Abstract
Proteins and their complexes in structures solved by X-ray crystallography or cryo-EM look rigid. While these structures yield very detailed information, they do not capture critically important property of proteins, their dynamic nature. The very fact that proteins function indicates that they must have moving parts. Structural studies have additional caveats: to obtain structures, proteins are often drastically engineered and placed into highly non-physiological conditions. In contrast to structural studies, biophysical methods, such as EPR and NMR spectroscopy, reveal protein and complex dynamics. Importantly, minimally mutated, virtually wild-type proteins can be used. Here, this issue is discussed using GPCRs and their signal transducers, G proteins and arrestins, as examples. To understand how proteins actually work in living cells, we must keep in mind the limitations of different methods and synthesize the information obtained by all of them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Protein structures, obtained by X-ray crystallography or cryo-EM, yield very detailed snapshots, which are extremely useful for the follow-up mutagenesis [1,2,3,4,5,6]. But these structures are misleading in several ways. One, the proteins look rigid, essentially “set in stone”. Two, proteins are often mercilessly engineered to obtain structures. Three, to enable structure determination, proteins and their complexes are often placed in highly non-physiological environment. Proteins that nature created and honed during millions of years of evolution are highly dynamic “living and breathing” molecules. Structures show “one frame of a movie” with high resolution; whereas, the life of a protein is the whole movie that consists of many frames. Proteins have to be dynamic to perform various tasks in the cell. To give an obvious example, enzymes cannot do their job without being dynamic shapeshifting entities. Such a thing as an enzyme lacking moving parts cannot exist. Protein dynamics are captured by the biophysical methods, such as nuclear magnetic resonance (NMR) and electron paramagnetic resonance (EPR). As an added bonus, these methods do not require heavy engineering: NMR can use essentially wild-type (WT) proteins where “silent” carbon-12 is replaced with “reporting” carbon-13 [7, 8]; while for EPR, one only needs to introduce a single point mutation (or two for double electron–electron resonance (DEER) distance measurements) to attach the spin label [9,10,11,12,13,14,15,16,17,18,19,20,21]. Here, we will illustrate these points using G protein-coupled receptors (GPCRs) and their main signal transducers, G proteins and arrestins, as examples. These are only three among many classes of proteins Dr. Hubbell studied during his long illustrious career.
2 GPCRs—Signal Transducing Machines
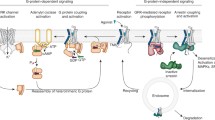
GPCRs are the largest family of receptors, with ~ 800 subtypes expressed in humans. All GPCRs share a core consisting of seven transmembrane α-helices, with extracellular N-terminus and cytoplasmic C-terminus (Fig. 1A). While this core is conserved, the sizes of the N- and C-termini, as well as the length of the loops between the helices, vary widely in the GPCR family. Activation of GPCRs by a variety of extracellular stimuli initiates conserved signaling and desensitization mechanisms. Active GPCRs interact with cognate heterotrimeric G proteins, catalyzing GDP–GTP exchange on their α-subunits (Fig. 1B). Activated G proteins dissociate from the GPCR, freeing the space for another G protein molecule that can be activated by the same receptor. GTP-liganded α-subunit dissociates from βγ-dimer, whereupon both parts of active G protein interact with their effectors, propagating the signal (Fig. 1B). Active GPCRs are phosphorylated by GPCR kinases (GRKs). Active phosphorylated receptors are the preferred target of arrestin proteins (Fig. 1C) [22]. Bound arrestins shield the cytoplasmic side of GPCRs (Fig. 1C), precluding their coupling to G proteins, thereby terminating G protein-mediated signaling.
Branches of GPCR signaling. A Inactive GPCRs do not interact with G proteins and arrestins. B Upon activation with an agonist (magenta circle), receptors bind inactive heterotrimeric G proteins, which consist of GDP-liganded α-subunit (has Ras- and helical domains) and βγ-dimer. GPCRs act as guanyl nucleotide exchange factors, activating G proteins by facilitating the release of bound GDP, whereupon G protein binds GTP abundant in the cytoplasm. Activated G proteins dissociate from GPCRs, and GTP-liganded α-subunit dissociates from βγ-dimer. Both parts of active G proteins interact with effectors, propagating the signal. C Active GPCRs are phosphorylated by GPCR kinases (GRKs) (receptor-attached phosphates are shown as red circles). Arrestins selectively bind to active phosphorylated GPCRs. Arrestin–GPCR complex facilitates several branches of signaling
For a long time, GPCRs were thought to be on–off switches with two conformations, active and inactive. Classification of orthosteric ligands (molecules that bind at the same sites as the endogenous ligands) reflects this view. Agonists were believed to push receptors into the active conformation, inverse agonists—into the inactive one (suppressing constitutive activity of unoccupied receptors), while neutral antagonists were thought to occupy the ligand-binding site without affecting the conformational state of the receptor (hence the term “neutral”). The classical ternary complex model that describes GPCR interactions with G proteins is based on this view [23]. Early crystal structures of a prototypical non-visual GPCR, β2-adrenergic receptor (β2AR), solved using “empty”, liganded, or stabilized by G protein-mimicking nanobody receptor, appeared to be consistent with this notion [4, 24,25,26]. A groundbreaking discovery that GPCR activation requires rigid-body motion of transmembrane helices relative to each other made by Dr. Hubblell’s lab using EPR in 1996 [10] and subsequently confirmed by numerous structural studies was also consistent with the idea that GPCRs are two-state molecules. However, a study of the β2AR using biophysical methods, NMR and EPR/DEER, revealed a totally different picture [16]. Receptor was shown to exist in a complex equilibrium of several conformations (Fig. 2A) even in the presence of a bound agonist and a G protein-mimicking nanobody, although the nature of the ligand and the presence of the interacting protein significantly affected this equilibrium [16]. Basically, in every case, the receptor explores a wide conformational space. The binding of an agonist or a signal transducer changes the set of conformations the receptor explores, but the sets of conformations sampled by unliganded, antagonist-occupied, and agonist-occupied receptor in complex with signal transducer overlap. The use of DEER at different pressures revealed that ligand-free “empty” β2AR samples active-like conformations, which are suppressed by inverse agonists [27]. This revealed the mechanistic basis of constitutive activity, which is relatively low in case of β2AR, but much higher in case of many other GPCRs. Another prototypical GPCR, rhodopsin, the founding member of the family [28], was also analyzed by DEER [18]. DEER distance measurements in the dark (inactive) and light-activated rhodopsin, in the presence and absence of its cognate G protein transducin, yielded similar results: complex conformational equilibrium was revealed in all cases, which was shifted but never fully suppressed by covalently bound inverse agonist 11-cis-retinal, activating light, and bound G protein [18].
