Abstract
Increased intracellular cysteine poses a potential danger to cells due to the high ability of cysteine to reduce free iron and promote the Fenton reaction. Here, we studied ways to maintain cysteine homeostasis in E. coli cells while inhibiting protein synthesis with valine or chloramphenicol. When growing wild-type bacteria on minimal medium with sulfate, an excess of cysteine resulting from the inhibition of protein synthesis is mainly incorporated into glutathione (up to 90%), which, therefore, can be considered as cysteine buffer. The share of hydrogen sulfide, which is the product of cysteine degradation by cysteine synthase B (CysM), does not exceed 1–3%, the rest falls on free cysteine, exported from cells. As a result, intracellular free cysteine is maintained at a low level (about 0.1 mM). The lack of glutathione in the gshA mutant increases H2S production and excretion of cysteine and leads to a threefold increase in the level of intracellular cysteine in response to valine and chloramphenicol. The relA mutants, exposed to valine, produce more H2S, dramatically accelerate the export of glutathione and accumulate more cysteine in the cytoplasm than their parent, which indicates that the regulatory nucleotide (p)ppGpp is involved in maintaining cysteine homeostasis. Disruption of cysteine homeostasis in gshA and relA mutants increases their sensitivity to peroxide stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
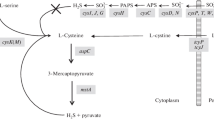
In living cells, l-cysteine is used for protein and glutathione (GSH) synthesis and serves as the primary source of reduced sulfur for many other organic molecules. The high redox activity of sulfhydryl groups of cysteine residues in these molecules plays an important role in protein conformation changes, enzyme activity, redox signaling, and other metabolic processes. However, due to its high ability to reduce intracellular iron, free cysteine can promote the Fenton reaction in the presence of H2O2 to form toxic hydroxyl radicals that cause oxidative damage to DNA and different cellular structures (Park and Imlay 2003; Imlay et al. 2015). Furthermore, even subtoxic cysteine concentrations can inhibit several enzymes of amino acid synthesis and impair bacterial growth (Reitzer 2005). To prevent these negative phenomena, cells should exercise strict regulatory control and maintain low levels of free intracellular cysteine (0.1–0.2 mM) (Park and Imlay 2003). Intracellular levels of cysteine in E. coli are controlled by synthesis and possibly transport and degradation. The expression of genes of the cysteine regulon, which comprises the genes for biosynthesis of the amino acid from l-serine and those for the uptake and reduction of oxidized sulfur sources, is under the control of the transcriptional activator CysB (Kredich 1992, 2008). Under sulfate-limiting conditions, CysB requires an inducer for activity. Earlier reports suggested that a signal sensed by CysB is N-acetyl-serine (NAS), which is spontaneously produced from O-acetyl-serine (OAS) (Kredich 2008). Recent studies, however, have shown that under physiological conditions that trigger the expression of the oatA gene, OatA N-acetyltransferase synthesizes N,O-diacetyl-serine (DAS) from OAS, and that DAS increases the expression of the cys regulon (VanDrisse and Escalante-Semerena 2018). In addition, sulfide and thiosulfate act as anti-inducers of CysB (Kredich 1992, 2008). Cysteine also down-regulates its own synthesis by inhibiting the activity of serine transacetylase, and therefore, cysteine biosynthesis ceases almost entirely when cells are grown on cysteine or cystine. Although several systems for cysteine export (EamA, EamB, Bcr, CydDC) and cysteine degradation (TnaA, CysK, CysM, MalY, MetC, SseA, IscS, CyuA) are known in E. coli (Daẞler et al. 2000; Pittman et al. 2002; Awano et al. 2005; Shimada et al. 2016; Nonaka and Takumi 2017; Loddeke et al. 2017), the role of these processes in maintaining cysteine homeostasis remains poorly understood. TnaA, CysK, CysM, MetC, MalY, and CyuA can degrade cysteine to sulfide and 2-aminoacrilate, which further decomposes to pyruvate and ammonia. SseA can cleave 3-mercaptopyruvate, which is a product of cysteine deamination by aspartate transaminase, to sulfide and pyruvate (Shatalin et al. 2011). Most of these enzymes mediate other primary physiological functions via distinctive enzymatic reactions; therefore, their functional significance in cysteine degradation is controversial. Some cysteine-degrading enzymes and cysteine transporters, including CcdA and CefA in Pantoea ananatis, aerobic CdsH in S. enterica and anaerobic CyuA and CyuP in E. coli and S. enterica, have been shown to be induced by cysteine, mediated by specific transcription regulators and involved in a specific mechanism coupling with cysteine toxicity (Takumi and Nonaka 2016; Nonaka and Takumi 2017; Loddeke et al. 2017).
It is assumed that the functions of many of cysteine transporters and cysteine-degrading enzymes only emerge under certain stress conditions or in situations, where an excess of intracellular cysteine may occur. The addition of cystine to E. coli grown on sulfate has been shown to cause uncontrolled import of cystine, excessive accumulation of cysteine within cells, followed by export of cysteine and release of sulfide into the medium (Imlay et al. 2015; Korshunov et al. 2016). Cysteine excretion was also observed in E. coli cells that overproduced the cysteine efflux pump (Daẞler et al. 2000) or lacked cysteine desulfhydrases and desulfidases (Awano et al. 2005; Nonaka and Takumi 2017). It has been proposed that the hydrogen peroxide-inducible l-cysteine/l-cystine shuttle system is involved in the detoxification of H2O2 in the periplasm (Ohtsu et al. 2010). In LB medium, the addition of cysteine or hydrogen peroxide to E. coli cells stimulated H2S production, which was essential for antibiotic and oxidative stress tolerance (Shatalin et al. 2011; Mironov et al. 2017). Recently, we showed that sulfate-grown E. coli produces H2S under growth cessation caused by glucose exhaustion, amino acid starvation, or exposure to several antibiotics, including chloramphenicol, tetracycline, and high doses of ciprofloxacin (Tyulenev et al. 2018). Simultaneously, changes in cysteine and GSH levels in starving cells were detected. We supposed that all these changes are associated with the need to maintain cysteine homeostasis under conditions when protein synthesis abruptly stops. Here, we studied in detail the changes in the concentrations of intracellular and extracellular cysteine and glutathione, as well as the production of hydrogen sulfide in the inhibition of protein synthesis by valine or chloramphenicol. We also analyzed the role of the regulatory nucleotide guanosine-(penta) tetraphosphate (p)ppGpp in these processes, which is a key player in the metabolic reprogramming of bacteria under various stresses (Hauryliuk et al. 2015). Our study presents the first comprehensive analysis of the different ways used by E. coli to maintain cysteine homeostasis under stress conditions that inhibit protein synthesis.
Materials and methods
Bacterial strains and growth conditions
A parental strain of Escherichia coli BW25113 (hereafter referred as wild type or wt) and single-knockout mutants used in this study were from the Keio collection (Baba et al. 2006) (Table S1). AN2343 (cydD1) was a generous gift from Prof. R. Poole (Cruz-Ramos et al. 2004). The double mutant NM4861 (ΔrelAΔgshA) was constructed by P1 transduction using Keio strains JW2663 (ΔgshA) and JW2755 (ΔrelA).
