Abstract
Natural and nutrient substances for cardiovascular disease are promising and capture researchers’ minds. Two kinds of novel bioactive peptides (high Fischer’s ratio oligopeptides and anticoagulant peptides) were obtained from Whitmania pigra protein via enzymatic hydrolysis. An oligopeptide (MW<874.0 Da) named as HF2 was obtained via chromatography purification procedures with a high Fischer’s ratio of 31.92 ± 1.36 and low phenylalanine + tyrosine content of 0.98 ± 0.04 %. Another peptide (WA3-1), prepared by alcalase AF 2.4 L-catalyzed hydrolysis and then purified by DEAE Sepharose FF, gel Sephadex G-15 chromatography, exhibited high anticoagulant activity with prolonging significantly plasma clotting time on activated partial thromboplastin time, prothrombin time, thrombin time (p < 0.01) and powerful thrombolytic activity. Amino acid composition and MALDI-TOF/TOF MS analysis showed that WA3-1 contained 11 amino acids (MW: 1422.0 Da) with the sequence as NH2–His-Asp-Phe-Leu-Asn-Asn-Lys-Leu-Glu-Tyr-Glu–COOH. Abundant negatively charged amino acids in C-terminal, as well as the special residue Lys contribute to its anticoagulant capacity. This research provided a novel natural candidate for the manufacture of nutrient oligopeptides with high branched chain amino acid, and anticoagulant thrombolytic agent in pharmaceutical industry with helping prevent from thrombosis and related cardiovascular diseases.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological active peptides have drawn great attention on account of various benefits for human health for many years. Among them, food-derived peptides are safe and nutrient for oral intake as components in nutraceuticals (Vercruysse et al. 2005; Nasria et al. 2012). Recently, a large amount of bioactive peptides with multifarious functions from various materials have been reported, such as antioxidant peptides (Adesso et al. 2015; Carrasco-Castilla et al. 2012), angiotensin-converting enzyme inhibitory peptide (Liu et al. 2010), and immunomodulating peptides (Hou et al. 2012), etc. Among these peptides, anticoagulant peptides and high Fischer’s ratio oligopeptides can be of great significance for guarding against atherothrombotic diseases and liver diseases, respectively. (Nasri and Nasri 2013; Hemeth et al. 1998).
Anticoagulant peptides could be utilized in the treatment of myocardial infarction and cardiovascular diseases that have been increasing in prevalence especially in many developed countries. These diseases usually result from blood coagulation in different degrees in vessels. Plasma serine proteases known as coagulation factors involved in blood clotting process, whenever an abnormal vascular condition and exposure to non-endothelial surfaces at sites of vascular injury occurs (Jung and Kim 2009). Intrinsic and extrinsic path ways constitute the whole coagulation system, which converge at the formation point of factor Xa (FXa) by the factor IXa (FIXa) and FVIIa, respectively. Subsequently, soluble fibrinogen converses into fibrin in the presence of thrombin action (Fig. 5). An accident during this process will lead to the formation of thrombus (Allford and Machin 2004). However, once endogenous or exogenous anticoagulants emerge, the coagulation factors will be interfered and blood coagulation can be inhibited or even stopped (Anastasopoulos et al. 2014). Conventional anticoagulants, such as heparin (a polysaccharide) and warfarin (a coumarin derivative), have been proven an efficacy in the treatment of thromboembolism, but still showed adverse effects and a narrow pharmaceutical window (Agnelli et al. 1990). Therefore, there is considerable incentive for the development of safer and more efficient anticoagulants. Over past decades, several kinds of anticoagulant peptides have been prepared from foods and some agricultural materials, such as egg white protein hydrolysate (Yang et al. 2007), papain-hydrolyzed peptides (Shimizu et al. 2009). Meanwhile, amino acid sequences of a few anticoagulant peptides have been identified. However, the structure–function relationship of anticoagulant peptides still remains largely ambiguous.
Another important bioactive peptide, high Fischer’s ratio oligopeptide, is a kind of small peptide mixture with the mole ratio of branched chain amino acids (BCAA) to aromatic amino acid (AAA) higher than 20 and phenylalanine (Phe) + tyrosine (Tyr) content lower than 2 % (Okita et al. 1985), which are widely used in clinical nutrition approaches for the treatment of liver disease, diabetes and digestive enzymes deficiency. They also can be added into fortified food for athletes and high strength laborers (Karabatas et al. 2000; Di Pasquale 2008; Mero 1999). Further researches are greatly encouraged in developing more new anticoagulants and high Fischer’s ratio peptides to meet the requirements for good clinical test results.