Signaling molecules are dynamic. A GPCRs exist in equilibrium of several conformations including active-like conformations. B Free arrestins explore a wide conformational space. C Activated GPCRs and GPCR-bound G proteins are also dynamic. In particular, in receptor-bound G protein the two domains of the α-subunit move relative to each other. D In the receptor–arrestin complex, both proteins remain dynamic, so that different “flavors” of this complex co-exist in the cell
In the early days, it was generally accepted that each GPCR couples to a particular class of heterotrimeric G proteins. Mammals have four main classes: Gs-like G proteins, which activate adenylyl cyclase; Gi-like, which inhibit adenylyl cyclase; Gq-like, which activate phospholipase C; and G12-like, which activate guanyl nucleotide exchange factors of Rho-like small G proteins [29]. Numerous subsequent studies showed that the coupling of the same receptor to several classes of G proteins is a rule, rather than an exception. In addition, arrestins (which most GPCRs bind) are widely viewed as another type of signal transducers (reviewed in [30,31,32]). To complicate matters further, virtually every cell in vertebrate animals expresses two homologous, but not identical non-visual arrestins, arrestin-2 and -3 (a.k.a. β-arrestin-1 and -2, respectively) [33]. Considerable effort is directed today to the search for ligands that would selectively direct GPCR signaling to G proteins or arrestins, i.e., demonstrate “biased agonism” (recently reviewed in [34]). As both G proteins and arrestins preferentially bind active GPCRs, the phenomenon of signaling bias clearly indicates that each GPCR must have more than one active conformation [35]. Biophysical and structural evidence supports the existence of multiple active conformations [16, 18, 36]. Thus, biophysical data revealing complex GPCR dynamics are consistent with known receptor biology (reviewed in [9]); whereas, the view of a rigid GPCR, or a receptor as a two-position on–off switch, directly contradicts experimental evidence.
3 G Proteins—the First Discovered GPCR Signal Transducers
GPCRs are so named because G proteins were the first discovered type of signal transducers of this receptor family. GPCRs couple to heterotrimeric G proteins consisting of three subunits, with the contacts largely mediated by the α-subunit [4] (Fig. 1B). Therefore, the α-subunit determines receptor specificity of a G protein. All G proteins, small and heterotrimeric, are active when GTP is bound and inactive after it is hydrolyzed to GDP by their internal GTPase activity (in most cases enhanced by specialized GTPase activating proteins). The G protein activation (replacement of bound GDP with GTP) requires guanyl nucleotide exchange factors (GEFs). GPCRs are most common (but not the only) GEFs of heterotrimeric G proteins.
The first step of G protein-mediated signaling is GDP/GTP exchange on cognate G proteins catalyzed by an activated GPCR, which triggers G protein transition from inactive to active conformation (Fig. 2C). Upon activation G protein dissociates from the GPCR and its GTP-liganded α-subunit dissociates from βγ-dimer. Both parts of activated G proteins interact with effectors, thereby propagating the signal (Fig. 1B). The α-subunits of heterotrimeric G proteins (~ 40 kDa) are about twice as large as small G proteins, consisting of one domain highly homologous to small G proteins (Ras domain) and another half unique for this family (helical domain). Nucleotide binding site is localized in a cleft between the two domains [1]. The structures of GTP- and GDP-liganded α-subunit of visual G protein transducin (belonging to the Gi subfamily) were solved back in 1994 [1]. The comparison revealed significant structural differences in three elements exposed on the surface, which were termed switch regions [1]. These switches were subsequently shown to participate in the interactions of activated α-subunits of different G proteins with their effectors.
A critical question in GPCR signaling via G proteins is how GPCR binding facilitates GDP/GTP exchange on the α-subunit, as the distance between GPCR-G protein interface and nucleotide-binding pocket is large (~ 30A) [4]. Thus, receptor action must be mediated by internal conformational changes in the bound α-subunit. The first study using EPR by Dr. Hubbell’s lab of the conformational effects on the G protein α-subunit of binding to the active receptor showed significant rearrangements [19]. A combination of contiguous wave EPR with DEER distance measurements revealed that the engagement of the C-terminus of the α-subunit by the active receptor results in the large movement (rotation and translation) of the C-terminal α-helix 5, which perturbs the loop between α-helix 5 and β-strand 6 containing TCAT motif conserved in the G protein family. The residues of this motif interact with the guanyl ring of the nucleotide [1], which explained how receptor facilitates GDP release [17]. Importantly, the introduction of a flexible (five glycines) linker between receptor-binding Gα C-terminus and α-helix 5 yields transducin mutant that still binds light-activated rhodopsin but does not demonstrate enhanced nucleotide exchange as the result of this interaction [37]. Extensive distance measurements by DEER revealed that receptor binding also induces the separation of the two domains of Gα, opening the inter-domain cleft where nucleotide is bound [20] (Fig. 2C). This suggested yet another possible molecular mechanism of the receptor-induced facilitation of the GDP dissociation. A combination of the molecular modeling and DEER showed that even in the absence of the receptor, the α-subunit is in equilibrium between conformations where the two domains are close together (as in the crystal [1]) or separated [38]. Mutations that prevented domain separation suppressed nucleotide exchange [38]. Thus, domain separation appears to be necessary, but insufficient for the release of the bound nucleotide, which highlighted critical role of the receptor-induced perturbation of the nucleotide-binding site discovered earlier [17]. Thus, the molecular mechanism of the GPCR-dependent activation of the bound G protein, a critical step in GPCR-initiated signaling, was largely elucidated using EPR and DEER with the key contribution of Dr. Hubbell’s lab.