Bacteria were grown in M9 minimal medium with glucose (8.5 mM) (Miller 1972). To adapt E. coli to aerobic conditions, cells grown overnight were precultured in 100 ml of fresh medium at 37 °C in 250-ml flasks with shaking at 150 rpm from an initial optical density at 600 nm (hereafter referred as OD600) of 0.1 to OD600 of 0.5. Then cultures were diluted with prewarmed fresh medium to OD600 of 0.1 and were further grown under the same conditions to OD600 of 0.4. Thereafter, 4.3 mM valine or 77 µM chloramphenicol was added to stop protein synthesis, and growth was monitored for 2 h. The specific growth rate (µ) was calculated by equation µ = Δln OD600/Δt, where t is the time in hours.
Real-time monitoring of dissolved oxygen, Eh, and extracellular sulfide (S2−)
Dissolved oxygen (dO2) in E. coli cultures was continuously measured directly in the flasks using a Clarke oxygen electrode InPro 6800 (Mettler Toledo). The dO2/pH controller of a BioFlo 110 fermentor (New Brunswick Scientific Co., USA) was used for data recording.
Redox potential (Eh) in E. coli cultures was continuously measured directly in the flasks using platinum and reference electrodes and Mettler Toledo SevenCompact™ pH/Ionmeters S220.
Extracellular sulfide was also detected directly in the flasks using the system of sulfide-specific ion-selective XC-S2−-001 (Sensor Systems Company, Russia) and reference electrodes and a computer pH/ion meter cpX-2 (IBI Pushchino, Russia). The sulfide concentration in the medium was calculated using a standard curve prepared with known amounts of Na2S.
Determination of H2S, sulfite, cysteine, and glutathione
The formation of gaseous H2S was estimated using lead acetate [Pb(Ac)2], which reacts specifically with H2S to form a brown lead sulfide stain. Lead acetate–soaked paper strips were affixed in culture flasks above the level of the liquid culture. These strips were successively replaced every 15 min, which made it possible to monitor the dynamics of H2S release. Spots were photographed and quantified using ImageJ. The mean value of the histogram of color intensity of each paper strip was measured. Data were presented as the ratio of the value before stress exposure to the values obtained at appropriate intervals during exposure, expressed as a percentage. All experiments were performed 3–6 times on separate days.
Extracellular sulfite was assayed in 2.5-ml samples taken at intervals by rapid filtration through 0.45 μm-pore-size membrane filters (Vladipor, Russia). The filtrates were then mixed with 400 µl of freshly prepared fuchsin reagent, incubated for 15 min at room temperature, and measured at OD580 (Leinweber and Monty 1987; Bastiat et al. 2012). The results were quantified using a calibration curve with a lower detection limit about 1 µM.
For the determination of intracellular l-cysteine, 40 ml samples of culture were harvested by centrifugation (8000×g for 5 min) at different timepoints, suspended in 4 ml of cold 20 mM EDTA, and lysed by sonification at 0 °C, using a 30 s pulse for six cycles. Perchloric acid (the final concentration 0.5 mM) was added to the lysate to precipitate proteins. After 30 min, the suspension was centrifuged (8000×g for 5 min), and supernatant was adjusted to pH 8.5 with KOH, frozen, centrifuged to eliminate the potassium perchlorate, and evaporated using a rotary evaporator RV 10 (IKA, Germany) at 65 °C to 0.5 ml and then treated with 0.25 ml of dithiothreitol (100 mM) for 10 min.
Extracellular l-cysteine was determined in 40-ml samples taken by rapid filtration through 0.45 μm-pore-size filters. The filtrates were concentrated to 1 ml using a rotary evaporator and then treated as described above to precipitate protein and reduce cystine to cysteine. The amount of l-cysteine in reduced supernatants was determined according to the method of Gaitonde (1967). The method is based on a specific reaction of the acid ninhydrin reagent with cysteine to form a pink product (Emax 560 nm). This method is highly sensitive, allows rapid and qualitative measurements of cysteine in the presence of other amino acids and glutathione, and is often used to determine cysteine in bacterial supernatants (Daẞler et al. 2000; Ohtsu et al. 2010). Standard curves were prepared with known amounts of cysteine, which were treated as samples of cell suspensions.
Extracellular reduced (GSH) and oxidized (GSSG) glutathione was determined in samples, which were removed at 15-min intervals by rapid filtration through 0.45 μm-pore-size filters. One portion of this filtrate was assayed for total glutathione, the other portion after treatment with 2 mM N-ethylmaleimide for 1 h and sevenfold ether extraction to remove GSH was used for the measurement of GSSG.
For the determination of intracellular glutathione, 10 ml samples of cell culture were harvested by centrifugation (8000×g for 5 min) at different timepoints and prepared as described previously (Smirnova et al. 2012). GSH was measured using the DTNB-glutathione reductase recycling method (Tietze 1969) modified as described previously (Smirnova et al. 2012). Due to the presence of glutathione reductase in the incubation medium, the method is highly specific and sensitive and allows determining total glutathione directly in the filtrates of the medium without their prior processing.
Study of cell viability, membrane potential, and accumulation of extracellular H2O2
For colony-forming studies, culture samples were removed before and at 30-min intervals after the addition of valine, chloramphenicol or H2O2, washed, serially diluted in 0.9% NaCl, mixed with molten soft LB-agar (0.8%) at 42 °C, and poured onto plates with solid LB-agar (1.5%). Colonies were counted over 24-h incubation at 37 °C.
Changes in the membrane potential (∆ψ) were evaluated using ∆ψ-sensitive fluorescent dye DiBAC4(3) (Wickens et al. 2000) as described elsewhere (Smirnova et al. 2015). Samples of log-phase cells treated with protonophore carbonylcyanide m-chlorophenylhydrasone (CCCP, 20 µM) were used as positive control. Fluorescent cells were counted using a Leica DM2000 microscope as earlier described (Smirnova et al. 2015). Total cell number was counted in transmitted light. About 1000 cells were counted for every sample and all experiments were conducted 3–6 times on separate days.
For H2O2 determination, 2-ml aliquots of culture were removed at intervals, passed through membrane filters and H2O2 was measured by an Amplex Red-horseradish peroxidase detecting system (AR/HRP) (Seaver and Imlay 2001) using a Shimadzu RF-1501 spectrofluorimeter (λex 563 nm and λem 587 nm).
Statistical analysis of the data
Each result is indicated as the mean value of three to six independent experiments ± the standard error of the mean (SEM). Significant difference was analyzed by Student’s t test. A P value of 0.05 was used as the cutoff for statistical significance. Results were analyzed by means of the program packet Statistica 6 (StatSoft Inc. 2001).
Results
Inhibition of protein synthesis leads to the release of H2S from E. coli cells
To inhibit protein synthesis, two conventional approaches were used: amino acid starvation and chloramphenicol addition. Amino acid starvation was induced by the addition of valine, the excess of which is known to inhibit isoleucine biosynthesis in E. coli K-12 strains (Traxler et al. 2008). Chloramphenicol binds to the 50S ribosomal subunit, thereby directly interfering with the binding of aminoacyl tRNA to the peptidyltransferase domain and preventing protein chain elongation. In these situations, opposite changes in (p)ppGpp have been reported. During amino acid starvation, RelA ((p)ppGpp synthetase I), a ribosome-associated protein, directly inspects the aminoacylation status of the incoming tRNA molecule in the ribosomal A-site (Brown et al. 2016; Loveland et al. 2016) and, upon recognition of deacylated tRNA, dramatically activates (p)ppGpp production, which peaks within the time scale of a few minutes (Lagosky and Chang 1980; Tian et al. 2016). ΔrelA mutants exhibit a “relaxed” phenotype and cannot elevate (p)ppGpp in response to valine (Lagosky and Chang 1980). In contrast to amino acid starvation, the addition of chloramphenicol strongly reduces cellular level of (p)ppGpp (Boehm et al. 2009).