Dry Whitmania pigra (WP), a protein-rich material, has been used orally to activate blood and remove stasis, thus gaining a large-scale commercial farming in China. Nevertheless, oral intake of WP body has low effect, due to the limited degradation of WP proteins (WPP) via enzymatic digestion inside our bodies. Hence, it is valuable to introduce appropriate in vitro enzymes to release bioactive peptides from WP protein before oral administration, which has been rarely reported previously.
In this research, two original peptides with anticoagulant activity and high Fischer’s ratio value were successfully prepared from the same WP protein by enzymatic hydrolysis, respectively. Thrombolytic activity assay was implemented. The separation and purification of WPP-derived peptides were conducted and the purified peptides were further identified. In addition, the dose-activity relationship and the amino acid compositions of the peptides were also investigated.
Materials and methods
Materials and chemicals
WP was supplied by Guangzhou Agricultural Institute, China. Flavourzyme (EC 3.4.11.1), alcalase AF 2.4L (alcalase) (EC 3.4.21.62) and neutrase (EC 3.4.24.28) were purchased from Novozymes A/S Co., Denmark. Papain (EC 3.4.22.2) was purchased from AoBo Bio-pharmaceutical Tech. Co., China. Trypsin (EC3.4.21.4) and carboxypeptidase A (EC 3.4.17.1), bacitracin, reduced glutathione (GSH) and gel Sephadex G-15 were purchased from Sigma Co., America. Bivalirudin was purchased from GL Biochem. (Shanghai) Co., China. Reagent for measuring activated partial thromboplastin time (APTT), prothrombin time (PT), thrombin time (TT), and fibrinogen (FIB) were purchased from Shanghai Sunbio Co., China. Blood was provided by healthy, euglycemic and normolipidemic volunteers. Superfine activated carbon was purchased from Longxin (Gongyi) Purifying Water Co., China. DEAE Sepharose FF was purchased from GE Co., America. All other chemicals and reagents were of analytical grade and commercially available.
Preparation of WPP
Cleaned WP bodies were dehydrated completely at 50 °C until they became dry completely (the moisture <2 %) and then were shattered into powder in a mincing machine (RRHP-200, Oklife Co., China). The powder was dispersed in distilled water with a ratio of 1:20 (w/v), followed by pH adjustment to 9.0 and a 1 h incubation in a water-bath shaker (37 °C, 150 r/min). The mixture was centrifuged at 8000g for 15 min and the supernatant was adjusted to pH 4.6 with 1 M HCl. After being centrifuged at 8000g for 15 min, the resulting precipitate was washed three times and the pH was adjusted to 7.0 with 1 M NaOH. And then it was freeze-dried using a freeze dryer (Christ Alpha 1-2, Christ Co., Germany) and was collected for the following enzymatic hydrolysis. The content of protein sample was determined by Kjeldahl method (Beljkas et al. 2010).
Two various enzymatic hydrolysis processes
WPP was dissolved in distilled water with the final concentration of 50 mg/ml and pretreated at 95 °C for 20 min (pH 9.5) (Ren et al. 2014). Enzymes (flavourzyme, alcalase, neutrase, papain and trypsin) were added to initiate the hydrolysis involving in WPP solution samples with a dosage of 104 U/g of WPP at 200 r/min in a water-bath shaker (each enzyme was employed under its optimum reaction condition), respectively. Then the enzymatic reactions were terminated by inactivating the biocatalyst at 95 °C for 10 min. After being adjusted to pH 7.0, the hydrolysates were centrifuged at 10,000g for 10 min at 4 °C, freeze-dried and collected for anticoagulant activity assay. For a two-step enzymatic procedure, after the first enzymatic process was terminated via heating, it was cooled down to the optimal reaction temperature for the second enzyme (carboxypeptidase A) which was then added immediately with following the procedure as mentioned above.
Analysis of DH value
For reactions mediated by flavourzyme, alcalase, neutrase, papain, trypsin or carboxypeptidase A, the reaction DH values were measured by “pH–stat” method using the following equation, due to which the pH values were ≥7.0 (Jens 1986):
where B is the volume of consumed NaOH solution (ml), Nb is the concentration of consumed NaOH solution (mmol/ml), α is the degree of dissociation of α-amino acid, α = [10(pH − pK)]/[1 + 10(pH − pK)] (the average pK of the amino, 7.0; pH, the value of the initial reaction). MP is the total mass of the substrate protein (g), while h tot is the total quantity of peptide linkage unit of substrate protein (mmol/g), which was calculated from amino acid analysis by summing the molar concentrations of each individual amino acid (mmoles) per gram of WPP.