4 Arrestins—Multi-functional Regulators of Cell Signaling.
Arrestins are another family of proteins where biologically important dynamics were revealed by biophysical methods, with an outstanding contribution of Dr. Hubbell’s lab. Arrestins were discovered as proteins that specifically bind active and phosphorylated by GRKs [39], or other kinases [40], receptors, precluding their coupling to cognate G proteins ([22, 41] and references therein). Subsequent studies revealed that both non-visual arrestins interact with a variety of other proteins and regulate many important signaling pathways in cells ([42, 43] and references therein). Crystal structures of all four vertebrate arrestin subtypes were solved [2, 44,45,46,47,48,49]. They revealed a relatively compact fold remarkably conserved across subtypes, leaving one to wonder how so many functions can be packed into these average-sized ~ 45 kDa proteins [42]. Arrestins were probed by both EPR [11, 12, 21, 50,51,52,53,54] and NMR [7, 8]. These methods revealed that virtually every part of the arrestin molecule is dynamic (Fig. 2B, D). So, multiplicity of functions is likely explained by an extensive conformational space arrestin molecule can explore. Indeed, EPR and DEER revealed at least three groups of conformations of arrestins: basal, GPCR bound, and microtubule bound [12, 21, 50, 52]. As each conformation has distinct functional capabilities [51, 55,56,57], this helps to explain how shapeshifting arrestin molecule can do so many different things in the cell [58].
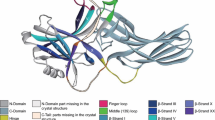
Oligomerization is another aspect of arrestin biology where structures proved misleading, while DEER data revealed the real picture. Three out of four vertebrate subtypes oligomerize: arrestin-1 [2, 11, 13, 48, 59,60,61], -2 [62, 63], and -3 [62, 64]. Each subtype forms a distinct type of oligomer: dimers and tetramers in case of arrestin-1 from different species [13, 14], “infinite” chains with no obvious limit in case of arrestin-2 [62, 63], and trimers in case of arrestin-3 [64]. Two groups crystallized arrestin-1 in different conditions and obtained virtually identical tetramers [2, 48]. This suggested that the tetramers observed in these structures might be what arrestin-1 naturally forms. However, experiments using DEER showed that the solution tetramer of arrestin-1 has a very different shape [11, 13]: a diamond with two interfaces involving the N-domains of the protomers (NN) and the other two involving the C-domains (CC) (Fig. 3). DEER results were confirmed by disulfide cross-linking and mutagenesis. Two residues in different protomers in the CC interface are very close to each other in the model, Phe197 and Ala348. Their substitution by cysteines resulted in efficient cross-linking [11]. Similarly, cysteine substitution of the two residues in the NN interface coming into close proximity in the model, Thr157 and Asp162, which are > 20 A apart in the crystal structure, also yielded cross-linking [11]. Finally, replacement of two phenylalanines located in the inter-protomer interfaces in the structure deduced from DEER data, which are not involved in protomer interactions in the crystal structure, yielded oligomerization-deficient mutants of both bovine and mouse arrestin-1 [13, 14, 65]. Non-oligomerizing mouse arrestin-1 was later transgenically expressed in rod photoreceptors of arrestin-1 knockout mice and tested in vivo [65]. It was shown to be biologically different from oligomerizing WT protein [65].
Solution tetramer of visual arrestin-1. In contrast to crystal, solution tetramer of arrestin-1 is a closed diamond. The four protomers are shown in different colors. In the tetramer the protomers interact via their N-domains (NN interface) and C-domains (CC interface). Both interfaces in the solution tetramer were confirmed by disulfide cross-linking
The DEER was instrumental in revealing receptor-bound conformation of arrestin-1 long before the structure of the arrestin-1–rhodopsin complex was solved [15]. As arrestin-1 readily oligomerizes, a single spin label per molecule yielded clear distances, reflecting the assembly of protomers into oligomers [13]. However, when enough phosphorylated light-activated rhodopsin was added to the sample to bind all arrestin-1 present, every one of these distances disappeared. This proved beyond reasonable doubt that only monomeric arrestin-1 can bind rhodopsin [13]. This finding is consistent with the model of the solution tetramer of arrestin-1 [11] (Fig. 3), which showed that rhodopsin-binding elements are shielded by sister protomers in the tetramer and both possible dimers. Recently this finding was confirmed yet again by the demonstration (using X-ray scattering) that the rate of arrestin-1 association with rhodopsin reconstituted into nanodiscs is directly proportional to the concentration of the monomeric form and does not depend on the concentration of the tetramer [66]. Normal ability of the monomer to bind rhodopsin was also confirmed in photoreceptors in vivo: oligomerization-deficient arrestin-1 mutant transgenically expressed in arrestin-1 knockout mice quenched light-induced rhodopsin signaling pretty much like WT protein [65]. Biochemical evidence obtained with purified proteins [67], in vivo studies of light-induced arrestin-1 translocation to the rhodopsin-containing rod outer segments in mouse lines expressing rhodopsin and arrestin-1 at different levels [68], as well as solved structure of the complex [6, 69], all tell the same story: one molecule of arrestin-1 binds one molecule of rhodopsin. Exactly the same mode of interaction of non-visual arrestin-2 with several GPCRs was revealed by the structures of complexes solved thus far [70,71,72,73,74,75], even though the orientation of the arrestin-2 molecule relative to the bundle of seven transmembrane helices of bound GPCR core was dramatically different in complexes with different GPCRs. Thus, this one-to-one binding appears to be the general rule of the arrestin-GPCR interaction.
5 Different Flavors of the Arrestin–GPCR Complex
Based on unusually high Arrhenius activation energy of arrestin-1 binding to rhodopsin, it was suggested more than 30 years ago that arrestin-1 must undergo a global conformational change in the process of receptor binding [76]. One element of this rearrangement, the release of the arrestin-1 C-terminus, was described soon thereafter [77]. Indeed, the structures solved later revealed that in the basal state the C-termini of all arrestins are anchored to the body of the N-domain [2, 44,45,46]. However, direct binding data at different temperatures suggested that there must be other receptor binding-induced rearrangements in the molecule that contribute to the activation energy [78]. As could be expected, WT arrestin-1 demonstrated virtually no binding to light-activated phosphorylated rhodopsin at 0 °C. In contrast, two “enhanced” mutants showed significant binding at this temperature. In one of these mutants, the C-terminus was detached by alanine substitutions of the three hydrophobic residues that anchor it to the body of the N-domain [2], i.e., this mutant had the C-terminus pre-released. In the other, the “polar core”, an arrangement of five interacting charged side chains in the middle of the molecule between the two domains [2], was destabilized by the reversal of one of the charges (Arg175Glu). Importantly, the mutant where these two substitutions were combined showed higher binding than either single mutant at all temperatures tested, 0 °C, 20 °C, and 37 °C [78]. Both enhanced mutants demonstrate much higher binding to unphosphorylated light-activated rhodopsin than the WT arrestin-1 [78,79,80]. Double mutant demonstrated higher binding to this form of rhodopsin than either single mutant at all three temperatures [78]. Thus, arrestin-1 binding involves both the release of the C-terminus and destabilization of the polar core. As the binding to unphosphorylated light-activated rhodopsin of the double mutant was several-fold higher at 20 °C and 37 °C than at 0 °C [78], there must be additional conformational rearrangements contributing to high activation energy of arrestin-1 binding.
