Abstract
The absorption of dietary proteins affects the anabolic response, among others protein synthesis. For elderly, optimal amino acid absorption is warranted to preserve the amino acid pool of the body, especially skeletal muscle proteins. Therefore, the aim of this study was to characterize if hydrolyzing meat protein (HMP) would improve the amino acid absorption after ingestion of meat compared to equal amounts of the same meat proteins but present in a different structure; steak or minced meat. With a crossover study design on 12 healthy older adults (> 65 years of age, BMI 18.5–30), the amino acid absorption kinetics were explored by ingesting 0.55 g protein/kg LBM as a mixed meal together with intrinsically [2H5]phenylalanine labeled meat proteins prepared as a STEAK, MINCED meat, or HMP. Plasma [2H5]phenylalanine enrichment as well as AA concentrations were measured by mass spectrometry from blood samples drawn during a 5-h postprandial period. After HMP ingestion, [2H5]phenylalanine was faster absorbed in the initial 2 h compared to STEAK and MINCED. The peak time in AA concentrations was faster in HMP compared to STEAK and MINCED. However, the peak AA concentrations were not different between STEAK, MINCED, and HMP. Although HMP showed to have the fastest initial amino acid appearance in older adults, the peak EAA concentrations were similar after ingesting meal with either STEAK, MINCED, or HMP in the 5-h postprandial period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With increasing age, a general deterioration of the body’s protein mass, in particular the skeletal muscle mass is seen (Janssen et al. 2000). This may compromise metabolic health as well as the ability to perform everyday activities due to an impaired muscle function. The etiology of this sarcopenic development is complex (Rosenberg 1997; Baumgartner et al. 1998; Cruz-Jentoft et al. 2010). However, a combination of age-related cellular changes and lifestyle changes such as poor diet and physical inactivity are important factors (Cruz-Jentoft et al. 2010; Fielding et al. 2011; Morley et al. 2011). In regards to nutrition, a sufficient dietary protein intake is needed to maintain the amino acid pool of the body, of which skeletal muscle constitutes the major pool (Houston et al. 2008). However, for some elderly people, it may be difficult to meet the recommended daily protein intake of 1.2 g protein/kg body weight (Nordic Council of Ministers 2008; Pedersen and Cederholm 2014; Deutz et al. 2014). Furthermore, it is well known that the protein digestibility is of major importance for the amino acid absorption rate and hence the ability to stimulate the body’s protein turnover (Pennings et al. 2011a, 2013). A dietary supplement of protein for this group of older adults can therefore be extremely important to meet the recommended daily protein intake, but also to provide easily digestible dietary proteins.
The amino acid absorption rate after intake of a protein is decisive for the ability to regulate protein metabolism (He and Giuseppin 2014). Previous studies have shown that especially high-quality proteins, i.e., proteins rich in essential amino acids and with a fast absorption rate, show an immediate increase in availability of important amino acids in the bloodstream (Dangin et al. 2001). This increased amino acid availability is important to achieve a state of positive protein balance; greater protein synthesis than protein breakdown, in the initial postprandial phase. From an aging perspective, the positive protein turnover at a whole body level is important to spare the breakdown of tissue proteins such as skeletal muscle proteins (Gwin et al. 2020). Numerous studies looking at the effect of protein intake on the protein turnover in humans have been made with milk proteins as the protein source. The proteins in milk are divided into two protein categories: casein proteins and whey proteins, which due to their structure and solubility in the digestive system are characterized as slow and fast absorbable (Boirie et al. 1997; Reitelseder et al. 2011). Meat proteins appear in a far more complex matrix, which is more difficult to degrade and thereby absorb. Only a limited number of studies have looked at the effect of meat-protein intake on the body’s protein metabolism. It has recently been shown that the uptake of the amino acids from meat extends beyond several hours (Bax et al. 2013). However, modulating the structure of the meat may alter the protein digestibility and hence amino acid absorption. Chopping the meat seems to improve the digestive process by increasing the gastric absorption and thereby increase the amino acid absorption rate (Pennings et al. 2013). An even greater improvement of the meat-protein digestibility is made from hydrolyzing the meat to a hydrolyzed meat product (HMP) (Reitelseder et al. 2020). Such hydrolyzed meat appears to result in a comparable time to peak in postprandial amino acid concentrations as for hydrolyzed whey and blood proteins (Bendtsen et al. 2019).