Determination of anticoagulant activity
Anticoagulant activities of the hydrolysates were evaluated by APTT, PT, TT and FIB assays. The assays were performed using a semi-automatic blood coagulation analyzer (AYW8003, Ruimai Co., China). Blood was centrifuged at 3000 r/min for 10 min at 4 °C, and the isolated plasma was collected in anticoagulant tubes. Sodium citrate with the final concentration of 3.2 % (w/v) was used as an anticoagulant. Saline and 0.01 M bivalirudin were used as negative and positive control, respectively: (1) APTT assay A 100 μl aliquot of thawed plasma was mixed equably with 100 μl of APTT reagent in a cuvette. Then, a 20 μl aliquot of sample was added, and the mixture was incubated for 5 min at 37 °C. The reaction was immediately initiated by adding a 100 μl aliquot of 25 mM CaCl2. The clotting time was recorded. (2) TT assay A 100 μl aliquot of TT reagent and 20 μl of sample were added together in a cuvette. Then, the reaction was immediately initiated by adding 100 μl of the plasma at 37 °C. The TT value was recorded. (3) PT assay A 200 μl aliquot of PT reagent and 20 μl of sample were added in a cuvette and incubated for 3 min at 37 °C. The reaction was immediately initiated with the addition of 100 μl of plasma preheated at 37 °C and incubated for 5 min at 37 °C. The PT value was recorded. (4) FIB assay the thawed plasma was diluted in saline at a ratio of 1:9 (v/v), 200 μl of diluted plasma and 20 μl of sample were added in a cuvette. The reaction was immediately initiated with the addition of 100 μl of FIB reagent. The FIB value was recorded.
Activated carbon adsorption
The hydrolysates prepared by alcalase-carboxypeptidase A treatment were dissolved in 100 ml of distilled water with the final concentration of 50 mg/ml, adjusted to pH 5.0 with 1 M HCl, and was then added with 2 % of activated carbon, cultivated at 30 °C for 90 min at the speed of 150 r/min. After being centrifuged at 10,000g for 15 min, the supernatant was isolated, freeze-dried and collected for the following gel Sephadex G-15 filtration.
Ion-exchange chromatography
A 0.5 g dose of the dry hydrolysates from above one-step enzymatic process was dissolved in 10 ml of 10 mM Tris–HCl butter (pH 7.6) and then loaded onto an ion-exchange column (2.6 × 50 cm) packed with the pretreated DEAE Sepharose FF at a flow rate of 50 ml/h continually. The effluent was monitored at 214 nm using a spectrophotometer (UV 752S, Lengguang Co., China). When the absorbance of the effluent passed 0.1, the addition of the sample was stopped. The column was previously equilibrated with 10 mM Tris–HCl buffer (pH 7.6), and then 0–1.0 M NaCl solution in 10 mM Tris–HCl buffer (pH 7.6) passed through the chromatography column (2.6 × 60 cm) at a flow rate of 50 ml/h with a linear gradient. Each fraction peak monitored at 214 nm was freeze-dried and collected for anticoagulant activity assay, and the fraction with the highest anticoagulant activity (namely A) would be purified further through a gel Sephadex G-15 filtration column.
Gel filtration chromatography
Two different kinds of peptides obtained by separation using the above-mentioned ion-exchange column or activated carbon adsorption were further purified with gel Sephadex G-15 filtration, respectively. 5 ml of samples (50 mg/ml) or reference solution including 25 mg/ml bacitracin and GSH was first loaded onto a gel Sephadex G-15 chromatography column (1.6 × 100 cm) and eluted at a flow rate of 20 ml/h by ultrapure water achieved from a Milli-Q Water Purification System (18.2 M < OMEG A > *cm at 25 °C, Millipore, MA, USA). The absorbance of the effluent was monitored at 214 nm, and the relevant peak curves were plotted. Each fraction peak isolated from fraction A was freeze-dried and collected for anticoagulant capacity assay. Likewise, the fractions exhibiting observable absorbance at 214 nm from the product after activated carbon adsorption was also freeze-dried and collected for determining the mole ratio of BCAA to AAA.
Thrombolytic activity assay
Thrombolytic activity was measured in terms of the size of the thrombolytic area (S TA) of clotting plasma, which derived from “Fossum” method with some improvements (Fossum and Hoem 1996). A 0.15 g dose of agarose was dissolved in 50 ml of distilled water, boiled for 10 min with only about 30 ml of solution remained, and then cooled to 40 °C. 4 ml of 0.05 M Tris–HCl buffer solution (pH 7.4) and 3 ml of thawy human plasma filtered through a filter membrane (0.22 μm) were equably put in the solution followed by the addition of 3 ml of 25 mM CaCl2 solution finally. The above mixture was uniformly poured into a glass culture dish (Dia.: 10 cm) at 40 °C. After the mixture cooled and clotted, six ostioles were picked in Fig. 4a. 20 μl of sample with four different concentrations of 0.5, 1, 5 and 20 mg/ml dissolved in saline were added into the ostioles, and labeled as “1”, “2”, “3” and “4”, respectively. 20 μl of 5 mg/ml nattokinase replaced the sample as a positive control and was labeled as “5”, while the same volume of saline was added as a negative control and labeled as “0”. The glass dish covered well was incubated at 37 °C for 20 h. The maximum and minimum diameters of thrombolytic circles in the glass dish were measured and S TA were calculated per 4 h.
where D max is the maximum diameter of thrombolytic circle, D min is the minimum diameter of thrombolytic circle, 0.159 is the size of the area of the ostiole.