DEER intra-molecular distance measurements in free and receptor-bound arrestin-1, -2, and -3 confirmed the release of the C-terminus upon receptor binding. The data showed that while the released C-terminus of the receptor-bound arrestin-1 does not have a fixed position, but likely “flops around”, yielding wide range of distances [12, 50], in case of non-visual arrestin-2 and -3 it occupies a certain preferred position upon its release [21]. Recent single-molecule fluorescence analysis suggested that there might be more than one preferred position of the arrestin-2 C-terminus released upon receptor binding [81]. DEER distance measurements revealed a number of additional conformational rearrangements in receptor-bound arrestin [15], which were later confirmed by the structure of arrestin-2 complex with multi-phosphorylated C-terminal peptide of the vasopressin V2 receptor [82], arrestin-1 bound to rhodopsin [6, 69], and arrestin-2 bound to β2AR [73], neurotensin NTSR1 [72, 75], other purified GPCRs [70, 71, 74], as well as arrestin-2 bound to parathyroid hormone receptor in the natural intracellular environment [83]. The orientation of the bound arrestin relative to the receptor core in the structures of its complex with various GPCRs was dramatically different. This is often explained by the difference among bound receptors and/or by the insertion of the receptor into roundish detergent micelle, as compared to relatively planar lipid bilayer in nanodiscs or the cell membrane. However, DEER measurements of the inter-molecular distances between certain points in the two proteins [6, 21] suggest a different explanation. In the first paper describing the structure of the arrestin-1 complex with rhodopsin, the distances between Y74 in the second transmembrane helix of rhodopsin (Ballesteros-Weinstein [84] numbering 2.41) and three points in different elements of bound arresin-1 molecule were measured [6]. In each case several distances were detected. The most populated distances in all three distributions matched the crystal structure [6]. However, the existence of other distances suggests that there are alternative forms of the complex that were not resolved in the structure. In a study describing higher resolution structure of the same complex the distances between arrestin-1 α-helix [2] and several residues in the rhodopsin C-terminus were measured by DEER [69]. Again, in each case the most populated distances matched the structure [69], but other distances were also detected, suggesting that rhodopsin C-terminus can occupy different positions relative to the bound arrestin-1. These data, often overlooked and underappreciated, convey a very important message: the same arrestin forms several distinct complexes with the same receptor (Fig. 2D). Recent in-cell cross-linking with unnatural amino acids (inserted by ribosomes when the cell expresses specialized engineered tRNA) of the complex of arrestin-2 with parathyroid hormone receptor PTRH1 suggests the same thing: no single shape of the complex could have possibly yielded all proximity points detected by cross-linking, indicating that several “flavors” of this complex co-exist in the natural environment of the living cell [83]. Molecular dynamics simulations support this conclusion: arrestin-2-PTHR1 complex is highly dynamic, changing its shape. Only the sum total of all shapes this complex can assume explains all detected proximity points between the two proteins [83].
In fact, widely accepted “barcode hypothesis” [85, 86], which posits that functional capabilities of arrestin bound to the same differentially phosphorylated GPCR are different, implies exactly that: the same arrestin forms several distinct complexes with the same differentially phosphorylated receptor, and each type of complex has unique set of functional capabilities. As experimental evidence supports the barcode hypothesis (e.g., [85, 87,88,89]), this implication should be taken seriously.
6 Conclusions
Numerous lines of evidence show that proteins and their complexes are dynamic. Their dynamic nature cannot be captured by structural studies, which yield very detailed information, but reveal only one of many possible conformational states. Biophysical and cell-based methods reveal a much more realistic picture of highly dynamic proteins and their complexes, which is consistent with known biology. Thus, we cannot rely solely on structures: they can be misleading without the information obtained by methods that detect and measure the movement of protein parts and of the proteins in the complex relative to each other. Naturally, every method has inherent limitations. Only synthesis of the information obtained by different methods can provide a clear insight into protein function, as illustrated here using GPCRs and their signal transducers as an exampleFootnote 1.
Availability of Data and Materials
No new data are presented and no materials were used.
Change history
04 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00723-023-01627-7
Notes
We use systematic names of arrestin proteins, where the number after the dash indicates the order of cloning: arrestin-1 (historic names S-antigen, 48 kDa protein, visual or rod arrestin), arrestin-2 (β-arrestin or β-arrestin1), arrestin-3 (β-arrestin2 or hTHY-ARRX), and arrestin-4 (cone or X-arrestin).