In minimal M9 medium with glucose, exponentially growing wild-type cells and mutants ΔrelA, ΔgshA, and ΔrelAgshA showed specific growth rates (μmax) 0.65 ± 0.01, 0.72 ± 0.01, 0.70 ± 0.01, and 0.72 ± 0.01 h−1, respectively. Valine addition rapidly reduced growth to 0.16 h−1 in the parent and to 0.12 h−1 in gshA cells, in contrast to the slower μ decrease in the relA and relAgshA mutants (Fig. 1a). Chloramphenicol caused a rapid decrease in growth below 0.1 h−1, regardless of the strain tested (Fig. 1b). In accordance with our earlier data (Smirnova et al. 2018), the addition of valine to wild type and gshA cells was accompanied by a sharp increase in dO2 due to inhibition of respiration (Fig. 1c). This sharp phase of dO2 increase was absent in the relA and relAgshA cells after valine treatment (Fig. 1c). After the addition of chloramphenicol, a more gradual increase in dO2 was observed than with valine, and there were no differences between all the strains studied (Fig. 1d).
Changes in the specific growth rates (a, b) and dissolved oxygen (c, d) in E. coli exposed to valine (a, c) or chloramphenicol (b, d). E. coli BW25113 (wt), JW2663 (ΔgshA), JW2755 (ΔrelA), and NM4861 (ΔrelAΔgshA) were grown in M9 medium with glucose to OD600 of 0.4, and then valine (4.3 mM) or chloramphenicol (77 μM) was added. The arrow indicates the time when valine (Val) or chloramphenicol (Cam) was added. a, b Values are the means and standard error (vertical bars) from at least three independent experiments. c, d Each experiment was repeated at least three times. The data shown are representative
Real-time monitoring of redox potential (hereafter referred as Eh) directly in the flasks revealed a sharp reversible drop in Eh immediately after the addition of valine or chloramphenicol (Fig. 2a, b). Recently, we showed that these changes in Eh are caused by an increase in the concentration of sulfide in the medium (Tyulenev et al. 2018). The release of sulfide from E. coli cells in response to treatment with valine and chloramphenicol was also directly recorded by a sulfide sensor (Fig. 2c, d). The time profiles of rapid reversible changes in the potential of the sulfide sensor were very close to those obtained from the Eh sensor for all strains tested. During isoleucine starvation, the mutations relA and gshA significantly increased both the amplitudes of these changes and the time required for the potential to return to the basal level, compared to those of the parent (Fig. 2c). The average amplitudes were 15.5 ± 1.2 mV, 34.5 ± 2 mV, 38.6 ± 2.4 mV, and 66 ± 2 mV for the wild type, gshA, relA, and gshArelA strains, respectively, which corresponded to sulfide concentrations in the medium of 42 ± 5 nM, 120 ± 8 nM, 145 ± 5 nM, and 520 ± 50 nM. Treatment with chloramphenicol increased the amplitude of changes in the potential of the sulfide sensor in the wild type and gshA strains by about 2 times compared with those with valine (Fig. 2d). However, unlike valine, the relA mutation did not cause significant additional sulfide formation when exposed to chloramphenicol. The average amplitudes of changes in sulfide sensor potential were 43 ± 4 mV, 67.5 ± 2.5 mV, 46 ± 2 mV, and 75 ± 2 mV for the wild-type, gshA, relA, and gshArelA strains, respectively, which corresponded to sulfide concentrations of 180 ± 20 nM, 550 ± 50 nM, 205 ± 10 nM, and 680 ± 40 nM (Fig. 2d). Values of maximum sulfide production per biomass unit (OD600) are shown in Fig. 2e. The data obtained using a sulfide sensor were confirmed by determining H2S in the gas phase using Pb(Ac)2 strips (Fig. 3, Fig. 1S). The results obtained by these two methods were similar, although the sulfide sensor was more sensitive and made it possible to record small changes in sulfide caused by the presence of valine in the wild-type strain that were not detected by the paper strip method. In all cases, the maximum sulfide production was observed within 10–15 min after the addition of valine or chloramphenicol, and then, the sulfide concentration decreased both in the liquid and gas phases, which indicates the cessation of its production and partial absorption by the cells. Together, our results show that the normal stringent response and the ability to synthesize GSH significantly limit the formation of H2S induced by inhibition of protein synthesis.
Inhibition of protein synthesis caused by valine or chloramphenicol leads to a sharp reversible reduction in the potential of the platinum (Eh) electrode (a, b) and sulfide sensor (c, d) due to an increase in sulfide production by E. coli cells. E. coli BW25113 (wt), JW2663 (ΔgshA), JW2755 (ΔrelA), and NM4861 (ΔrelAΔgshA) were grown in M9 medium with glucose to OD600 of 0.4, and then valine (4.3 mM) (a, c) or chloramphenicol (77 µM) (b, d) was added. Representative changes in the potential of the Eh and sulfide sensors are shown. The arrow indicates the time when valine (Val) or chloramphenicol (Cam) was added. e The maximum concentrations of sulfide accumulated in the medium of various E. coli strains are shown. Values are the means and standard error (vertical bars) from at least three independent experiments. Statistical differences compared to the wild-type strain (P < 0.05) are noted with asterisk
Addition of valine (a) and chloramphenicol (b) leads to a reversible increase in the concentration of H2S in the gas phase, which depends on the gshA and relA mutations. E. coli BW25113 (wt), JW2663 (ΔgshA), JW2755 (ΔrelA), and NM4861 (ΔrelAΔgshA) were grown in M9 medium with glucose to OD600 of 0.4, and then valine (4.3 mM) or chloramphenicol (77 µM) was added. The arrow indicates the time when valine (Val) or chloramphenicol (Cam) was added. H2S was estimated using lead acetate–soaked paper strips. All the strains studied did not produce H2S during growth without treatments (control curve). Results are expressed as a percentage compared to the value before stress, as described in “Materials and methods”. Values are the means and standard error (vertical bars) from 3 to 6 independent experiments. *Indicates P ≤ 0.05, **indicates P < 0.005
Mutants lacking the EamA, EamB, and Bcr cysteine export systems showed increased H2S production in response to valine compared to the parent (Fig. 4a). Sulfide formation in the cydD mutant was close to the parent. The maximum sulfide concentrations reached in the medium were 105 ± 10 nM, 115 ± 25 nM, 240 ± 50 nM, 50 ± 10, and 42 ± 5 nM in eamA, eamB, bcr, cydD and wild-type cultures, respectively. Increased sulfide production in the eamA, eamB, and bcr mutants may be the result of an increase in intracellular cysteine due to a decrease in its export and an acceleration of its degradation. Unlike other strains, the cydD mutant had a reduced growth rate (μ = 0.5 h−1) and, consequently, less steep growth inhibition with valine, which complicates the interpretation of the data and does not allow us to judge the involvement of the CydDC transporter in cysteine export in this situation.