The present project utilized intrinsically labeled meat sources. Intrinsically labeled protein sources have previously been used to explore the amino acid absorption kinetics and effect on protein turnover from milk proteins (Reitelseder et al. 2011), but the method can also be applied to explore the effect of meat proteins (Pennings et al. 2011b). In the present study, the meat was intrinsically labeled with stable amino acid isotope ([2H5]phenylalanine) (Reitelseder et al. 2020). The appearance of the [2H5]phenylalanine in the blood circulation reflects the direct amino acid absorption from the dietary protein. Notably, serving a dietary protein either alone or in a mixed meal could influence the protein digestion and absorption (Gwin et al. 2020). Therefore importantly and in contrary to previous studies (Pennings et al. 2013; Burd et al. 2015; Buffière et al. 2017), the meat products in question in the present study were served with standardized meal to provide a protein, carbohydrate, and fat content of a regular mixed meal.
The purpose of the present study was to explore HMP derived amino acid absorption into the blood circulation compared to two common meat-protein sources; steak and minced-beef when ingested as part of a mixed meal. We hypothesized that the absorption rate and maximal amino acid concentration in the blood circulation would be in the order HMP, MINCED, and STEAK, with HMP showing the quickest absorption.
Materials and methods
Ethical approval
All eligible subjects were informed about the design of the study, the risks of tests and investigations, and their rights according to the Declaration of Helsinki II before volunteering to participate in the project. The study protocol was approved by the Research Ethics Committee Region Hovedstaden (H-17021471, September 12, 2017), Danish Data Protection Agency (2012-58-0004, July 21, 2017), and registered at ClinicalTrials.gov (NCT03301337, September 28, 2017). All participants gave written informed consent before enrollment to the study.
Subjects and study design
Twelve healthy older adults (69 ± 5 years of age; 6 females, 6 males) were enrolled to the study (Table 1). Exclusion criteria were BMI > 30 and < 18.5, smokers, vegetarians, metabolic diseases (e.g., diabetes), gastrointestinal disorders, impaired renal or hepatic function, inflammatory condition, medical treatment affecting protein absorption and protein turnover, and frequent intense exercise. At the inclusion, a dual-energy X-ray absorptiometry (DXA) scan was performed to measure the lean body mass (LBM), which was used for calculating protein intake from the meal.
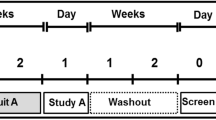
The study was designed as a randomized single-blinded (test-personnel) crossover study. All subjects completed three trials ingesting either a beef-steak (STEAK), chopped steak (MINCED), or hydrolyzed meat protein (HMP) in a randomized order together with a standardized meal. Each trial was performed with at least a week washout in between.
Intrinsically labeled meat
The [2H5]phenylalanine intrinsically labeled meat was produced by infusing a Holstein cow with L-[2H5]phenylalanine for 72 h (Reitelseder et al. 2020). Subsequently, the cow was slaughtered and the meat were stored at − 40 °C until further use. The steaks, minced beef, and HMP were produced from the same cut of meat, to ensure an identical isotope labeling in all three meat products. The steak and minced-meat were sous vide pre-cooked at 90 °C and stored at − 40 °C until usage.
HMP production
The meat used for hydrolysate was minced and mixed up in water and under constant stirring heated to 60 °C. Hereafter, enzymes (0.1% of meat weight of both the endoprotease Protamex® and the exopeptidase Flavourzyme®, Novozymes, Bagsvaerd, Denmark) were added and the solution was heated under constant stirring: 60 °C for 1 h and subsequently 90 °C for 15 min. The slurry was drained and the pellet (mainly connective tissue proteins) was discarded. The watery hydrolysate was portioned and stored at − 40 °C until usage.