References
D.G. Lambright, J.P. Noel, H.E. Hamm, P.B. Sigler, Structural determinants for activation of the alpha-subunit of a heterotrimeric G protein. Nature 369(6482), 621–628 (1994)
J.A. Hirsch, C. Schubert, V.V. Gurevich, P.B. Sigler, The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell 97(2), 257–269 (1999)
K. Palczewski, T. Kumasaka, T. Hori, C.A. Behnke, H. Motoshima, B.A. Fox, I. Le Trong, D.C. Teller, T. Okada, R.E. Stenkamp, M. Yamamoto, M. Miyano, Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289(5480), 739–745 (2000)
S.G. Rasmussen, H.J. Choi, D.M. Rosenbaum, T.S. Kobilka, F.S. Thian, P.C. Edwards, M. Burghammer, V.R. Ratnala, R. Sanishvili, R.F. Fischetti, G.F. Schertler, W.I. Weis, B.K. Kobilka, Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450(7168), 383–387 (2007)
S.G. Rasmussen, B.T. DeVree, Y. Zou, A.C. Kruse, K.Y. Chung, T.S. Kobilka, F.S. Thian, P.S. Chae, E. Pardon, D. Calinski, J.M. Mathiesen, S.T. Shah, J.A. Lyons, M. Caffrey, S.H. Gellman, J. Steyaert, G. Skiniotis, W.I. Weis, R.K. Sunahara, B.K. Kobilka, Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477(7366), 549–555 (2011)
Y. Kang, X.E. Zhou, X. Gao, Y. He, W. Liu, A. Ishchenko, A. Barty, T.A. White, O. Yefanov, G.W. Han, Q. Xu, P.W. de Waal, J. Ke, M.H. Tan, C. Zhang, A. Moeller, G.M. West, B.D. Pascal, N. Van Eps, L.N. Caro, S.A. Vishnivetskiy, R.J. Lee, K.M. Suino-Powell, X. Gu, K. Pal, J. Ma, X. Zhi, S. Boutet, G.J. Williams, M. Messerschmidt, C. Gati, N.A. Zatsepin, D. Wang, D. James, S. Basu, S. Roy-Chowdhury, C.E. Conrad, J. Coe, H. Liu, S. Lisova, C. Kupitz, I. Grotjohann, R. Fromme, Y. Jiang, M. Tan, H. Yang, J. Li, M. Wang, Z. Zheng, D. Li, N. Howe, Y. Zhao, J. Standfuss, K. Diederichs, Y. Dong, C.S. Potter, B. Carragher, M. Caffrey, H. Jiang, H.N. Chapman, J.C. Spence, P. Fromme, U. Weierstall, O.P. Ernst, V. Katritch, V.V. Gurevich, P.R. Griffin, W.L. Hubbell, R.C. Stevens, V. Cherezov, K. Melcher, H.E. Xu, Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523(7562), 561–567 (2015)
T. Zhuang, Q. Chen, M.-K. Cho, S.A. Vishnivetskiy, T.I. Iverson, V.V. Gurevich, W.L. Hubbell, C.R. Sanders, Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc. Nat. Acad. Sci. USA 110(3), 942–947 (2013)
T. Zhuang, S.A. Vishnivetskiy, V.V. Gurevich, C.R. Sanders, Elucidation of inositol hexaphosphate and heparin interaction sites and conformational changes in arrestin-1 by solution nuclear magnetic resonance. Biochemistry 49(49), 10473–10485 (2010)
M. Elgeti, W.L. Hubbell, DEER Analysis of GPCR conformational Heterogeneity. Biomolecules 11(6), 778 (2021)
D.L. Farrens, C. Altenbach, K. Yang, W.L. Hubbell, H.G. Khorana, Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274(5288), 768–770 (1996)
S.M. Hanson, E.S. Dawson, D.J. Francis, N. Van Eps, C.S. Klug, W.L. Hubbell, J. Meiler, V.V. Gurevich, A model for the solution structure of the rod arrestin tetramer. Structure 16, 924–934 (2008)
S.M. Hanson, D.J. Francis, S.A. Vishnivetskiy, E.A. Kolobova, W.L. Hubbell, C.S. Klug, V.V. Gurevich, Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc. Natl. Acad. Sci. USA 103, 4900–4905 (2006)
S.M. Hanson, N. Van Eps, D.J. Francis, C. Altenbach, S.A. Vishnivetskiy, V.Y. Arshavsky, C.S. Klug, W.L. Hubbell, V.V. Gurevich, Structure and function of the visual arrestin oligomer. EMBO J. 26, 1726–1736 (2007)
M. Kim, S.M. Hanson, S.A. Vishnivetskiy, X. Song, W.M. Cleghorn, W.L. Hubbell, V.V. Gurevich, Robust self-association is a common feature of mammalian visual arrestin-1. Biochemistry 50, 2235–2242 (2011)
M. Kim, S.A. Vishnivetskiy, N. Van Eps, N.S. Alexander, W.M. Cleghorn, X. Zhan, S.M. Hanson, T. Morizumi, O.P. Ernst, J. Meiler, V.V. Gurevich, W.L. Hubbell, Conformation of receptor-bound visual arrestin. Proc. Nat. Acad. Sci. USA 109(45), 18407–18412 (2012)
A. Manglik, T.H. Kim, M. Masureel, C. Altenbach, Z. Yang, D. Hilger, M.T. Lerch, T.S. Kobilka, F.S. Thian, W.L. Hubbell, R.S. Prosser, B.K. Kobilka, Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 161(5), 1101–1111 (2015)
W.M. Oldham, N. Van Eps, A.M. Preininger, W.L. Hubbell, H.E. Hamm, Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat. Struct. Mol. Biol. 13(9), 772–777 (2006)
N. Van Eps, L.N. Caro, T. Morizumi, A.K. Kusnetzow, M. Szczepek, K.P. Hofmann, T.H. Bayburt, S.G. Sligar, O.P. Ernst, W.L. Hubbell, Conformational equilibria of light-activated rhodopsin in nanodiscs. Proc. Natl. Acad. Sci. USA 114(16), E3268–E3275 (2017)
N. Van Eps, W.M. Oldham, H.E. Hamm, W.L. Hubbell, Structural and dynamical changes in an alpha-subunit of a heterotrimeric G protein along the activation pathway. Proc. Natl. Acad. Sci. USA 103(44), 16194–16199 (2006)
N. Van Eps, A.M. Preininger, N. Alexander, A.I. Kaya, S. Meier, J. Meiler, H.E. Hamm, W.L. Hubbell, Interaction of a G protein with an activated receptor opens the interdomain interface in the alpha subunit. Proc. Natl. Acad. Sci. USA 108(23), 9420–9424 (2011)
Y. Zhuo, S.A. Vishnivetskiy, X. Zhan, V.V. Gurevich, C.S. Klug, Identification of receptor binding-induced conformational changes in non-visual arrestins. J. Biol. Chem. 289(30), 20991–21002 (2014)
V.V. Gurevich, E.V. Gurevich, The molecular acrobatics of arrestin activation. Trends Pharmacol. Sci 25, 59–112 (2004)