Amino acid composition
Samples were digested in the solution of 6 M HCl and 1 % phenol at 105 °C for 24 h, vacuum-dried at 50 °C, dissolved in a loading buffer and centrifuged at 10,000g for 15 min. A dose of the supernatant was analyzed by HPLC on a “PICO-TAG” amino acid column (Hitachi Co., Japan) at 254 nm using a mobile phase (A solution: acetic acid/sodium acetate buffer solution (pH6.4), B solution: 60 % (v/v) acetonitrile) at a flow rate of 1.0 ml/min at 38 °C. Tryptophan content was determined after alkaline hydrolysis (Landry and Delhaye 1992).
Characterization of purified high Fischer’s ratio oligopeptides by ESI–MS
A 10 μl aliquot of sample was injected into a high capacity ion trap mass spectrometer, and detected using electron spray ionization tandem mass spectrometry (Bruker, Co., Germany). Ionization methods: electrospray ionization; detection mode: positive ion mode; capillary voltage: 3.5 kV; cone voltage: 30 V; source temperature: 80 °C; drying air temperature: 350 °C; collision activated dissociation voltage: 35 V; mass scan range m/z: 50–1500; dry gas and aerosol: N2; flow rate: 5.0 L/min; collision gas: Ar.
Identification of anticoagulant peptides by MALDI-TOF/TOF MS
Molecular mass and amino acid sequence of the purified anticoagulant peptides were analyzed by a MALDI-TOF/TOF mass spectrometer (Autoflex III, Bruker Daltonics, Inc., Germany) equipped with a UV nitrogen laser (337 nm) and a dual microchannel plate detector. The samples were prepared by mixing 1 μl of analyte and 1 μl of matrix solution (sinapic acid saturated in 0.1 % trifluoroacetic acid and acetonitrile, 1:1, v/v), deposited on the probe plate and dried at room temperature. The spectra were obtained in the positive reflector mode at an accelerating voltage of 25 kV. An external calibration was performed for each measurement, using adequate standard peptides (Bruker Daltonics, Bremen, Germany). The peptide sequencing of the major peak was performed by processing the ion series in the spectra using BioTools (version 3.0, Bruker Daltonics) and manual interpretation.
Statistical analysis
All experiments were carried out at least three times to check the reproducibility. Statistical calculation was performed by one-way analysis of variance (ANOVA) using SPSS, version 19.0 (SPSS Inc., Chicago, IL). Data was expressed as mean ± standard deviation (SD) of triplicate determinations. Differences were considered to be significant at p < 0.05 or 0.01.
Results and discussion
Bioactivities of different hydrolysates from WPP
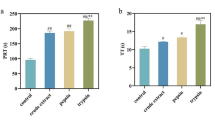
Peptides prepared via different enzymes treatment have different bioactivities, since endopeptidases can release different peptides by cleaving internal peptide bonds. In this research, five endopeptidases were employed for a one-step enzymatic hydrolysis of WP. However, Fischer’s ratios of the hydrolysates were unsatisfactory. Furthermore, a consecutively two-step enzymolysis was designed and results showed that the combined employment of alcalase-catalyzed hydrolysis and then carboxypeptidase A-catalyzed reaction, namely alcalase-carboxypeptidase A, generated the hydrolysate (HF) with the highest Fischer’s ratio of 31.92 ± 1.36 (p < 0.01) at degree of hydrolysis (DH) of 28 %, followed by trypsin-carboxypeptidase A, papain-carboxypeptidase A, flavourzyme-carboxypeptidase A and neutrase-carboxypeptidase A (p < 0.05; Fig. 1a). It was inferred that the present two-step enzymatic hydrolysis could remove more AAA residues and AAA-rich peptides than the one-step procedure, thus endowing the production of high mole ratio of BCAA. For the reactions involved by alcalase-carboxypeptidase A, the protein was degraded by alcalase first, and thus more amino acid residues was exposed. As an exopeptidase, carboxypeptidase A can cut preferentially the residues including Phe, Tyr, and alanine (Ala) off C-terminal. Therefore, when it was employed as the second catalyst, the AAA level of the hydrolysates may be lowered. This consecutively two-step enzymatic hydrolysis was thus confirmed, for the first time, as an efficient route for producing high Fischer’s ratio oligopeptides with low content of AAA (Pedroche et al. 2006; Udenigwe and Aluko 2010).