Trial day
On each trial day, the subjects arrived at the Institute of Sports Medicine Copenhagen at 8.00 A.M. in the morning after overnight fasting from 9.00 P.M. the evening before. An antecubital catheter was inserted for venous blood sampling. After a postabsorptive baseline blood sample, the subjects consumed the meal in a separate room and returned to the trial room immediately hereafter. During the 5-h postprandial period, blood sampling was performed at 20, 40, 60, 90, 120, 150, 180, 240, and 300 min after finishing the meal. Subjects were not allowed to eat and only allowed to drink water during the postprandial period. Throughout the trial period, the subjects rested by lying or sitting in a bed.
All plasma samples were obtained from venous blood samples collected in EDTA tubes. After the blood draw, the samples were put on ice for 10–30 min and subsequently spun at 3200×g for 10 min at 4 °C. The plasma was put into aliquots (500 μl Eppendorf tubes) and stored at − 80 °C until further analysis.
Meal intake
Each meat product was served with a protein amount of 0.55 g protein/kg LBM, in a standardized meal, giving a mixed meal with approximately 22% protein, 59% carbohydrates, and 19% fat. The serving size of the standardized meal was also adjusted to the LBM. The standardized meal consisted of mashed potatoes, baked carrots, and sauce, all pre-cooked at the hospital kitchen (Bispebjerg Hospital, Denmark) from whole food products, and stored at − 20 °C until usage. Importantly, this meal was low in protein to be able to exclusively evaluate the amino acid absorption from the meat product in the mixed meal and to avoid any plasma dilution of the amino acid tracer. The protein amount from 100 g of each meat product was 24.0 g, 23.4 g, and 10.5 g, STEAK, MINCED, and HMP, respectively. The pre-cooked meals and meat were warmed in a microwave oven prior to consumption. The subjects were instructed to consume the meal at a regular pace, and to consume the meat product in conjunction with the standardized meal. The ingestion time of the meal was measured as the time from intake of the first bite to the time of swallowing the last bite. The end of the meal intake was set as time point 0 min.
Mass spectrometry analysis
Liquid chromatography–tandem mass spectrometry (LC–MS/MS) (TSQ Quantiva; Thermo Fisher Scientific, San Jose, CA) was used to measure the [2H5]phenylalanine enrichment as well as amino acid concentrations as described elsewhere (Bornø and van Hall 2014). For the analysis, 200 μL of plasma was used. Importantly, each of the individual 19 amino acids was quantified using their own stable isotopically labeled internal standard (uniformly labeled-13C/15N), which was added to the plasma in a 50% acetic acid solution. Hereafter, the solution was poured over cation exchange columns with resin (AG 50 W-X8 resin, Bio-Rad Laboratories), which had been prepared by adding 3 × 2 mL 1 M HCl. The resin columns were washed 5 times with 3 mL deionized water before the amino acids were eluted into collection vials by adding 2 × 2 mL 4 M NH4OH. The solvent was evaporated under a stream of N2 flow at 70 °C and samples were derivatized into their PITC derivative with phenylisothiocyanate (PITC). Ten microliters of the derivatized samples were loaded and analyzed by LC–MS/MS.
Insulin
Plasma samples had to be treated with thrombin (T6884, Sigma-Aldrich), before analyzed for insulin concentration. Briefly, 5 μL of thrombin was added to 200 μL of plasma, immediately vortexed, and left at room temperature for 10 min. Samples were spun at 1600×g for 10 min and subsequently a coagulate clog was removed and the remaining supernatant used for analysis.