P. Samama, S. Cotecchia, T. Costa, R.J. Lefkowitz, A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 268(7), 4625–4636 (1993)
V. Cherezov, D.M. Rosenbaum, M.A. Hanson, S.G. Rasmussen, F.S. Thian, T.S. Kobilka, H.J. Choi, P. Kuhn, W.I. Weis, B.K. Kobilka, R.C. Stevens, High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318(5854), 1258–1265 (2007)
S.G. Rasmussen, H.J. Choi, J.J. Fung, E. Pardon, P. Casarosa, P.S. Chae, B.T. Devree, D.M. Rosenbaum, F.S. Thian, T.S. Kobilka, A. Schnapp, I. Konetzki, R.K. Sunahara, S.H. Gellman, A. Pautsch, J. Steyaert, W.I. Weis, B.K. Kobilka, Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature 469(7329), 175–180 (2011)
D.M. Rosenbaum, C. Zhang, J.A. Lyons, R. Holl, D. Aragao, D.H. Arlow, S.G. Rasmussen, H.J. Choi, B.T. Devree, R.K. Sunahara, P.S. Chae, S.H. Gellman, R.O. Dror, D.E. Shaw, W.I. Weis, M. Caffrey, P. Gmeiner, B.K. Kobilka, Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature 469(7329), 236–240 (2011)
M.T. Lerch, R.A. Matt, M. Masureel, M. Elgeti, K.K. Kumar, D. Hilger, B. Foys, B.K. Kobilka, W.L. Hubbell, Viewing rare conformations of the β2 adrenergic receptor with pressure-resolved DEER spectroscopy. Proc. Natl. Acad. Sci. USA 117(50), 31824–31831 (2020)
R.A. Dixon, B.K. Kobilka, D.J. Strader, J.L. Benovic, H.G. Dohlman, T. Frielle, M.A. Bolanowski, C.D. Bennett, E. Rands, R.E. Diehl, R.A. Mumford, E.E. Slater, I.S. Sigal, M.G. Caron, R.J. Lefkowitz, C.D. Strader, Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature 321, 75–79 (1986)
G. Milligan, E. Kostenis, Heterotrimeric G-proteins: a short history. Br J. Pharmacol. 147, S46-55 (2006)
J.W. Wisler, K. Xiao, A.R. Thomsen, R.J. Lefkowitz, Recent developments in biased agonism. Curr. Opin. Cell Biol. 27, 18–24 (2014)
C.M. Costa-Neto, L.T. Parreiras-E-Silva, M. Bouvier, A pluridimensional view of biased agonism. Mol. Pharmacol. 90(5), 587–595 (2016)
S.M. DeWire, J.D. Violin, Biased ligands for better cardiovascular drugs: dissecting G-protein-coupled receptor pharmacology. Circ. Res. 109(2), 205–216 (2011)
H. Indrischek, S.J. Prohaska, V.V. Gurevich, E.V. Gurevich, P.F. Stadler, Uncovering missing pieces: duplication and deletion history of arrestins in deuterostomes. BMC Evol. Biol. 17(1), 163 (2017)
D.S. Eiger, U. Pham, J. Gardner, C. Hicks, S. Rajagopal, GPCR systems pharmacology: a different perspective on the development of biased therapeutics. Am. J. Physiol. Cell Physiol. 322(5), C887-c895 (2022)
V.V. Gurevich, E.V. Gurevich, Biased GPCR signaling: possible mechanisms and inherent limitations. Pharmacol. Ther. 211, 107540 (2020)
L.M. Wingler, C. McMahon, D.P. Staus, R.J. Lefkowitz, A.C. Kruse, Distinctive activation mechanism for angiotensin receptor revealed by a synthetic nanobody. Cell 176(3), 479–490 (2019)
M. Natochin, M. Moussaif, N.O. Artemyev, Probing the mechanism of rhodopsin catalyzed transducin activation. J. Neurochem. 77, 202–210 (2001)
R.O. Dror, T.J. Mildorf, D. Hilger, A. Manglik, D.W. Borhani, D.H. Arlow, A. Philippsen, N. Villanueva, Z. Yang, M.T. Lerch, W.L. Hubbell, B.K. Kobilka, R.K. Sunahara, D.E. Shaw, SIGNAL TRANSDUCTION. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science 348(6241), 1361–1365 (2015)
E.V. Gurevich, J.J. Tesmer, A. Mushegian, V.V. Gurevich, G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol. Ther. 133(1), 40–46 (2012)
A.D. Tóth, S. Prokop, P. Gyombolai, P. Várnai, A. Balla, V.V. Gurevich, L. Hunyady, G. Turu, Heterologous phosphorylation-induced formation of a stability lock permits regulation of inactive receptors by β-arrestins. J. Biol. Chem. 293(3), 876–892 (2018)
C.V. Carman, J.L. Benovic, G-protein-coupled receptors: turn-ons and turn-offs. Curr. Opin. Neurobiol. 8, 335–344 (1998)
V.V. Gurevich, E.V. Gurevich, Plethora of functions packed into 45 kDa arrestins: biological implications and possible therapeutic strategies. Cell. Mol. Life Sci. 76(22), 4413–4421 (2019)
Y.K. Peterson, L.M. Luttrell, The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol. Rev. 69(3), 256–297 (2017)
M. Han, V.V. Gurevich, S.A. Vishnivetskiy, P.B. Sigler, C. Schubert, Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure 9(9), 869–880 (2001)
R.B. Sutton, S.A. Vishnivetskiy, J. Robert, S.M. Hanson, D. Raman, B.E. Knox, M. Kono, J. Navarro, V.V. Gurevich, Crystal structure of cone arrestin at 2.3Å: evolution of receptor specificity. J. Mol. Biol. 354, 1069–1080 (2005)
X. Zhan, L.E. Gimenez, V.V. Gurevich, B.W. Spiller, Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual arrestins. J. Mol. Biol. 406, 467–478 (2011)
S.K. Milano, H.C. Pace, Y.M. Kim, C. Brenner, J.L. Benovic, Scaffolding functions of arrestin-2 revealed by crystal structure and mutagenesis. Biochemistry 41(10), 3321–3328 (2002)
J. Granzin, U. Wilden, H.W. Choe, J. Labahn, B. Krafft, G. Buldt, X-ray crystal structure of arrestin from bovine rod outer segments. Nature 391(6670), 918–921 (1998)
C.L. Sander, J. Luu, K. Kim, D. Furkert, K. Jang, J. Reichenwallner, M. Kang, H.J. Lee, B.T. Eger, H.W. Choe, D. Fiedler, O.P. Ernst, Y.J. Kim, K. Palczewski, P.D. Kiser, Structural evidence for visual arrestin priming via complexation of phosphoinositols. Structure 30(2), 263–277 (2022)