Comparison of the highest Fischer’s ratios of various hydrolysates from Whitmania pigra protein (WPP) prepared by five two-step enzymolysis. A trypsin-carboxypeptidase A; B papain-carboxypeptidase A; C flavourzyme-carboxypeptidase A; D alcalase-carboxypeptidase A; E neutrase-carboxypeptidase A (a) and the highest anticoagulant activities on APTT, TT, PT by five single enzymes treatment, respectively (b). Data are expressed as the mean value (±SD) of three independent experiments. Different upper case letters indicate the significant difference of APTT, different lower case letters indicate the significant difference of PT, and different Arabic numerals indicate the significant difference of TT (p < 0.05)
Anticoagulant activities of the hydrolysates prepared by different enzymes were evaluated by detection of their APTT, PT, TT and FIB values. The peptides (WA) prepared by alcalase at DH of 19 % exhibited strong anticoagulant capacity in terms of APTT (116.6 ± 7.3 s), TT (58.3 ± 5.7 s), PT (51.7 ± 1.8 s), and FIB (0.87 ± 0.07 g/L) at the concentration of 0.1 mg/ml (Table 1), which is much higher than that prepared by trypsin or papain (p < 0.01; Fig. 1b). When flavourzyme or neutrase was employed as the catalyst, only poor anticoagulant capacity was observed (p < 0.01; Fig. 1b). The experimental results showed one-step enzymatic hydrolysis by alcalase treatment could produce high enough anticoagulant peptides. Therefore, HF and WA were chosen for further isolation and purification process.
Separation and purification of two bioactive peptides
Two kinds of the above hydrolysates were purified to collect higher Fischer’s ratio oligopeptides and higher anticoagulant peptides. Flow chart was showed in Fig. 2.
Preparation of high Fischer’s ratio oligopeptides by activated carbon adsorption and gel filtration
Table 2 showed that Fischer’s ratio of the hydrolysates (namely HF1) purified by activated carbon adsorption was evidently enhanced from 6.13 ± 0.32 to 23.64 ± 1.45, and its Phe + Tyr content dropped to 1.30 % (p < 0.01). Activated carbon adsorption may effectively carry off free AAA, AAA-rich peptides and recover BCAA-rich peptides, thereby increasing Fischer’s ratio and reducing Phe + Tyr content of the WPP-derived hydrolysates observably (Udenigwe and Aluko 2010). Subsequently, six fractions (namely P1, P2, P3, P4, P5 and P6) were obtained through a gel Sephadex G-15 column (Fig. 3a). The major fraction P4 (also namely HF2) had the highest Fischer’s ratio of 31.92 ± 1.36 (Fig. 3b; Table 2) (p < 0.01). Table 2 showed that this fraction has a much lower Phe + Tyr content of only 0.98 ± 0.04 % than HF1. Hence, the combination of activated carbon adsorption and gel Sephadex G-15 filtration not only purified the hydrolysates but also efficiently carried off AAA thus improving Fischer’s ratio of the fraction.
Elution profile of HF1 separated by gel filtration chromatography on Sephadex G-15 column (a), Fischer’s ratios of the six fractions isolated (b), elution profile of WA separated by ion-exchange chromatography on DEAE Sepharose FF column (c) and elution profile of WA3 separated by gel filtration chromatography on Sephadex G-15 column (d). Data are expressed as the mean value (±SD) of three independent experiments. Different letters (a, b, c, d) present significant differences at p < 0.01
Purification of anticoagulant peptides by ion-exchange and gel filtration
Anticoagulant peptides released from WPP had different charges, which could bond with ion-exchange filler and then were eluted down by different charged eluents. Figure 3a showed three fractions (Fa, Fb and Fc) were well isolated from the parent hydrolysates by ion-exchange DEAE Sepharose FF chromatography. As shown in Table 1, the third fraction Fc (WA3) exhibited the highest anticoagulant activity in terms of APTT (155.4 ± 10.5 s), TT (68.2 ± 6.4 s), PT (72.7 ± 3.7 s), and FIB (0.40 ± 0.04 g/L) at the concentration of 0.1 mg/ml (p < 0.01), followed by Fb and Fa. Since DEAE Sepharose FF is an anion-exchanger that can adsorb negatively charged substance, the fraction WA3 was considered as an acidic peptide fraction with negative charge, which was further confirmed by analysis of its amino acid composition. As showed in Table 2, the negatively charged amino acids content of WA3 increased greatly from 26.26 ± 1.52 to 42.65 ± 2.25 % (p < 0.01). Negatively charged peptides were very important in the implement of anticoagulant action due to the bind to positively charged thrombin (Mao et al. 1988). The coagulation mechanism in the presence of WA3-1 was observed in Fig. 5.