The insulin concentration was analyzed by a commercial ELISA-kit according to the manufacture’s instruction (80-INSHU-E01.1, ALPCO, NH, USA). Briefly, 50 μL of samples and insulin standards were loaded in replicates on a pre-coated 96-well plate, and 200 μL of insulin primary antibody was added to each well before incubation on an ELISA plate shaker at room temperature for 60 min. Subsequently, the samples were discarded and the plate was washed 6 times. 200 μL of HRP streptavidin solution was added to each well followed by 15 min incubated on an ELISA plate shaker at room temperature. Next, 200 μL of stop solution was added to each well, and immediately hereafter the intensity was measured at 450 nm on a 96-well plate reader (Multiscan FC, Thermo-Fisher Scientific). Finally, the insulin concentrations were calculated on basis of the standard curve.
Statistics
Subject characteristics are shown as mean ± standard deviation (SD), and sex comparisons were performed by a two-tailed unpaired t test.
Meal fat content, total kcal, meal consumption time, amino acid and insulin concentration data, and peak enrichment/concentration were checked for normality (Shapiro–Wilk normality test) before subjected to parametric testing. Insulin concentration data were log-transformed before normality testing. Meal macronutrient composition, total kcal, meal consumption, and peak enrichment/concentration data were analyzed by one-way ANOVA with repeated measures for group. Whenever a significant group effect was seen, a subsequent post hoc analysis was run (Tukey’s multiple comparisons test). Amino acid and log-transformed insulin concentration data were subjected to two-way ANOVA testing with repeated measures for time and group. Whenever significant effects of time, group, or interaction appeared, a subsequent post hoc test was performed (Tukey’s multiple comparisons test). For the log-transformed insulin concentration data, a Dunnett’s post hoc test was also performed to show changes from baseline. All trial data are shown as mean ± standard error mean (SEM) unless otherwise stated.
Peak times for [2H5]phenylalanine, phenylalanine, EAA, and total AA were analyzed by non-parametric Friedman test followed by Dunn’s multiple comparisons test. These data are shown as median with interquartile range (IQR).
All statistical tests were performed on GraphPad Prism 8 (GraphPad Software, CA, USA). Significant level was set to p < 0.05 and 0.10 < p < 0.05 were seen as tendencies.
Results
Subject characteristics
An equal number of women and men were included in the study (N = 6 for both men and women) (Table 1). The women and men were of the same age and had similar BMI, but the men had a greater height (p < 0.001), weight (p = 0.003), and lean body mass (LBM) (p < 0.001) compared to women, whereas women had a greater fat mass (p = 0.008) compared to men (Supplementary data 1).
Meal composition and meal consumption
The protein amount for the meal was set at 0.55 g protein/kg LBM, and thus, an equal amount of protein was ingested in all three trials (Table 2). This gave an average protein intake of 29.5 ± 5.4, of which 25.4 ± 4.6 g originated from the meat product and 4.2 ± 0.8 g originated from the standardized meal. Although the three meat products were made from the same pieces of meat, the fat percentage differed slightly. Therefore, although the standardized meal was identical through all three trials for each subject, the amount of fat was significantly different between the three trials (p < 0.001) (Table 2). On basis hereof the total amount of kcal was also significantly different between the three trials (p < 0.001).
The time for ingesting the meals showed a tendency towards a difference (p = 0.093), with STEAK showing the longest ingestion time and MINCED showing the shortest ingestion time (Table 2).
Plasma phenylalanine kinetics
At baseline (−30 min), the plasma [2H5]phenylalanine enrichments were not different. After intake of the meal with HMP, the plasma [2H5]phenylalanine enrichment increased immediately and was significantly greater compared to STEAK and MINCED at time points 20, 40, and 60 min (Fig. 1a) and peaked at 75 min post-meal intake (Table 3). At 150, 180, 240, and 300 min time points, the plasma [2H5]phenylalanine enrichments were greater for STEAK and MINCED compared to HMP, and at 300 min time point, the plasma [2H5]phenylalanine enrichments were greater in STEAK compared to MINCED (Fig. 1a). Thus, after the meals with STEAK and MINCED, a delayed increase in plasma [2H5]phenylalanine enrichments were seen peaking at 180 min for both STEAK and MINCED, which were significantly later time points compared to the peak at 75 min time for HMP (Table 3). In contrast, the peak plasma [2H5]phenylalanine enrichments were greater for STEAK and MINCED compared to HMP (Table 3).