S.A. Vishnivetskiy, D.J. Francis, N. Van Eps, M. Kim, S.M. Hanson, C.S. Klug, W.L. Hubbell, V.V. Gurevich, The role of arrestin alpha-helix I in receptor binding. J. Mol. Biol. 395, 42–54 (2010)
S.M. Hanson, W.M. Cleghorn, D.J. Francis, S.A. Vishnivetskiy, D. Raman, X. Song, K.S. Nair, V.Z. Slepak, C.S. Klug, V.V. Gurevich, Arrestin mobilizes signaling proteins to the cytoskeleton and redirects their activity. J. Mol. Biol. 368(2), 375–387 (2007)
S.M. Hanson, D.J. Francis, S.A. Vishnivetskiy, C.S. Klug, V.V. Gurevich, Visual arrestin binding to microtubules involves a distinct conformational change. J. Biol. Chem. 281, 9765–9772 (2006)
N. Wu, S.M. Hanson, D.J. Francis, S.A. Vishnivetskiy, M. Thibonnier, C.S. Klug, M. Shoham, V.V. Gurevich, Arrestin binding to calmodulin: a direct interaction between two ubiquitous signaling proteins. J. Mol. Biol. 364, 955–963 (2006)
S.A. Vishnivetskiy, L.E. Gimenez, D.J. Francis, S.M. Hanson, W.L. Hubbell, C.S. Klug, V.V. Gurevich, Few residues within an extensive binding interface drive receptor interaction and determine the specificity of arrestin proteins. J. Biol. Chem. 286, 24288–24299 (2011)
X. Song, D. Raman, E.V. Gurevich, S.A. Vishnivetskiy, V.V. Gurevich, Visual and both non-visual arrestins in their : “inactive” conformation bind JNK3 and Mdm2 and relocalize them from the nucleus to the cytoplasm. J. Biol. Chem. 281, 21491–21499 (2006)
M. Breitman, S. Kook, L.E. Gimenez, B.N. Lizama, M.C. Palazzo, E.V. Gurevich, V.V. Gurevich, Silent scaffolds: inhibition OF c-Jun N-terminal kinase 3 activity in cell by dominant-negative arrestin-3 mutant. J. Biol. Chem. 287(23), 19653–19664 (2012)
M.R. Ahmed, X. Zhan, X. Song, S. Kook, V.V. Gurevich, E.V. Gurevich, Ubiquitin ligase parkin promotes Mdm2-arrestin interaction but inhibits arrestin ubiquitination. Biochemistry 50, 3749–3763 (2011)
V.V. Gurevich, E.V. Gurevich, Extensive shape shifting underlies functional versatility of arrestins. Curr. Opin. Cell Biol. 27, 1–9 (2014)
C. Schubert, J.A. Hirsch, V.V. Gurevich, D.M. Engelman, P.B. Sigler, K.G. Fleming, Visual arrestin activity may be regulated by self-association. J. Biol. Chem. 274, 21186–21190 (1999)
S.M. Hanson, S.A. Vishnivetskiy, W.L. Hubbell, V.V. Gurevich, Opposing effects of inositol hexakisphosphate on rod arrestin and arrestin2 self-association. Biochemistry 47, 1070–1075 (2008)
S.A. Vishnivetskiy, Q. Chen, M.C. Palazzo, E.K. Brooks, C. Altenbach, T.M. Iverson, W.L. Hubbell, V.V. Gurevich, Engineering visual arrestin-1 with special functional characteristics. J. Biol. Chem. 288(17), 11741–11750 (2013)
Q. Chen, Y. Zhuo, P. Sharma, I. Perez, D.J. Francis, S. Chakravarthy, S.A. Vishnivetskiy, S. Berndt, S.M. Hanson, X. Zhan, E.K. Brooks, C. Altenbach, W.L. Hubbell, C.S. Klug, T.M. Iverson, V.V. Gurevich, An eight amino acid segment controls oligomerization and preferred conformation of the two non-visual arrestins. J. Mol. Biol. 433(4), 166790 (2021)
S.K. Milano, Y.M. Kim, F.P. Stefano, J.L. Benovic, C. Brenner, Nonvisual arrestin oligomerization and cellular localization are regulated by inositol hexakisphosphate binding. J. Biol. Chem. 281, 9812–9823 (2006)
Q. Chen, N.A. Perry, S.A. Vishnivetskiy, S. Berndt, N.C. Gilbert, Y. Zhuo, P.K. Singh, J. Tholen, M.D. Ohi, E.V. Gurevich, C.A. Brautigam, K.S. Klug, V.V. Gurevich, T.M. Iverson, Structural basis of arrestin-3 activation and signaling. Nat. Commun. 8(1), 1427 (2017)
S. Samaranayake, S.A. Vishnivetskiy, C.R. Shores, K.C. Thibeault, S. Kook, J. Chen, M.E. Burns, E.V. Gurevich, V.V. Gurevich, Biological role of arrestin-1 oligomerization. J. Neurosci. 40(42), 8055–8069 (2020)
Y. Imamoto, K. Kojima, R. Maeda, Y. Shichida, T. Oka, Role of monomer/tetramer equilibrium of rod visual arrestin in the interaction with phosphorylated rhodopsin. Int. J. Mol. Sci. 24(5), 4963 (2023)
T.H. Bayburt, S.A. Vishnivetskiy, M. McLean, T. Morizumi, C.-C. Huang, J.J. Tesmer, O.P. Ernst, S.G. Sligar, V.V. Gurevich, Rhodopsin monomer is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J. Biol. Chem. 286, 1420–1428 (2011)
S.M. Hanson, E.V. Gurevich, S.A. Vishnivetskiy, M.R. Ahmed, X. Song, V.V. Gurevich, Each rhodopsin molecule binds its own arrestin. Proc. Nat. Acad. Sci. USA 104, 3125–3128 (2007)
X.E. Zhou, Y. He, P.W. de Waal, X. Gao, Y. Kang, N. Van Eps, Y. Yin, K. Pal, D. Goswami, T.A. White, A. Barty, N.R. Latorraca, H.N. Chapman, W.L. Hubbell, R.O. Dror, R.C. Stevens, V. Cherezov, V.V. Gurevich, P.R. Griffin, O.P. Ernst, K. Melcher, H.E. Xu, Identification of phosphorylation codes for arrestin recruitment by G protein-coupled receptors. Cell 170(3), 457–469 (2017)
J. Bous, A. Fouillen, H. Orcel, S. Trapani, X. Cong, S. Fontanel, J. Saint-Paul, J. Lai-Kee-Him, S. Urbach, N. Sibille, R. Sounier, S. Granier, B. Mouillac, P. Bron, Structure of the vasopressin hormone-V2 receptor-β-arrestin1 ternary complex. Sci. Adv. 8(35), eabo7761 (2022)
C. Cao, X. Barros-Álvarez, S. Zhang, K. Kim, M.A. Dämgen, O. Panova, C.M. Suomivuori, J.F. Fay, X. Zhong, B.E. Krumm, R.H. Gumpper, A.B. Seven, M.J. Robertson, N.J. Krogan, R. Hüttenhain, D.E. Nichols, R.O. Dror, G. Skiniotis, B.L. Roth, Signaling snapshots of a serotonin receptor activated by the prototypical psychedelic LSD. Neuron 110(19), 3154–3167 (2022)