Lack of systems of cysteine export (a) and degradation (b) affects H2S production. E. coli BW25113 (wt), JW5250 (ΔeamA), JW2562 (ΔeamB), JW5363 (Δbcr), JW2414 (ΔcysM), JW2505 (ΔsseA), JW1614 (ΔmalY), JW5518 (ΔcyuA), JW2514 (ΔiscS), JW3686 (ΔtnaA) and AN2343 (cydD1) were grown in M9 medium with glucose to OD600 of 0.4, and then valine (4.3 mM) was added at the moment indicated by the arrow. The data shown are representative
To identify the pathway of cysteine degradation under our conditions, we used mutants that lacked various enzymes, which can exhibit cysteine-degrading activity: 3-mercaptopyruvate sulfurtransferase (SseA), cysteine synthases A and B (CysK and CysM), cystathionine β-lyases A and B (MetC and MalY), cysteine desulfurase (IscS), cysteine desulfidase (CyuA), and tryptophanase (TnaA) (Awano et al. 2005; Shimada et al. 2016; Nonaka and Takumi 2017; Loddeke et al. 2017). Mutants lacking the metC and cysK genes grew very poorly in M9. Other mutants lacking cysteine degradation enzymes (sseA, malY, cyuA, iscS, tnaA) grew at the same rate as the wild-type strain and generated H2S in response to valine (Fig. 4b). The only exception was the cysM mutant, which had a growth rate close to the parent (0.7 h−1), but did not generate H2S when growth was inhibited by valine (Fig. 4b) or chloramphenicol, as well as under the action of ciprofloxacin and with glucose exhaustion (not shown). This indicates that CysM is the main enzyme that is responsible for the production of H2S under our conditions. It should be noted that the mutants malY, cyuA, iscS, and tnaA produced more sulfide when treated with valine than wild-type cells. Further more detailed studies are needed to clarify the causes of this phenomenon.
The first product of sulfate reduction, which precedes H2S, is sulfite (SO32−). Bacterial cells can accumulate endogenous sulfite in the culture medium (Benov et al. 1996; Bastiat et al. 2012). Sulfite levels have been shown to increase when Sinorhizobium meliloti enters the stationary phase (Bastiat et al. 2012). Under our conditions, in exponentially growing E. coli, an increase in OD600 from 0.4 to 0.8 was accompanied by a gradual increase in the concentration of sulfite in the medium from about 1.5 to 3 μM. There were no statistically significant changes in sulfite concentration per OD600 unit 20 min after valine was added, whereas chloramphenicol treatment decreased sulfite levels by 12% and 22% in the relA and gshA mutants, respectively (not shown).
Inhibition of protein synthesis leads to an increase in the intracellular and extracellular pools of glutathione
The main low molecular weight thiol in living cells, glutathione, is found at the millimolar level in the cytoplasm of E. coli and can accumulate in the medium at the micromolar level (Owens and Hartman 1986). The best-studied transporter, which exports GSH from the cytoplasm to periplasm, is the ATP-binding cassette-type transporter CydDC (Pittman et al. 2005). Under our conditions, exponentially growing wild-type E. coli BW25113 contained 4.3 ± 0.4 µM/OD600 (about 5 mM) of intracellular GSH (GSHin), the level of which gradually increased during growth (Fig. 5a). The concentration of extracellular GSH (GSHout) per biomass unit was maintained at about a constant level (1.1 ± 0.06 µM/OD600) (Fig. 5b). In the relA mutant, GSHin and GSHout were 1.2 and 1.4 times lower, respectively, than in the parent (Fig. 5a, b). The cydD mutation did not affect GSHin and slightly reduced (1.2 times) GSHout (Fig. 5a, b).
Inhibition of protein synthesis leads to an increase in the levels of intracellular (a, c) and extracellular (b, d) glutathione. E. coli BW25113 (wt), JW2755 (ΔrelA), and AN2343 (cydD1) were grown in M9 medium with glucose to OD600 of 0.4, and then valine (4.3 mM) (a, b) or chloramphenicol (77 µM) (c, d) was added. Values are the means and standard error (vertical bars) from at least three independent experiments
The addition of valine and chloramphenicol stimulated glutathione accumulation both in the cytoplasm and in the medium in all strains tested (Fig. 5). However, if isoleucine starvation predominantly increased the concentration of intracellular glutathione (Fig. 5a, b), then the exposure to chloramphenicol significantly accelerated the export of GSH from cells to the medium (Fig. 5c, d). An exception was the relA mutant, which, in response to valine, accumulated 4 times more extracellular GSH than the parent (Fig. 5b). In the case of chloramphenicol, there was no difference in the GSHout level between these strains (Fig. 5d). We suggest that increased GSH efflux in relA cells during valine exposure and in all strains after adding chloramphenicol, that is, in situations, where (p)ppGpp cannot increase in response to stress, indicates the involvement of (p)ppGpp in the regulation of GSH export. According to these data, increased (p)ppGpp limits GSH efflux from E. coli cells. The lack of the CydDC exporter of GSH did not prevent the exit of glutathione from cells treated with valine or chloramphenicol (Fig. 5b, d), which indicates that another as yet unknown transporter may be involved in the export of glutathione under these conditions.
Even in the gor mutant, which is devoid of glutathione reductase and, therefore, cannot reduce oxidized glutathione (GSSG) to GSH, most of the intracellular and extracellular glutathione was presented in reduced form (Fig. 6). The addition of valine and chloramphenicol led to changes in GSHin and GSHout, similar to those in wild-type bacteria (Fig. 6a, b). In this mutant, 15 min after valine addition a 1.4 times increase in the intracellular concentration of GSSG (GSSGin) compared to its initial level was observed, which decreased gradually over the next 60 min (Fig. 6c). Chloramphenicol treatment did not cause significant changes in GSSGin, but its level maintained higher than in the untreated control. Extracellular GSSG (GSSGout) almost linearly increased 2.4 and 4.4 times within 90 min after the addition of valine and chloramphenicol, respectively (Fig. 6d). However, despite the increase in the level of oxidized glutathione inside and outside the cell, the GSH/GSSG ratio after these treatments became even higher than in the control culture due to the more rapid increase in reduced glutathione (Fig. 6e, f).
Changes in intracellular and extracellular reduced and oxidized glutathione and the ratio of GSH/GSSG in E. coli JW3467 (Δgor) devoid of glutathione reductase activity after the addition of valine and chloramphenicol. E. coli JW3467 (Δgor) was grown in M9 medium with glucose to OD600 of 0.4, and then valine (4.3 mM) or chloramphenicol (77 µM) was added. a, b Intracellular and extracellular GSH. c, d Intracellular and extracellular GSSG. e, f GSH/GSSG ratio inside and outside the cells. The arrow indicates the time when valine or chloramphenicol was added. Values are the means and standard error (vertical bars) from at least three independent experiments
Inhibition of protein synthesis stimulates cysteine export as a way to maintain cysteine homeostasis
Exponentially growing cultures of the parent and the mutants relA, gshA, and relAgshA showed similar levels of intracellular cysteine (0.13 ± 0.02 µM/OD600, corresponding intracellular concentration to about 100 µM), which remained constant during growth (not shown). In wild-type cells, exposure to valine or chloramphenicol did not cause significant changes in the concentration of free intracellular cysteine (Fig. 7a, b). In the relA, gshA, and relAgshA mutants, the level of intracellular cysteine increased 1.6, 2.9, and 2.5 times, respectively, under the action of valine and 1.4, 2.9, and 2.1 times under the action of chloramphenicol (Fig. 7a, b).
gshA and relA deficiency disrupts the intracellular cysteine homeostasis in E. coli (a, b) and affects the export of cysteine (c) when exposed to valine and chloramphenicol. E. coli BW25113 (wt), JW2663 (ΔgshA), JW2755 (ΔrelA), and NM4861 (ΔrelAΔgshA) were grown in M9 medium with glucose to OD600 of 0.4, and then valine (4.3 mM) (a) or chloramphenicol (77 µM) (b) was added. Values are the means and standard error (vertical bars) from at least three independent experiments
The concentration of extracellular cysteine per biomass unit maintained at a constant level during growth of all strains tested (not shown). The addition of valine accelerated cysteine export, which was more pronounced in the gshA and relAgshA mutants (Fig. 7c). After 1 h of incubation with valine, the extracellular level of cysteine increased 4.4, 4.7, 5.5, and 6.5 times compared to the initial level in the wild-type, relA, relAgshA, and gshA strains, respectively. Chloramphenicol also stimulated the efflux of cysteine from cells, but to a lesser extent than valine (Fig. 7c). Thus, the presence of glutathione is most important for maintaining cysteine homeostasis during the inhibition of protein synthesis when E. coli grows in minimal medium with glucose and sulfate. The lack of relA also disrupts cysteine homeostasis, but to a lesser extent than gshA.