Plasma [2H5]phenylalanine enrichment and phenylalanine, essential amino acids (EAA), and total amino acid concentrations for steak beef (STEAK), minced beef (MINCED), and hydrolyzed meat protein (HMP) trials, respectively, shown at basal fasting levels (−30 min) and 300 min into the postprandial period after the meal was finished at time point 0 min. a Difference between HMP and MINCED (p < 0.05), b difference between HMP and STEAK (p < 0.05), and c difference between STEAK and MINCED (p < 0.05). Data are shown as mean ± SEM
For plasma phenylalanine concentrations, a similar pattern was seen. After intake of HMP, an immediate increase in plasma phenylalanine was seen with greater concentrations compared to STEAK and MINCED at time points 20, 40, and 60 min post-meal intake (Fig. 1b). Plasma phenylalanine concentrations were greater after intake of meals with STEAK and MINCED compared to HMP at 150, 180, and 240 min (Fig. 1b). Thus, the plasma phenylalanine concentrations peaked at significantly later time point for STEAK (165 min) and MINCED (180 min) compared to the peak at 30 min for HMP (Table 3). However, the peak phenylalanine concentrations were not different between STEAK, MINCED, and HMP (Table 3).
Plasma total AA and EEA concentrations
After intake of meal with HMP, an immediate increase in plasma AA concentration was seen being significantly greater than STEAK (at 20, 40, and 60 min) and MINCED (at 20 and 40 min) (Fig. 1c). Plasma AA concentrations showed a delayed increase for STEAK and MINCED, thus, being significantly greater compared to HMP at 150, 180, 240, and 300 min. At 300 min, the plasma AA concentrations were greater for STEAK compared to MINCED (Fig. 1c). The peak time was faster in HMP (40 min) compared to STEAK and MINCED (both 180 min) (Table 3). However, there were no difference in plasma AA peak concentrations between STEAK, MINCED, and HMP (Table 3). Similar patterns as for total AA were seen in individual plasma concentrations of aspartic acid, serine, asparagine, histidine, threonine, proline, arginine, tyrosine, valine, methionine, isoleucine, leucine, tryptophan, and lysine (Supplementary table 2).
Plasma EAA concentrations increased immediately after intake of the meal with HMP, with significantly greater concentrations compared to STEAK and MINCED at 20, 40, and 60 min (Fig. 1d). Plasma EAA concentrations increased later flowing meal with STEAK or MINCED being significantly greater compared to HMP at 150, 180, 240, and 300 min. At 300 min, the plasma EAA concentrations were greater for STEAK compared to MINCED. The peak time was faster in HMP (40 min) compared to STEAK and MINCED (both 180 min) (Table 3). However, there were no difference in plasma EAA peak concentrations between STEAK, MINCED, and HMP (Table 3). A similar pattern in plasma BCAA concentrations were seen (Supplementary table 2).
Plasma insulin
An immediate increase in plasma insulin concentration was seen after the meal intake, but at no time points were any differences between the three trials seen (Fig. 2). The change over time in the postprandial period was analyzed by a Dunnett’s post hoc test to describe at which time points the insulin concentration was elevated above basal fasting levels. This showed that at all postprandial time points, except for 240 and 300 min, the insulin concentrations were significantly elevated above −30 min (all time points, p < 0.001).