W. Huang, M. Masureel, Q. Qianhui, J. Janetzko, A. Inoue, H.E. Kato, M.J. Robertson, K.C. Nguyen, J.S. Glenn, G. Skiniotis, B.K. Kobilka, Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579(7798), 303–308 (2020)
Y. Lee, T. Warne, R. Nehmé, S. Pandey, H. Dwivedi-Agnihotri, M. Chaturvedi, P.C. Edwards, J. García-Nafría, A.G.W. Leslie, A.K. Shukla, C.G. Tate, Molecular basis of β-arrestin coupling to formoterol-bound β(1)-adrenoceptor. Nature 583(7818), 862–866 (2020)
D.P. Staus, H. Hu, M.J. Robertson, A.L.W. Kleinhenz, L.M. Wingler, W.D. Capel, N.R. Latorraca, R.J. Lefkowitz, G. Skiniotis, Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc. Nature 579(7798), 297–302 (2020)
W. Yin, Z. Li, M. Jin, Y.L. Yin, P.W. de Waal, K. Pal, Y. Yin, X. Gao, Y. He, J. Gao, X. Wang, Y. Zhang, H. Zhou, K. Melcher, Y. Jiang, Y. Cong, X. Edward Zhou, X. Yu, Xu. Eric, A complex structure of arrestin-2 bound to a G protein-coupled receptor. Cell Res. 29(12), 971–983 (2019)
A. Schleicher, H. Kuhn, K.P. Hofmann, Kinetics, binding constant, and activation energy of the 48-kDa protein-rhodopsin complex by extra-metarhodopsin II. Biochemistry 28(4), 1770–1775 (1989)
K. Palczewski, J. Buczyłko, N.R. Imami, J.H. McDowell, P.A. Hargrave, Role of the carboxyl-terminal region of arrestin in binding to phosphorylated rhodopsin. J. Biol. Chem. 266, 15334–15339 (1991)
V.V. Gurevich, S.M. Hanson, X. Song, S.A. Vishnivetskiy, E.V. Gurevich, The functional cycle of visual arrestins in photoreceptor cells. Prog. Retin. Eye Res. 30(6), 405–430 (2011)
V.V. Gurevich, J.L. Benovic, Visual arrestin binding to rhodopsin: diverse functional roles of positively charged residues within the phosphorylation-recognition region of arrestin. J. Biol. Chem. 270(11), 6010–6016 (1995)
V.V. Gurevich, The selectivity of visual arrestin for light-activated phosphorhodopsin is controlled by multiple nonredundant mechanisms. J. Biol. Chem. 273, 15501–15506 (1998)
W.B. Asher, D.S. Terry, G.G.A. Gregorio, A.W. Kahsai, A. Borgia, B. Xie, A. Modak, Y. Zhu, W. Jang, A. Govindaraju, L.Y. Huang, A. Inoue, N.A. Lambert, V.V. Gurevich, L. Shi, R.J. Lefkowitz, S.C. Blanchard, J.A. Javitch, GPCR-mediated β-arrestin activation deconvoluted with single-molecule precision. Cell 185(10), 1661–1675 (2022)
A.K. Shukla, A. Manglik, A.C. Kruse, K. Xiao, R.I. Reis, W.C. Tseng, D.P. Staus, D. Hilger, S. Uysal, L.Y. Huang, M. Paduch, P. Tripathi-Shukla, A. Koide, S. Koide, W.I. Weis, A.A. Kossiakoff, B.K. Kobilka, R.J. Lefkowitz, Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 497(7447), 137–141 (2013)
Y. Aydin, T. Böttke, J.H. Lam, S. Ernicke, A. Fortmann, M. Tretbar, B. Zarzycka, V.V. Gurevich, V. Katritch, I. Coin, Structural details of a class B GPCR-arrestin complex revealed by genetically encoded crosslinkers in living cells. Nat. Commun. 14(1), 1151 (2023)
J.A. Ballesteros, H. Weinstein, Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 (1995)
J. Kim, S. Ahn, X.R. Ren, E.J. Whalen, E. Reiter, H. Wei, R.J. Lefkowitz, Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc. Nat. Acad. Sci. USA 102, 1442–1447 (2005)
K.N. Nobles, K. Xiao, S. Ahn, A.K. Shukla, C.M. Lam, S. Rajagopal, R.T. Strachan, T.Y. Huang, E.A. Bressler, M.R. Hara, S.K. Shenoy, S.P. Gygi, R.J. Lefkowitz, Distinct phosphorylation sites on the {beta}2-adrenergic receptor establish a barcode that encodes differential functions of {beta}-arrestin. Sci. Signal 4, 51 (2011)
M. Choi, D.P. Staus, L.M. Wingler, S. Ahn, B. Pani, W.D. Capel, R.J. Lefkowitz, G protein-coupled receptor kinases (GRKs) orchestrate biased agonism at the β2-adrenergic receptor. Sci. Signal 11, 544 (2018)
A.I. Kaya, N.A. Perry, V.V. Gurevich, T.M. Iverson, Phosphorylation barcode-dependent signal bias of the dopamine D1 receptor. Proc. Nat. Acad. Sci. USA 117(25), 14139–14149 (2020)
X.R. Ren, E. Reiter, S. Ahn, J. Kim, W. Chen, R.J. Lefkowitz, Different G protein-coupled receptor kinases govern G protein and beta-arrestin mediated signaling of V2 vasopressin receptor. Proc. Nat. Acad. Sci. USA 102, 1448–1453 (2005)
Acknowledgements
This work was supported by NIH Grants EY011500, GM122491, and Cornelius Vanderbilt Endowed Chair (Vanderbilt University).
Funding
Supported in part by NIH RO1 EY011500, R35 GM122491, and Cornelius Vanderbilt Endowed Chair (Vanderbilt University).
Author information
Authors and Affiliations
Contributions
VVG wrote and edited the manuscript. EVG edited the manuscript and created figures.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no competing interests.
Ethical approval
No human or animal studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised as the second author’s name Eugenia V. Gurevich was incorrectly written as Eugenis V. Gurevich.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gurevich, V.V., Gurevich, E.V. Dynamic Nature of Proteins is Critically Important for Their Function: GPCRs and Signal Transducers. Appl Magn Reson 55, 11–25 (2024). https://doi.org/10.1007/s00723-023-01561-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-023-01561-8