WA3 was further purified with gel Sephadex G-15 filtration, isolating four fractions named F1, F2, F3 and F4 (Fig. 3b). Among them, the major fraction F1 (also namely WA3-1) displayed the highest anticoagulant capacity of APTT: 172.6 ± 15.8 s, TT: 79.6 ± 5.6 s, PT: 82.8 ± 5.7 s, and FIB: 0.32 ± 0.04 g/L at the concentration of 0.1 mg/ml, much higher than F3 of APTT: 133.5 ± 6.6 s, TT: 48.8 ± 3.5 s, PT: 53.4 ± 5.2 s, FIB: 0.56 ± 0.06 g/L (p < 0.01). The lowest capacity of F4 was attributed to the presence of a mass of free amino acids and saline ions. F1 was thus chosen for further investigation of amino acids composition and sequence.
Bioactivity-dose relationship of anticoagulant peptides
The three fractions WA, WA3 and WA3-1 revealed satisfactory anticoagulant capacities within the concentrations tested (Table 1). Four coagulation indicators (APTT, PT, TT and FIB) for the three fractions increased in a dose-dependant manner. And a small dose involvement may cause immense prolonging in clotting time. WA3-1 at the concentration of 0.01 mg/ml had the best performance in prolonging the normal clotting time up to 55.7 ± 3.2 s from 17.3 ± 1.2 s on PT and reducing the control of 3.41 ± 0.23 g/L down to 0.75 ± 0.05 g/L on FIB. WA3-1 (0.01 mg/ml) can also increase APTT and TT up to 116.6 ± 10.2 s and 52.6 ± 3.6 s (p < 0.01), respectively, which were close to bivalirudin. The significantly prolonged APTT and PT suggested that WA3-1 played a role in anticoagulation by inhibiting intrinsic and extrinsic coagulation factors. The increase of TT value also confirmed that the fraction WA3-1 could efficiently inhibit thrombin activity.
Thrombolytic activity of WA3-1
In the present research, the thrombolytic activity of WA3-1 was evaluated in terms of the degradation degree of fibrin in plasma, and thrombolytic circle was a direct expression. In the glass culture dish, the spread of thrombolytic circles were observed as the incubation time was prolonged to 20 h (Fig. 4a). Thrombolytic activities of WA3-1 at different concentrations were assessed in terms of S TA around six ostioles. All the samples at four tested concentrations exhibited various thrombolytic effect. Furthermore, as the concentration and incubation time increased, WA3-1 demonstrated stronger thrombolytic power, and the greatest S TA was also produced by WA3-1(20 mg/ml) at 20 h. Removing of existing thrombus in blood vessels is equally important as the retard of the formation of a thrombus. The results showed that the fraction WA3-1 not only could prolong the clotting time of plasma, but also possessed thrombolytic activity. Hence, WA3-1, which shows both anticoagulant and thrombolytic activity, may be more effective in preventing the formation of thrombus and degrading existing thrombus. The thrombolytic mechanism involving in WA3-1 could be observed in Fig. 5.
Evolution photographs of thrombolytic circles pretreated with saline, WA3-1 at different concentrations of 0.5, 1.0, 5.0, 20 mg/ml or nattokinase (5.0 mg/ml) at different incubation time, labeled as 0, 1, 2, 3, 4 and 5, respectively, (a) and thrombolytic curves of WA3-1 at different concentrations and incubation time (b)
Characterization of HF2 and WA3-1
ESI–MS analysis showed that the peptide HF2 is an oligopeptide fraction with the molecule weight (MW) less than 874.0 Da (Fig. 6a). High Fischer’s ratio oligopeptide is rich in branched chain amino acids that can be easily absorbed and transferred in blood restraining, simultaneously the delivery of aromatic amino acids, and can improve secondary metabolism of the patients during advanced liver disease (Clemente 2000). Meanwhile, it also can enhance the quality and strength of muscle, thereby, is welcome in athlete’s diet (Tipton and Wolfe 2004).
WA3-1 was analyzed by MALDI-TOF/TOF MS for its molecular weight and amino acid sequence (Fig. 6b, c). A purified peptide, namely WA3-1-0, was further identified by MS/MS showing a major ion with m/z of 1423.0 in its fragmentation spectrum. And its molecular weight was known as 1422.0 Da. The major products generated upon collision-induced dissociation were well in accord with all the b-type ions and some y-type ions. Each mass signal and corresponding fragmentation spectra could be matched to a single peptide fragment via manual calculation. As shown in Fig. 6c, WA3-1-0 contained eleven amino acids with its sequence of NH2-His-Asp-Phe-Leu-Asn–Asn-Lys-Leu-Glu-Tyr-Glu-COOH (HDFLNNKLEYE). It was a novel bioactive peptide, showing no same primary structural to any of the known anticoagulant peptides (Rojas et al. 2012). Moreover, extensive homology searches from the protein databanks could not yield any other peptide homologous with WA3-1-0.