Plasma insulin concentrations for steak beef (STEAK), minced beef (MINCED), and hydrolyzed meat-protein (HMP) trials, respectively, shown at basal fasting levels (−30 min) and 300 min into the postprandial period after the meal was finished at time point 0 min. *denote different from basal levels at −30 min. Data are shown as mean ± SEM
Discussion
The present study explored how the structure of meat affects the AA appearance into the circulation when ingested together with a mixed meal. The application of intrinsically [2H5]phenylalanine meat allowed for assessing changes in plasma enrichments, which reflects the digestion and absorption kinetics of the meat products. By a randomized crossover study, it is shown that hydrolyzed meat was much more rapidly absorbed leading to peaking plasma AA concentrations immediately after intake, whereas AA from steak and minced meat is more slowly absorbed as hypothesized. However, in contrary to our hypothesis, similar peak AA concentrations were reached. Previous study by Pennings and colleagues has shown that ingestion of minced meat compared to beef steak was followed by a more rapid protein digestion and amino acid absorption (Pennings et al. 2013). However, these temporal differences between minced meat and beef steak were, although present, very small. The present study extends with an even easier digested protein structure the hydrolyzed meat, in a realistic setting of a mixed meal based on whole foods.
The level of plasma EAA concentrations in the postprandial phase is known to positively affect the rate of muscle protein synthesis (Bohé et al. 2003; Pennings et al. 2011a). The present study was designed to solely explore the AA absorption into the circulation when altering the meat structure in a mixed meal, without measuring protein turnover. Peak concentrations of plasma EAA were identical for all types of meat. Thus, it is possible that protein synthesis rates across the 5-h postprandial phases were equal, irrespective of protein structure. At least from studies on whey and casein, fast and slow-absorbed dietary proteins, respectively, have shown that although whey is much faster absorbed, the protein synthesis throughout the entire postprandial period is similar between whey and casein (Reitelseder et al. 2011). Furthermore, in a study comparing ingestion of beef vs. whey, which is normally thought to give an immediate increase in protein synthesis, the 5-h postprandial muscle protein synthesis rate was similar (Burd et al. 2015), but in the initial 2-h period, the faster absorbed whey protein induced a greater synthesis rate. Likewise from previous studies on milk proteins, it is known that a fast peak in circulating AA concentrations results in a quick stimulation of MPS (Burd et al. 2012). In the present study, the protein structure resulted in a clear difference in time to peak concentrations between HMP vs. STEAK and MINCED. Therefore, it could be speculated that a temporal shift in protein synthesis response between HMP vs. STEAK and MINCED would be seen.
During synthesis or uptake of AA into cells, both the [2H5]phenylalanine and unlabeled phenylalanine is removed from the circulation. Thus, the cellular AA uptake does not affect the plasma enrichment of [2H5]phenylalanine. If plasma [2H5]phenylalanine enrichment is dropping, it is caused by unlabeled phenylalanine being released into circulation from protein degradation within the cells, as no subsequent meal intake is applied in the present study, which could also affect the [2H5]phenylalanine enrichment. The drop in [2H5]phenylalanine in HMP from 180 min and onwards could therefore be caused by either an increase in protein degradation (Fig. 1a) or that the meat phenylalanine is taken up, thereby decreasing exogenous rate of appearance of phenylalanine from the meat. STEAK and MINCED [2H5]phenylalanine enrichments are peaking at 180 min, thereby indicating a slower AA absorption compared to HMP peaking at 75 min (Table 3). Most likely, the difference in peak time is caused by a need for enzymatic degradation of the proteins and peptides within the STEAK and MINCED meat products, whereby AA are released into the circulation at a slower rate initially after the meal intake. However, the peak enrichment in the circulation of [2H5]phenylalanine is greater in STEAK and MINCED compared to HMP (Table 3). Thus, a lesser plasma [2H5]phenylalanine dilution by unlabeled phenylalanine occurs. It can be speculated that this is caused by a greater suppression of protein degradation after intake of STEAK and MINCED meat. However, total and thereby also endogenous phenylalanine rate of appearance was not measured in the current study, so no specific conclusion can be drawn in this regard.