Disruption of cysteine homeostasis is crucial for E. coli survival only at high levels of H2O2
Elevated levels of intracellular cysteine in gshA and relA mutants during the inhibition of protein synthesis can be dangerous for cells because of the ability of cysteine to reduce Fe3+ to Fe2+, thereby contributing to the Fenton reaction between H2O2 and Fe2+, which generates toxic hydroxyl radicals. The question arises whether the observed disturbances in cysteine homeostasis in these mutants can affect their survival under stressful conditions. Analysis of the CFU data did not reveal a significant effect of mutations on the change in the number of CFU in both exponentially growing cultures (control) and 1 h after the addition of valine (Fig. 8a) or chloramphenicol (Fig. 8b). Another indicator of cell viability is their ability to maintain membrane potential. To estimate changes in membrane potential (∆ψ), the ∆ψ-sensitive fluorescent dye DiBAC4(3) was used. Cells stained with DiBAC4 (3) are considered to have lost their membrane potential. Only about 1% of exponentially growing populations of all E. coli strains tested were stained with DiBAC4(3) (Fig. 8c). Samples of log-phase culture treated with protonophore CCCP (positive control) increased the number of fluorescent cells to 54% for 20 min (data not shown). After a 1-h exposure to valine or chloramphenicol, the number of fluorescent cells increased 1.8 and 2.4 times, respectively, but in both cases, the statistical difference between the strains was absent (Fig. 8c). The absence of pronounced negative effects of the disruption of cysteine homeostasis may indicate that under these conditions the Fenton reaction is not accelerated, probably due to the low level of H2O2. Recently, we showed that the addition of valine led to a transient increase in H2O2 accumulation in the medium of wild type and relA cells (Smirnova et al. 2018). This was also true for the gshA mutant when exposed to valine and chloramphenicol (Fig. 8d, e). However, the increase in H2O2 was very low and ranged from 0.1 to 0.3 µM. Apparently, under these conditions, the activity of antioxidant systems in all strains was sufficient to prevent severe damage, although, as shown by our previous studies, the relA mutant had 1.25 times lower total catalase activity and 1.4 times lower expression of the SOS-controlled sulA gene compared to the parent (Smirnova et al. 2018). The gshA mutant showed the same total catalase activity and expression of the katG and sulA genes as the wild-type strain (Smirnova et al. 2017).
Changes in CFU (a, b), membrane potential (c) and H2O2 accumulation (d, e), caused by the addition of valine or chloramphenicol to various E. coli strains. E. coli BW25113 (wt), JW2663 (ΔgshA), JW2755 (ΔrelA), and NM4861 (ΔrelAΔgshA) were grown in M9 medium with glucose to OD600 of 0.4, and then valine (4.3 mM) or chloramphenicol (77 µM) was added. The arrow indicates the time when valine (Val) or chloramphenicol (Cam) was added. Values are the means and standard error (vertical bars) from at least three independent experiments
In the next set of experiments, we examined the effect of the addition of H2O2 on the growth and survival of the parent and mutant strains. The addition of 2 mM H2O2 led to an immediate inhibition of the growth of all tested E. coli strains (Fig. 9a). As in the case of valine and chloramphenicol, H2O2-induced growth inhibition was accompanied by an increase in the formation of H2S, which was recorded by a sulfide sensor (not shown). After the initial fall in the specific growth rate, its gradual recovery was observed, which was significantly delayed in the mutant strains (Fig. 9a). This delay was also expressed in a slower increase in CFU in the mutants compared with the parent (Fig. 9b). To obtain a significant bactericidal effect, higher concentrations of H2O2 were needed. The number of CFU in the parent strain decreased 20 times after the addition of 10 mM H2O2 (Fig. 9c). All the tested mutants were more sensitive to this dose of H2O2 than the parent: CFU decreased in approximately 200, 4200, and 9500 times in the relA, relAgshA, and gshA strains, respectively. A high inverse correlation (r = − 0.98, p = 0.02) was found between the levels of intracellular cysteine during inhibition of protein synthesis and the number of CFU after treatment with 10 mM H2O2. These data indicate that the inability of mutants to maintain a low level of cysteine under stress may contribute to a sharp decrease in survival at high concentrations of H2O2.
Mutants lacking glutathione and (p)ppGpp synthetase RelA are more susceptible to peroxide stress. E. coli BW25113 (wt), JW2663 (ΔgshA), JW2755 (ΔrelA), and NM4861 (ΔrelAΔgshA) were grown in M9 medium with glucose to OD600 of 0.4, and then 2 mM H2O2 (a, b) or 10 mM H2O2 (c, d) were added. d M9 medium was additionally supplemented with 0.2% casamino acids and thiamine (10 µg ml−1). The arrow indicates the time when H2O2 was added. Values are the means and standard error (vertical bars) from at least three independent experiments
Our experiments were carried out in a minimal medium with sulfate as a sole sulfur source. To test whether glutathione is important for the survival of E. coli under peroxide stress in the presence of exogenous cystine, we studied the effect of 10 mM H2O2 in M9 medium supplemented with 0.2% casamino acids (CA) (final concentration about 25 µM cystine). We showed that the sensitivity of wild-type cells to H2O2 increased significantly, and the difference between the parent and gshA mutant was greatly reduced when they grew in the presence of CA (Fig. 9d).
Discussion
Our results show that inhibition of protein synthesis triggers a series of regulatory events aimed at preventing the increase in the level of intracellular cysteine (Fig. 10). The cysteine flux, previously involved in protein synthesis, is redirected to glutathione synthesis, which is limited by cysteine while growing on minimal medium with sulfate as a sole sulfur source (Loewen 1979). As a result, the GSH level grows inside and outside the cells. Part of cysteine, not included in the synthesis of glutathione, is exported from cells to the medium. Simultaneously, the level of the cysteine precursor O-acetylserine (OAS) may be reduced due to the inhibition of serine transacetylase activity by an excess of cysteine (Kredich 1992, 2008) or due to the export of OAS to the medium (Franke et al. 2003). The lack of OAS leads to a decrease in the level of its derivative N,O-diacetyl-serine (DAS), which is an inducer of the transcriptional activator CysB (VanDrisse and Escalante-Semerena 2018). In addition, the lack of O-acetylserine can probably promote the degradation of excess cysteine with the formation of hydrogen sulfide catalyzed by cysteine synthase B (CysM). The resulting H2S is an anti-inducer of the CysB transcriptional activator and contributes to the inhibition of CysB regulon. The maximum generation of hydrogen sulfide is reached after 10 min, while the release of cysteine from cells and a rapid increase in the concentration of intracellular glutathione lasts for 30 min. Apparently, during this period, the transition to a new steady state, characterized by a low rate of cysteine synthesis, is completed. Although at a slower rate, cysteine continues to be incorporated into glutathione over the next hour of observations. As a result of these events, occurring with an abrupt inhibition of protein synthesis, the concentration of free intracellular cysteine in the wild-type strain remains almost unchanged.
