Future research should specify which aspects of trauma exposure (e.g., type of trauma exposure (violent versus non-violent), timing of trauma exposure) are related to maternal affect and HPA axis activity during mother-child interactions. Second, our study focused on cortisol levels and maternal behavior observed in a laboratory context. It is unclear whether our findings would extend to cortisol or maternal behavior in a more naturalistic setting. Third, these results should be replicated in more demographically diverse samples in order to examine the generalizability of these findings. Fourth, although HPA axis dysregulation has been implicated in trauma exposure and depression, a consensus regarding the significance of inter-individual variations in cortisol has yet to be reached (Burke et al. 2005; Meewisse et al. 2007). Although our findings suggest that HPA axis dysregulation is one mechanism for differences in maternal affect during dyadic interactions, these findings should be interpreted cautiously.
For over a decade, researchers have reported intergenerational effects of early-life trauma. While our current findings are observational by nature, they support early experimental work (Champagne and Meaney 2001) showing that maternal early-life trauma exerts lasting effects on brain development that impact offspring, likely through maternal behavior in addition to other mechanisms. Identifying HPA axis dysregulation as a mediator that predicts affective differences highlights the important role of emotion regulation in intergenerational transmission. Interventions designed to treat postpartum psychiatric illness should give serious consideration to maternal trauma history.
References
Banyard VL (1997) The impact of childhood sexual abuse and family functioning on four dimensions of women’s later parenting. Child Abuse Negl 21:1095–1107
Banyard VL, Williams LM, Siegel JA (2003) The impact of complex trauma and depression on parenting: an exploration of mediating risk and protective factors. Child Maltreat 8:334–349
Barrett J, Fleming AS (2011) Annual research review: all mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry 52:368–397
Beck AT, Steer RA, Garbin MG (1988) Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 8:77–100
Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN (2010) The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology 35:686–693
Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe Z (2008) Maternal depression and infant cortisol: influences of timing, comorbidity and treatment. J Child Psychol Psychiatry 49:1099–1107
Briere J (1992) Child abuse trauma: theory and treatment of the lasting effects. Sage, Thousand Oaks
Burke HM, Davis MC, Otte C, Mohr DC (2005) Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 30:846–856
Champagne F, Meaney MJ (2001) Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. In: Russell JA (ed.) Progress in Brain Research, Vol 133. Elsevier. pp 287–302
Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA (2009) Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry 66:206–213
Elhai JD, Franklin CL, Gray MJ (2008) The SCID PTSD module’s trauma screen: validity with two samples in detecting trauma history. Depress Anxiety 25:737–741
First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, Patientth edn. Biometrics Research, New York
Forman DR, O’Hara MW, Stuart S, Gorman LL, Larsen KE, Coy KC (2007) Effective treatment for postpartum depression is not sufficient to improve the developing mother–child relationship. Dev Psychopathol 19:585–602
Franklin CL, Sheeran T, Zimmerman M (2002) Screening for trauma histories, posttraumatic stress disorder (PTSD), and subthreshold PTSD in psychiatric outpatients. Psychol Assess 14:467–471
Gil E (1988) Treatment of adult survivors of childhood abuse: launch press. Walnut Creek, CA
Gonzalez A, Jenkins JM, Steiner M, Fleming AS (2009) The relation between early life adversity, cortisol awakening response and diurnal salivary cortisol levels in postpartum women. Psychoneuroendocrinology 34:76–86
Gonzalez A, Jenkins JM, Steiner M, Fleming AS (2012) Maternal early life experiences and parenting: the mediating role of cortisol and executive function. J Am Acad Child Adolesc Psychiatry 51:673–682
Heim C, Newport DJ, Heit S et al (2000) Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. J Am Med Assoc 284:592–597
Johnson KC, Brennan PA, Stowe ZN, Leibenluft E, Newport DJ (2014) Physiological regulation in infants of women with a mood disorder: examining associations with maternal symptoms and stress. J Child Psychol Psychiatry 55:191–198
Krpan KM, Coombs R, Zinga D, Steiner M, Fleming AS (2005) Experiential and hormonal correlates of maternal behavior in teen and adult mothers. Horm Behav 47:112–122
Lutenbacher M, Hall LA (1998) The effects of maternal psychosocial factors on parenting attitudes of low-income, single mothers with young children. Nurs Res 47:25–34
Lyons-Ruth K, Block D (1996) The disturbed caregiving system: relations among childhood trauma, maternal caregiving, and infant affect and attachment. Infant Mental Health J 17:257–275
Marx BP, Sloan DM (2002) The role of emotion in the psychological functioning of adult survivors of childhood sexual abuse. Behav Ther 33:563–577
Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M (2007) Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. Br J Psychiatry 191:387–392
Mills-Koonce WR, Propper C, Gariepy JL et al (2009) Psychophysiological correlates of parenting behavior in mothers of young children. Dev Psychobiol 51:650–661
Moehler E, Biringen Z, Poustka L (2007) Emotional availability in a sample of mothers with a history of abuse. Am J Orthopsychiatry 77:624–628
Muzik M, Marcus SM, Flynn HA (2009) Psychotherapeutic treatment options for perinatal depression: emphasis on maternal-infant dyadic outcomes. J Clin Psychiatry 70:1318–9
Muzik M, Bocknek E, Broderick A et al (2013) Mother–infant bonding impairment across the first 6 months postpartum: the primacy of psychopathology in women with childhood abuse and neglect histories. Arch Womens Ment Health 16:29–38
Preacher K, Hayes A (2008) Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40:879–891
Roberts R, O’Connor T, Dunn J, Golding J (2004) The effects of child sexual abuse in later family life; mental health, parenting and adjustment of offspring. Child Abuse Negl 28:525–545
Schechter DS, Zeanah CH Jr, Myers MM et al (2004) Psychobiological dysregulation in violence-exposed mothers: salivary cortisol of mothers with very young children pre- and post-separation stress. Bull Menn Clin 68:319–336
Seng JS, Sperlich M, Low LK, Ronis DL, Muzik M, Liberzon I (2013) Childhood abuse history, posttraumatic stress disorder, postpartum mental health, and bonding: a prospective cohort study. J Midwifery Womens Health 58:57–68
Zuravin SJ, Fontanella C (1999) Parenting behaviors and perceived parenting competence of child sexual abuse survivors. Child Abuse Negl 23:623–632
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Juul, S.H., Hendrix, C., Robinson, B. et al. Maternal early-life trauma and affective parenting style: the mediating role of HPA-axis function. Arch Womens Ment Health 19, 17–23 (2016). https://doi.org/10.1007/s00737-015-0528-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00737-015-0528-x