Ingesting other macronutrients together with dietary protein does influence the AA absorption rate (Reitelseder et al. 2020). Therefore, the meat products in the present study were ingested in a mixed meal consisting of approximately 22% protein, 59% carbohydrates, and 19% fat, as the application of hydrolyzed meat protein could be a source for protein enrichment of food products, and not merely as a standalone supplement. Although HMP shows to be fast absorbed immediately after intake compared to STEAK and MINCED, it is doubtful that HMP would result in a greater protein synthesis and improved protein turnover net-balance throughout the whole postprandial period, as explained previously. Notably, HMP with the umami taste as a meat product can be easily masked in existing meat or food products. Therefore, HMP could be an additive to existing slow-absorbed food products, to induce an early rise in plasma EAA concentration that is then maintained by other sources throughout the whole postprandial period. However, future proof-of-concept studies would be needed to clarify such effect.
Importantly, the present study was conducted as a crossover design allowing the AA absorption kinetics of STEAK, MINCED, and HMP to be compared within the same individuals. Any difference in digestion and absorption between subjects would thereby to a lesser degree affect the outcome measure of AA appearing in the circulation after distinct dietary protein products. Moreover, the order of meat source for the three trials was randomized in order not to introduce any systematic error. With the application of an intrinsically stable isotope tracer ([2H5]phenylalanine) in a crossover design, a washout period is needed between trials as it is known that tracer amino acids are recycling (Holm et al. 2019). In the present study, we report that the baseline [2H5]phenylalanine had a very low variance, indicating that baseline measurements were similar, irrespective of the trial order. Thus, the washout period of 1 week between trials appeared sufficient (Fig. 1a).
Conclusion
Ingestion of a mixed meal with HMP as the meat-protein source results in a much more rapid AA appearance into circulation compared to STEAK and MINCED meat. Although a much more rapid peak concentration of plasma EAA concentration was reached in HMP, the peak EAA concentrations were identical for HMP, STEAK, and MINCED.
References
Baumgartner RN, Koehler KM, Gallagher D et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763. https://doi.org/10.1186/1471-2318-7-5
Bax M-L, Buffière C, Hafnaoui N et al (2013) Effects of meat cooking, and of ingested amount, on protein digestion speed and entry of residual proteins into the colon: a study in minipigs. PLoS ONE 8:e61252. https://doi.org/10.1371/journal.pone.0061252
Bendtsen LQ, Thorning TK, Reitelseder S et al (2019) Human muscle protein synthesis rates after intake of hydrolyzed porcine-derived and cows’ milk whey proteins-a randomized controlled trial. Nutrients 11:406. https://doi.org/10.3390/nu11050989
Bohé J, Low A, Wolfe RR, Rennie MJ (2003) Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol 552:315–324. https://doi.org/10.1113/jphysiol.2003.050674
Boirie Y, Dangin M, Gachon P et al (1997) Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci 94:14930–14935. https://doi.org/10.1073/pnas.94.26.14930
Bornø A, van Hall G (2014) Quantitative amino acid profiling and stable isotopically labeled amino acid tracer enrichment used for in vivo human systemic and tissue kinetics measurements. J Chromatogr B Analyt Technol Biomed Life Sci 951–952:69–77. https://doi.org/10.1016/j.jchromb.2014.01.019
Buffière C, Gaudichon C, Hafnaoui N, et al (2017) In the elderly, meat protein assimilation from rare meat is lower than that from meat that is well done. Am J Clin Nutr Nov 106:1257–1266. https://doi.org/10.3945/ajcn.117.158113
Burd NA, Yang Y, Moore DR et al (2012) Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr 108:958–962. https://doi.org/10.1017/S0007114511006271
Burd NA, Gorissen SH, van Vliet S et al (2015) Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am J Clin Nutr 102:828–836. https://doi.org/10.3945/ajcn.114.103184
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423. https://doi.org/10.1093/ageing/afq034