Cysteine homeostasis in Escherichia coli under inhibition of protein synthesis. The cysteine flux, previously involved in protein synthesis, is redirected to the synthesis of glutathione, part of which is exported to the medium. The part of cysteine, not included in the synthesis of GSH, is also exported to the medium. An excess of cysteine can inhibit the activity of serine transacetylase, reducing the production of the cysteine precursor O-acetylserine (OAS) and, therefore, the synthesis of cysteine. An excess of cysteine can also degrade to form H2S, which is an anti-inducer of CysB transcription activator and contributes to the inhibition of cysteine biosynthesis. Elevated levels of (p)ppGpp negatively regulate cysteine synthesis and limit the export of GSH
Under our conditions, excess cysteine, which occurs when protein synthesis is inhibited, is mainly incorporated into glutathione (Table 1). In wild-type cells, the proportion of glutathione in newly synthesized cysteine derivatives is over 80% when treated with valine and over 90% when treated with chloramphenicol. The share of hydrogen sulfide does not exceed 1–3%, the rest falls on cysteine, exported from cells. The proportion between the degradation of cysteine with the formation of sulfide, the export of cysteine from cells and its inclusion in glutathione can vary considerably depending on the activity of one or another way of utilization of excess cysteine. Restriction of cysteine export in eamA, eamB, and bcr mutants increased sulfide production. The absence of glutathione in the gshA mutant increased H2S production and the concentration of extracellular cysteine. However, more intensive formation of H2S and efflux of cysteine is insufficient to prevent an increase in its intracellular concentration and disturbance of cysteine homeostasis in this mutant. Prolonged persistence of elevated intracellular levels of cysteine in mutants lacking glutathione may indicate that CysM and cysteine export systems have a relatively low affinity for cysteine. It should be noted that the total amount of newly synthesized cysteine products in the gshA mutant was 3 and 6.5 times lower compared to the parent after exposure to valine and chloramphenicol, respectively (Table 1), which indicates a higher extent of inhibition of the CysB regulon than in the parent strain. Thus, under conditions of bacterial growth on minimal medium with sulfate, glutathione can play the role of cysteine buffer and becomes an important factor in maintaining cysteine homeostasis when its intracellular level rises sharply. At similar reduction potentials, glutathione differs from cysteine by a strikingly diminished ability to restore free iron and the accumulation of glutathione in cells up to 2–4 mM is not accompanied by an increased vulnerability to Fenton chemistry (Park and Imlay 2003). This may be the reason that E. coli employs GSH to maintain cysteine homeostasis, while at the same time storing cysteine for subsequent rapid regrowth when conditions become more favorable. Therefore, the continued incorporation of cysteine into glutathione during the transition period can be beneficial to cells from a physiological point of view.
The use of relA mutants and two methods of inhibiting protein synthesis, which are accompanied by opposite changes in the level of guanosine tetraphosphate, made it possible to identify the role of (p)ppGpp in maintaining cysteine homeostasis. Treatment of the relA mutant with valine or exposure of both the parent and mutant strains to chloramphenicol increased the total production of thiol-containing compounds by 2 times compared with the wild-type strain treated with valine (Table 1). This increase was mainly due to an elevation in the proportion of extracellular glutathione. In addition, relA mutants exhibited an enhanced formation of hydrogen sulfide and increased level of intracellular cysteine during valine treatment. The increase in production of cysteine and its derivatives under conditions of low levels of guanosine tetraphosphate indicates that (p)ppGpp can negatively regulate cysteine biosynthesis. It is known that during periods of amino acid starvation, (p)ppGpp together with the transcription factor DksA interacts with the RNA polymerase (RNAP) and inhibits transcription from the promoters of rRNA and ribosomal protein genes, but it activates transcription from the promoters of amino acid biosynthesis genes (Paul et al. 2005; Potrykus and Cashel 2008; Hauryliuk et al. 2015). However, it has recently been shown that regulation of RNAP activity may be more complex due to the redox sensitivity of DksA and the ability of DksA/DnaJ redox couple to activate transcription of genes involved in the biosynthesis of amino acids in combination with low levels of (p)ppGpp or inhibit transcription of these genes at high concentrations of (p)ppGpp (Kim et al. 2018). These findings probably explain the higher production of cysteine and its derivatives in those our conditions when (p)ppGpp could not increase in response to inhibition of protein synthesis.
Under the same conditions (the action of chloramphenicol or the treatment of relA mutants with valine), a low level of (p)ppGpp can apparently be a reason for accelerated efflux of glutathione from E. coli cells. We suggest that the effect of guanosine tetraphosphate on the export of glutathione may be mediated by its influence on membrane functions. Recently, we have shown that the addition of valine leads to a dramatic RelA-dependent inhibition of respiration, followed by transient perturbations in the membrane potential (Δψ) and K+ fluxes, which were significantly altered in relA mutants (Smirnova et al. 2018). According to our previous data, changes in membrane potential and ATP synthase activity can affect the transmembrane cycling of glutathione (Smirnova et al. 2012). Thus, an increase in the (p)ppGpp level during inhibition of protein synthesis negatively regulates the synthesis of cysteine and limits the export of GSH, which contributes to maintaining the safe form of reduced sulfur inside the cells (Fig. 10).
Mutants lacking RelA or glutathione showed disruption of cysteine homeostasis during inhibition of protein synthesis. This disruption of cysteine homeostasis was not essential for cell survival if the concentrations of other participants in the Fenton reaction (H2O2 and iron) did not exceed the capabilities of antioxidant systems. However, at high concentrations of H2O2, the enhanced level of intracellular cysteine in the gshA and relA mutants significantly increased the vulnerability of cells to oxidative stress and accelerated cell death. It should be noted that hypersensitivity to peroxide stress in the relA mutant may be caused not only by impaired cysteine homeostasis, but also by the reduced activity of the defense systems. An increase in the level of (p)ppGpp has been shown to contribute to the induction of RpoS-controlled katG and katE genes encoding HPI and HPII catalases (Ivanova et al. 1994), as well as DNA repair systems, including the SOS response (Maslowska et al. 2019).
Earlier, it was reported that glutathione in E. coli is dispensable for resistance to H2O2 (Greenberg and Demple 1986). In these experiments, cells were grown in minimal medium with casamino acids. We also observed no significant differences in resistance to hydrogen peroxide between wild-type and gshA cells growing in the presence of CA, due to the increased sensitivity of the wild-type bacteria to H2O2 under these conditions. Concentration of intracellular glutathione in E. coli was shown to increase on addition into the medium of cysteine, cystine, or casamino acids (Loewen 1979). Cysteine-unlimited synthesis of GSH and its high level in cells growing in the presence of CA seem to reduce the ability of GSH to serve as cysteine buffer, thereby increasing cysteine concentration in cells and facilitating the bacterial killing mediated by H2O2.
Depending on the cultivation conditions and the activity of different metabolic pathways, E. coli cells can probably use different enzymes to degrade the excess cysteine. The major desulfhydrase that produced H2S under our conditions was CysM. It was previously reported that in LB medium, 3-mercaptopyruvate sulfurtransferase (3MST or SseA) is the major source of endogenous H2S in E. coli (Mironov et al. 2017), while cysteine desulfidase CyuA is the major anaerobic cysteine-catabolizing enzyme in both E. coli and Salmonella enterica (Loddeke et al. 2017). Cysteine desulfhydrases are common throughout bacterial species which inhabit the colon, including Clostridum, Enterobacter, Klebsiella, Streptococcus, Desulfovibrio, and Tissierella (Barton et al. 2017). The wide distribution of these enzymes suggests the necessity of cysteine homeostasis in various bacteria to prevent cytotoxic effects. In addition, H2S may be an important regulatory signal for intestinal tract bacteria (Barton et al. 2017) and can enhance bacterial tolerance to oxidative stress and some antimicrobials (Shatalin et al. 2011; Mironov et al. 2017; Weikum et al. 2018). We have previously shown that gshA mutants of E. coli are more sensitive to superoxide generated in the system with xanthine and xanthine oxidase, which can be used as a model of oxidative burst of macrophages (Smirnova et al. 2015). This suggests that cysteine homeostasis may be important for the survival of pathogenic bacteria during the attack of professional phagocytes that generate large amounts of superoxide by the NADPH-dependent NOX2 oxidase complex (Fang 2011).