Although rare studies have been reported about the structure–activity relationship of anticoagulant peptide, it was proven that negatively charged C-terminal may be very crucial for binding to positively charged thrombin for preventing coagulation process (Dodt et al. 1985; Mao et al. 1987). Two negatively charged Glu in the C-terminal of WA3-1-0 was believed to play a vital role in anticoagulant action, which was also similar to the segmental sequence in the C-terminal of bivalirudin (–Glu–Glu–Tyr–Leu–COOH), a well-known direct thrombin inhibitor. Simultaneously, WA3-1-0 possessed lysine (Lys) that may also be an important amino acid residue in performing anticoagulant activity (Mengwasser et al. 2005). The charged amino acids in C-terminal of the peptide were also considered to make contribution to its thrombin inhibition effect and/or to be a factor involving in the clotting process (Nasria et al. 2012). No evident relationship between the MW and anticoagulant activity of the peptide was found. And the types and sequences of amino acids in peptide may directly impact the coagulation process.
Conclusions
We reported here, for the first time, the successful preparation of two kinds of novel bioactive peptides (high Fischer’s ratio oligopeptides and anticoagulant peptides) from WPP by controlling enzymatic hydrolysis. Via separation and purification processes involving in activated carbon adsorption and gel Sephadex G-15 filtration, an oligopeptides (HF2) (MW <874.0 Da) was generated with high Fischer’s ratio of 31.92 ± 1.36 and low Phe + Tyr content of 0.98 ± 0.04 %. After successive DEAE Sepharose FF and gel Sephadex G-15 chromatography, the peptide (WA3-1) with high anticoagulant and thrombolytic activity was finally obtained. From WA3-1, a novel anticoagulant peptide (WA3-1-0) was further identified with the sequence of NH2–His-Asp-Phe-Leu-Asn-Asn-Lys-Leu-Glu-Tyr-Glu–COOH (MW: 1422.0 Da). Abundant negatively charged amino acids in C-terminal, as well as the special residue Lys, may play an important role in the express of anticoagulant capacity of the peptide. This research provides an efficient enzymatic route for simultaneously preparing two kinds of novel bioactive peptides from the same raw material WPP. The present anticoagulant peptide is meritorious template for developing novel agent helping preventing the formation of thrombus, degrading existing thrombus in cardiovascular disease.
References
Adesso S, Ventre G, Sommella E, Pepe G, Pagano F, Sansone F (2015) Potential antioxidant peptides released after simulated gastro-intestinal digestion of Stracchino soft cheese. Amino Acids 47(8):1674
Agnelli G, Pascucci C, Cosmi B, Nenci GG (1990) The comparative effects of recombinant hirudin (CGP39393) and standard heparin on thrombus growth in rabbits. Thromb Haemost 63(2):204–207
Allford SL, Machin SJ (2004) Haemostasis. Surgery (Oxford) 22:200a–200b
Anastasopoulos C, Sarigiannis Y, Stavropoulos G (2014) Cyclic peptide analogs of 558–565 epitope of A2 subunit of Factor VIII prolong aPTT. Toward a novel synthesis of anticoagulants. Amino Acids 46(4):1087–1096
Beljkas B, Matic J, Milovanovic I, Jovanov P, Misan A, Saric L (2010) Rapid method for determination of protein content in cereals and oilseeds: validation, measurement uncertainty and comparison with the Kjeldahl method. Accredit Qual Assur 15(10):555–561
Carrasco-Castilla J, Hernndez-lvar AJ, Jimnez-Martn C, Jacinto-Hernnde C, Alaiz M, Girn-Call J (2012) Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem 135(3):1789–1795
Clemente A (2000) Enzymatic protein hydrolysates in human nutrition. Trend Food Sci Tech 11(7):254–262
Di Pasquale MG (2008) Amino acids and proteins for the athlete: the anabolic edge, 2nd edn. CRC Press, Taylor & Francis Group, Boca Raton, p 434
Dodt J, Seemuller U, Maschler R, Fritz H (1985) The complete covalent structure of hirudin. Localization of the disulfide bonds. Biol Chem Hoppe Seyler 366(4):379–385
Fossum S, Hoem NO (1996) Urokinase and non-urokinase fibrinolytic activity in protease-inhibitor-deprived plasma, assayed by a fibrin micro-plate method. Immunopharmacology 32:119–121
Hemeth AM, Steindl P, Ferenci P, Roth E, Hortnagl H (1998) Role of tryptophan in the elevated serotonin-turnover in hepatic encephalopathy. J Neural Trans 105(8–9):975–986