Dangin M, Boirie Y, Garcia-Rodenas C et al (2001) The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 280:340–348
Deutz NEP, Bauer JM, Barazzoni R et al (2014) Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 33:929–936. https://doi.org/10.1016/j.clnu.2014.04.007
Fielding RA, Vellas B, Evans WJ et al (2011) Sarcopenia: an undiagnosed condition in older adults. current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12:249–256. https://doi.org/10.1016/j.jamda.2011.01.003
Gwin JA, Church DD, Wolfe RR et al (2020) Muscle protein synthesis and whole-body protein turnover responses to ingesting essential amino acids, intact protein, and protein-containing mixed meals with considerations for energy deficit. Nutrients 12:1–15. https://doi.org/10.3390/nu12082457
He T, Giuseppin MLF (2014) Slow and fast dietary proteins differentially modulate postprandial metabolism. Int J Food Sci Nutr 65:386–390. https://doi.org/10.3109/09637486.2013.866639
Holm L, Dideriksen K, Nielsen RH et al (2019) An exploration of the methods to determine the protein-specific synthesis and breakdown rates in vivo in humans. Physiol Rep 7:1–14. https://doi.org/10.14814/phy2.14143
Houston DK, Nicklas BJ, Ding J et al (2008) Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the health, aging, and body composition (Health ABC) Study. Am J Clin Nutr 87:150–155. https://doi.org/10.1093/ajcn/87.1.150
Janssen I, Heymsfield SB, Wang ZM, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89:81–88. https://doi.org/10.1152/jappl.2000.89.1.81
Morley JE, Abbatecola AM, Argiles JM et al (2011) Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 12:403–409. https://doi.org/10.1016/j.jamda.2011.04.014
Nordic Council of Ministers (2008) Nordic nutrition recommendations 2012. Nord Nutr Recomm 2012(5):1–3. https://doi.org/10.6027/Nord2014-002
Pedersen AN, Cederholm T (2014) Health effects of protein intake in healthy elderly populations: a systematic literature review. Food Nutr Res 58:1–39. https://doi.org/10.3402/fnr.v58.23364
Pennings B, Boirie Y, Senden JMG et al (2011a) Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr 93:997–1005. https://doi.org/10.3945/ajcn.110.008102
Pennings B, Pellikaan WF, Senden JMG et al (2011b) The production of intrinsically labeled milk and meat protein is feasible and provides functional tools for human nutrition research. J Dairy Sci 94:4366–4373. https://doi.org/10.3168/jds.2011-4451
Pennings B, Groen BBL, van Dijk J-W et al (2013) Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am J Clin Nutr 98:121–128. https://doi.org/10.3945/ajcn.112.051201
Reitelseder S, Agergaard J, Doessing S et al (2011) Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab 300:E231–E242. https://doi.org/10.1152/ajpendo.00513.2010
Reitelseder S, Tranberg B, Agergaard J et al (2020) Phenylalanine stable isotope tracer labeling of cow milk and meat and human experimental applications to study dietary protein-derived amino acid availability. Clin Nutr 39:3652–3662. https://doi.org/10.1016/j.clnu.2020.03.017
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S–991S
Acknowledgements
Thanks to the subjects volunteering for the study, and thanks to Pia Søderberg and Maria Bækgaard Kjær for administrative work on the project.
Funding
The Danish Innovation Fund and Danish Crown Ingredients A/S have funded the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
At the time of the experiments, JA and EH were employed at the Danish Crown Ingredients A/S.
Informed consent
This research involved human participants, who were carefully informed of the procedures, risks, benefits of the study and that data would be published. The participants signed an informed consent approved by the Research Ethics Committee Region Hovedstaden (H-17021471, September 12, 2017) before participation.
Additional information
Handling editor: G. Wu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agergaard, J., Hansen, E.T., van Hall, G. et al. Postprandial amino acid availability after intake of intact or hydrolyzed meat protein in a mixed meal in healthy elderly subjects: a randomized, single blind crossover trial. Amino Acids 53, 951–959 (2021). https://doi.org/10.1007/s00726-021-03000-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-021-03000-z