The involvement of (p)ppGpp, along with CysB and other factors, in the regulation of cysteine homeostasis seems to be of great physiological significance, since various stresses are accompanied by inhibition of growth and protein synthesis, during which a transient cysteine overproduction can occur. The combined action of various regulators under stress conditions allows well-coordinated metabolism reprogramming in such a way as to prevent an increase in cysteine levels and to avoid the harmful effects of oxidative stress.
Conclusions
In summary, our study has shown that abrupt inhibition of protein synthesis is accompanied by transient perturbations in cysteine fluxes. In wild-type cells, free intracellular cysteine is maintained at a low level (about 100 µM), mainly due to the incorporation of its excess into glutathione and due to the export of cysteine from cells. A small part of the excess cysteine degrades by CysM with the formation of H2S, the production of which is short term and may serve as a signal to repress the CysB regulon. The regulatory nucleotide (p)ppGpp additionally inhibits the synthesis of cysteine and prevents the export of GSH from cells, thereby ensuring the maintenance of cysteine homeostasis while preserving its storage form within cells. Single and double mutants lacking the gshA and relA genes show disturbed cysteine homeostasis under inhibition of protein synthesis and are more vulnerable to oxidative stress. Cysteine homeostasis plays an important role in stress adaptation and can be considered as a potential target in the development of new antimicrobial drugs.
References
Awano N, Wada M, Mori H, Nakamori S, Takagi H (2005) Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl Environ Microbiol 71:4149–4152. https://doi.org/10.1128/AEM.71.7.4149-4152.2005
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2(2006):0008. https://doi.org/10.1038/msb4100050
Barton LL, Ritz NL, Fauque GD, Lin HC (2017) Sulfur cycling and the intestinal microbiome. Dig Dis Sci 62:2241–2257. https://doi.org/10.1007/s10620-017-4689-5
Bastiat B, Sauviac L, Picheraux C, Rossignol M, Bruand C (2012) Sinorhizobium meliloti sigma factors RpoE1 and RpoE4 are activated in stationary phase in response to sulfite. PLoS One 7:e50768. https://doi.org/10.1371/journal.pone.0050768
Benov L, Kredich NM, Fridovich I (1996) The mechanism of the auxotrophy for sulfur-containing amino acids imposed upon Escherichia coli by superoxide. J Biol Chem 271:21037–21040. https://doi.org/10.1074/jbc.271.35.21037
Boehm A, Steiner S, Zaehringer F, Casanova A, Hamburger F, Ritz D, Keck W, Ackerman M, Schirmer T, Jenal U (2009) Second messenger signaling governs Escherichia coli biofilm induction upon ribosomal stress. Mol Microbiol 72:1500–1516. https://doi.org/10.1111/j1365-2958.2009.06739.x
Brown A, Fernandez IS, Gordiyenko Y, Ramakrishnan V (2016) Ribosome-dependent activation of stringent control. Nature 534:277–280. https://doi.org/10.1038/nature17675
Cruz-Ramos H, Cook GM, Wu G, Cleeter MW, Poole RK (2004) Membrane topology and mutational analysis of Escherichia coli CydDC, an ABC-type cysteine exporter required for cytochrome assembly. Microbiology 150:3415–3427. https://doi.org/10.1099/mic.0.27191-0
Daẞler T, Maier T, Winterhalter C, Böck A (2000) Identification of a major facilitator protein from Escherichia coli involved in efflux of metabolites of the cysteine pathway. Mol Microbiol 36:1101–1112. https://doi.org/10.1046/j.1365-2958.2000.01924.x
Fang FC (2011) Antimicrobial actions of reactive oxygen species. mBio 2:e00141-11. https://doi.org/10.1128/mBio.00141-11
Franke I, Resch A, Daßler T, Maier T, Bӧck A (2003) YfiK from Escherichia coli promotes export of O-acetylserine and cysteine. J Bacteriol 185:1161–1166. https://doi.org/10.1128/JB.185.4.1161-1166.2003
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633. https://doi.org/10.1042/bj1040627
Greenberg JT, Demple B (1986) Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol 168:1026–1029. https://doi.org/10.1128/jb.168.2.1026-1029.1986
Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K (2015) Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol 13:298–309. https://doi.org/10.1038/nrmicro3448
Imlay KRC, Korshunov S, Imlay JA (2015) Physiological roles and adverse effects of the two cystine importers of Escherichia coli. J Bacteriol 197:3629–3644. https://doi.org/10.1128/JB.00277-15
Ivanova A, Miller C, Glinsky G, Eisenstark A (1994) Role of the rpoS(katF) in oxyR independent regulation of hydroperoxidase I in Escherichia coli. Mol Microbiol 12:571–578. https://doi.org/10.1111/j.1365-2958.1994.tb01043.x
Kim J-S, Liu L, Fitzsimmons LF, Wang Y, Crawford MA, Mastrogiovanni M, Trujillo M, Till JKA, Radi R, Dai S, Vázquez-Torres A (2018) DksA–DnaJ redox interactions provide a signal for the activation of bacterial RNA polymerase. Proc Natl Acad Sci USA 115:E11780–E11789. https://doi.org/10.1073/pnas.1813572115
Korshunov S, Imlay KRC, Imlay JA (2016) The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol Microbiol 101:62–77. https://doi.org/10.1111/mmi.13372
Kredich NM (1992) The molecular basis for positive regulation of cys promoters in Salmonella typhimurium and Escherichia coli. Mol Microbiol 6:2747–2753. https://doi.org/10.1111/j.1365-2958.1992.tb01453.x
Kredich N (2008) Biosynthesis of cysteine. EcoSal Plus. https://doi.org/10.1128/ecosalplus.3.6.1.11
Lagosky PA, Chang FN (1980) Influence of amino acid starvation on guanosine 5′-diphosphate 3′-diphosphate basal-level synthesis in Escherichia coli. J Bacteriol 144:499–508
Leinweber FJ, Monty KJ (1987) Sulfite determination: fuchsin method. Methods Enzymol 143:15–17. https://doi.org/10.1016/0076-6879(87)43006-1
Loddeke M, Schneider B, Oguri T, Mehta I, Xuan Z, Reitzer L (2017) Anaerobic cysteine degradation and potential metabolic coordination in Salmonella enterica and Escherichia coli. J Bacteriol 199(16):e00117-17. https://doi.org/10.1128/JB.00117-17
Loewen PC (1979) Levels of glutathione in Escherichia coli. Can J Biochem 57:107–111. https://doi.org/10.1139/o79-013
Loveland AB, Bah E, Madireddy R, Zhang Y, Brilot AF, Grigorieff N, Korostelev AA (2016) Ribosome *RelA structures reveal the mechanism of stringent response activation. eLife 5:e17029. https://doi.org/10.7554/eLife.17029
Maslowska KH, Makiela-Dzbenska K, Fijalkowska IJ (2019) The SOS system: a complex and tightly regulated response to DNA damage. Environ Mol Mutagen 60:368–384. https://doi.org/10.1002/em.22267