Hou H, Fan Y, Li BF, Xue CH, Yu GL, Zhang ZH (2012) Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chem 134(2):821–828
Jens AN (1986) Enzymatic hydrolysis of food protein. Elsevier Applied Science Publishers, New York
Jung WK, Kim SK (2009) Isolation and characterization of an anticoagulant oligopeptide from blue mussel, Mytilus edulis. Food Chem 117(4):687–692
Karabatas LM, de Bruno LF, Pastorale C, Lombardo YB, Basabe JC (2000) Branched-chain amino acid-enriched diet: effects on insulin secretion and cellular immune aggression. Proc Soc Exp Biol Med 224(3):159–165
Landry J, Delhaye S (1992) Simplified procedure for the determination of tryptophan of foods and feedstuffs from barytic hydrolysis. J Agric Food Chem 40(5):776–779
Liu JB, Yu ZP, Zhao WZ, Lin SY, Wang EL, Zhang Y (2010) Isolation and identification of angiotensin-converting enzyme inhibitory peptides from egg white protein hydrolysates. Food Chem 122(4):1159–1163
Mao SJT, Yates MT, Blankenship DT, Cardin AD, Krstenansky JL, Lovenberg W (1987) Rapid purification and revised amino-terminal sequence of hirudin: a specific thrombin inhibitor of the bloodsucking leech. Anal Biochem 161(2):514–518
Mao SJ, Yates MT, Owen TJ, Krstenansky JL (1988) Interaction hirudin with thrombin: identification of a minimal binding domain of hirudin that inhibits clotting activity. Biochem US 27(21):8170–8173
Mengwasser KE, Bush LA, Shih P, Cantwell AM, Di Cera E (2005) Hirudin binding reveals key determinants of thrombin allostery. J Biol Chem 280(29):26997–27003
Mero A (1999) Leucine supplementation and intensive training. Sports Med 27(6):347–358
Nasri R, Nasri M (2013) Marine-derived bioactive peptides as new anticoagulant agents: a review. Curr Protein Pept Sci 14(3):199–204
Nasria R, Amorb IB, Bougatefa A, Nedjar-Arroume N, Dhulster P, Gargouri J (2012) Anticoagulant activities of goby muscle protein hydrolysates. Food Chem 133(3):835–841
Okita M, Watanabe A, Nagashima H (1985) Nutritional treatment of liver cirrhosis by branched amino acids-enriched nutrient mixture. J Nutr Sci Vitaminol 31(3):291–303
Pedroche J, Yust MDM, Lqari H, Megias C, Ciron-Calle J, Alaiz M (2006) Production of Brassica carinata protein hydrolyzates with a high Fischer’s ratio using immobilized proteases. J Agric Food Chem 54(20):7621–7627
Ren Y, Wu H, Li XF, Lai FR, Zhao GL, Xiao XL (2014) A two-step, one-pot enzymatic method for preparation of duck egg white protein hydrolysates with high antioxidant activity. Appl Biochem Biotech 172(3):1227–1240
Rojas RR, Cruz GA, Flores NA, Rodriguez SG, Gomez RL (2012) Antithrombotic and angiotensin-converting enzyme inhibitory properties of peptides released from bovine casein by Lactobacillus casei Shirota. Int Dairy J 26(2):147–154
Shimizu M, Sawashita N, Morimatsu F, Ichikawa J, Taguchi Y, Ijiri Y (2009) Antithrombotic papain-hydrolyzed peptides isolated from pork meat. Thromb Res 123(5):753–757
Tipton KD, Wolfe RR (2004) Protein and amino acids for athletes. J Sport Sci 22(1):65–79
Udenigwe CC, Aluko RE (2010) Antioxidant and angiotensin converting enzyme-inhibitory properties of a flaxseed protein-derived high Fischer ratio peptide mixture. J Agric Food Chem 58(8):4762–4768
Vercruysse L, Camp JV, Smagghe G (2005) ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: a review. J Agric Food Chem 53(21):8106–8115
Yang WG, Wang Z, Xu SY (2007) A new method for determination of antithrombotic activity of egg white protein hydrolysate by microplate reader. Chin Chem Lett 18(4):449–451
Acknowledgments
The authors are grateful to Program for New Century Excellent Talents in University (NCET-12-0192), and Scientific Research Foundation for Young Teachers of Sichuan University (2016SCU11035) for the financial supports.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: M. S. Palma.
Rights and permissions
About this article
Cite this article
Ren, Y., Yang, Y., Wu, W. et al. Identification and characterization of novel anticoagulant peptide with thrombolytic effect and nutrient oligopeptides with high branched chain amino acid from Whitmania pigra protein. Amino Acids 48, 2657–2670 (2016). https://doi.org/10.1007/s00726-016-2299-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2299-8