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Mironov A, Seregina T, Nagornykh M, Luhachack LG, Korolkova N, Lopes LE, Kotova V, Zavilgelsky G, Shakulov R, Shatalin K, Nudler E (2017) Mechanism of H2S- mediated protection against oxidative stress in Escherichia coli. Proc Natl Acad Sci USA 114:6022–6027. https://doi.org/10.1073/pnas.1703576114
Nonaka G, Takumi K (2017) Cysteine degradation gene yhaM, encoding cysteine desulfidase, serves as a genetic engineering target to improve cysteine production in Escherichia coli. AMB Expr 7:90. https://doi.org/10.1186/s13568-017-0389-y
Ohtsu I, Wiriyathanawudhiwong N, Morigasaki S, Nakatani T, Kadokura H, Takagi H (2010) The l-cysteine/L-cystine shuttle system provides reducing equivalents to the periplasm in Escherichia coli. J Biol Chem 285:17479–17487. https://doi.org/10.1074/jbc.M109.081356
Owens RA, Hartman PE (1986) Export of glutathione by some widely used Salmonella typhimurium and Escherichia coli strains. J Bacteriol 168:109–114. https://doi.org/10.1128/jb.168.1.109-114.1986
Park S, Imlay JA (2003) High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J Bacteriol 185:1942–1950. https://doi.org/10.1128/JB.185.6.1942-1950.2003
Paul BJ, Berkmen MB, Gourse MB (2005) DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci USA 102:7823–7828. https://doi.org/10.1073/pnas.0501170102
Pittman MS, Corker H, Wu GH, Binet MB, Moir AJG, Poole RK (2002) Cysteine is exported from Escherichia coli cytoplasm by CydDC, an ATP-binding cassette-type transporter required for cytochrome assembly. J Biol Chem 277:49841–49849. https://doi.org/10.1074/jbc.M205615200
Pittman MS, Robinson HC, Poole RK (2005) A bacterial glutathione transporter (Escherichia coli CydDC) exports reductant to the periplasm. J Biol Chem 280:32254–32261. https://doi.org/10.1074/jbc.M503075200
Potrykus K, Cashel M (2008) (p)ppGpp: still magical? Annu Rev Microbiol 6:35–51. https://doi.org/10.1146/annurev.micro.62.081307.162903
Reitzer L (2005) Catabolism of amino acids and related compounds. EcoSal Plus. https://doi.org/10.1128/ecosalplus.3.4.7
Seaver LC, Imlay JA (2001) Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol 183:7173–7181. https://doi.org/10.1128/JB.183.24.7173-7181.2001
Shatalin K, Shatalina E, Mironov A, Nudler E (2011) H2S: a universal defense against antibiotics in bacteria. Science 334:986–990. https://doi.org/10.1126/science.1209855
Shimada T, Tanaka K, Ishihama A (2016) Transcription factor DecR (YbaO) controls detoxification of l-cysteine in Escherichia coli. Microbiology 162:1698–1707. https://doi.org/10.1099/mic.0.000337
Smirnova G, Muzyka N, Oktyabrsky O (2012) Transmembrane glutathione cycling in growing Escherichia coli cells. Microbiol Res 167:166–172. https://doi.org/10.1016/j.micres.2011.05.005
Smirnova GV, Muzyka NG, Ushakov VY, Tyulenev AV, Oktyabrsky ON (2015) Extracellular superoxide provokes glutathione efflux from Escherichia coli cells. Res Microbiol 166:609–617. https://doi.org/10.1016/j.resmic.2015.07.007
Smirnova GV, Tyulenev AV, Muzyka NG, Peters MA, Oktyabrsky ON (2017) Ciprofloxacin provokes SOS-dependent changes in respiration and membrane potential and causes alterations in redox status of Escherichia coli. Res Microbiol 168:64–73. https://doi.org/10.1016/j.resmic.2016.07.008
Smirnova GV, Tyulenev AV, Muzyka NG, Oktyabrsky ON (2018) The sharp phase of respiratory inhibition during amino acid starvation in Escherichia coli is RelA-dependent and associated with regulation of ATP synthase activity. Res Microbiol 169:157–165. https://doi.org/10.1016/j.resmic.2018.02.003
Takumi K, Nonaka G (2016) Bacterial cysteine-inducible cysteine resistance systems. J Bacteriol 198:1384–1392. https://doi.org/10.1128/JB.01039-15
Tian C, Roghanian M, Jørgensen MG, Sneppen K, Sørensen MA, Gerdes K, Mitarai N (2016) Rapid curtailing of the stringent response by toxin-antitoxin module-encoded mRNases. J Bacteriol 198:1918–1926. https://doi.org/10.1128/JB.00062-16
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522. https://doi.org/10.1016/0003-2697(69)90064-5
Traxler MF, Summers SM, Nguyen H-T, Zacharia VM, Smith JT, Conway T (2008) The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol Microbiol 68:1128–1148. https://doi.org/10.1111/j.1365-2958.2008.06229.x
Tyulenev A, Smirnova G, Muzyka N, Ushakov V, Oktyabrsky O (2018) The role of sulfides in stress-induced changes of Eh in Escherichia coli cultures. Bioelectrochem 121:11–17. https://doi.org/10.1016/j.bioelechem.2017.12.012
VanDrisse CM, Escalante-Semerena JC (2018) In Salmonella enterica, OatA (formerly YjgM) uses O-acetyl-serine and acetyl-CoA to synthesize N, O-diacetylserine, which upregulates cysteine biosynthesis. Front Microbiol 9:2838. https://doi.org/10.3389/fmicb.2018.02838
Weikum J, Ritzmann N, Jelden N, Klöckner A, Herkersdorf S, Josten M, Sahl H-G, Grein F (2018) Sulfide protects Staphylococcus aureus from aminoglycoside antibiotics but cannot be regarded as a general defense mechanism against antibiotics. Antimicrob Agents Chemother 62:e00602-18. https://doi.org/10.1128/AAC.00602-18
Wickens HJ, Pinney RJ, Mason DJ, Gant VA (2000) Flow cytometric investigation of filamentation, membrane patency and membrane potential in Escherichia coli following ciprofloxacin exposure. Antimicrob Agents Chemother 44:682–687. https://doi.org/10.1128/AAC.44.3.676-681.2000
Acknowledgements
This work was performed according to state assignment 01201353249, and was also supported by a grant from the Program of the Ural Branch of the Russian Academy of Sciences AAAA-A18-118041890005-1 and by a grant from the Russian Foundation of Basic Research 19-04-00888.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: P. Fechter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig.
1S Changes in the color of lead acetate–soaked paper strips in untreated culture (control) and after treatment of E. coli with valine (a) and chloramphenicol (b). E. coli BW25113 (wt), JW2663 (ΔgshA), JW2755 (ΔrelA), and NM4861 (ΔrelAΔgshA) were grown in M9 medium with glucose to OD600 of 0.4, and then 4.3 mM valine or 77 µM chloramphenicol was added. Lead acetate–soaked paper strips, which were affixed in culture flasks above the level of the liquid culture, were successively replaced every 15 min and photographed. Representative samples from 3-6 independent experiments are shown
Rights and permissions
About this article
Cite this article
Smirnova, G.V., Tyulenev, A.V., Bezmaternykh, K.V. et al. Cysteine homeostasis under inhibition of protein synthesis in Escherichia coli cells. Amino Acids 51, 1577–1592 (2019). https://doi.org/10.1007/s00726-019-02795-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-019-02795-2