Abstract
The aim of this work is to show that the bones with marrow, treated as a quasi-porous media, can be successfully used to study the effects of ovariectomy-induced osteoporosis. Proton one-dimensional (1D) nuclear magnetic resonance (NMR) T 2-distribution and two-dimensional (2D) T 2–T 2 exchange maps combined with histological images were used to measure the proximal part of the femoris, diaphysis and distal epiphysis of ovariectomized and non-ovariectomized Wistar albino rats. The 1D normalized T 2 distributions showed four peaks which were associated with protons in four major pools: (1) the protons from bounded water to collagenous matrix; (2) fluids in osteocyte lacunae and canaliculi channels; (3) fluids in secondary pores like Haversian and transverse Volkmann canals and (4) soft matter like bone marrow and fluids in primary pores like trabecular bone cavities. The peak’s association and hierarchical structure of pores in femoral bone were supported by a 2D T 2–T 2 exchange map and by a series of dehydration experiments monitored by NMR measurements. The bone marrow narrows the T 2-distributions, increasing resolution, but will not influence significantly the peaks positions; therefore, the NMR relaxometry is a valuable tool to characterize the pore distributions and effects of induced osteoporosis in diverse bones sections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Osteoporosis is the most common metabolic bone disease, causing annually 1.66 millions hip, wrist and vertebral fractures [1]. The two main characteristics of this disease are a decreased bone mass and the alteration of bone microarchitecture. In osteoporosis, interconnecting cavities corresponding to cancellous bone increase in size and the bony trabeculas become thicker. As the bone is a porous structure, these cavities are the largest, followed by the vascular, lacunar-canalicular and collagen-apatite porosities. Therefore, the bone is considered a composite material consisting of a collagenous matrix mineralized with nonstoichiometric calcium apatite [2]. In addition, it contains water, part of which is bound to collagen while a larger fraction occupies the spaces of the Haversian and lacuno-canalicular system.
There are many similarities between human and rat bone; therefore, rat models are frequently used for osteoporosis studies. In both species, the bone elongates by epiphyseal growth and increases in cross-sectional area by periosteal growth. The secondary spongiosa in rats undergoes sequential remodeling, similar to that observed in human cancellous bone [3, 4]. To obtain the equivalent of postmenopausal osteoporosis in women, an estrogen deficiency can be induced by means of rat surgical ovariectomy [5].
The bone morphological changes can be assessed using several approaches: standard histological techniques, immunohistochemistry, confocal microscopy, scanning and transmission electron microscopy [6], micro-CT [7], dual-energy X-ray absorptiometry [8] and more recently, by cryo-porometry and thermo-porometry [9]. To the best of our knowledge this is the first investigation related to the osteoporosis in rat femurs using 1D and 2D 1H nuclear magnetic resonance (NMR) transverse relaxometry. Just several articles discuss the subject of rat osteoporosis by NMR in general, or by NMR relaxometry in particular [10–12]. In this sense, Fantazzini et al. used the 1H transverse and longitudinal relaxation times distribution to study the L1 to L6 rat lumbar vertebrae [13]. Magnetic resonance imaging (MRI) has shown to be able to differentiate between postmenopausal women, to enhance the discrimination between osteoporotic subjects and age-matched controls when associated with bone mineral density analysis [13–15]. 31P and 1H-MRI techniques were used for the evaluation of treatment following ovariectomy in rats undergoing treatment with alendronate and the results were used for the estimation of bone mineral and matrix properties [16]. Nevertheless, there are a series of report on the human cortical bone measurement by 1D and 2D 1H NMR relaxometry [17–22], while the problem of water in bone was discussed from many points of view [16–28]. Horch et al. in Ref. [17] used the 1D (FID—free induction decay and CPMG—Carr–Purcell–Meiboom–Gill decays) and 2D (T 1–T 2 correlation and T 2–T 2 exchange maps) NMR to make the assignment of various biophysical proton sources like collagen, collagen bound water, pore space water and lipid methylene; furthermore, these authors discussed clinically compatible MRI strategies for discriminating bound water from pore water in human cortical bone based on multiple peaks observed in T 2-distributions [18]. Ni et al. used the T 2 relaxation data measured by CPMG pulse sequence to obtain the T 2-distribution which finally can be transformed to a pore size distribution [19]. Horch et al. [20] and Nyman et al. [21] use the 1D transverse relaxation time T 2-distributions (and high-resolution X-ray tomography [20]) to correlate the microscopic parameters with mechanic properties of bone.
The goal of this work is to use 1D and 2D 1H NMR transverse relaxation times distribution to compare the effects of the induced osteoporosis on the rat femoral bone considered as a quasi-porous media. First step to this goal was to clarify the association of experimental peaks from 1D T 2 distributions with proton species located in various bone pores, i.e., primary pores like the bone cavities, secondary osteonal canals like Haversian channels and transverse Volkmann canals, the space between the osteocyte and lacunar–canalicular wall, and protons from collagen or bounded water to collagen. Finally, the time evolution of the bone structure during 8 weeks post-ovariectomy was evaluated by NMR Laplace relaxometry, separately for the proximal part of femoris, diaphysis and distal epiphysis and the results are compared with some histological data.
2 Methods
2.1 Samples
We used 25 Albino Wistar adult female rats, of 16 months age (human correspondent: women from 47 years old [10–12, 29, 30]) at the beginning of the observation, with an average weight of 300 grams. One animal was measured at 1 day after sacrifice and the rest of 24 animals were divided into 8 groups of 3 rats each. Half of them have been ovariectomized (OVX) and half remained non-ovariectomized (NOVX). The surgical protocol applied on the rats that suffered ovariectomy was performed as previously described [10–12, 31]. After the ovariectomy at 2, 4, 6, and 8 weeks, the animals from each group were sacrificed with an overdose of ketamine and xiline (8–10 mg/kg). From each rat the right femoral bone was harvested and preserved in 10 % formalin until the NMR measurements. Before NMR measurement the rat femurs was wiped with an absorbent paper and then was cut in three parts. The first section was made under the trochanter and the second one above the intercondylar fossa, resulting three parts: proximal part of femoris (femoral head, femoral neck and proximal diaphysis), diaphysis and distal epiphysis. The animal investigation has been approved by the Ethics Committee of the University of Medicine and Pharmacy of Targu Mures, Romania.
2.2 NMR Measurements and Data Analysis
Proton NMR measurements were performed using the BRUKER MINISPEC mq20 spectrometer working at 19.7 MHz. The echo time in CPMG pulse sequence was 0.4 ms. The tipping pulse length was 9 µs and the refocusing pulses 18 µs. A total number of 3000 echoes were recorded (see Fig. 1a).
The 2D 1H NMR exchange spectrum was recorded using the T 2–M z(store)–T 2 pulse sequence [32] with a period of magnetization storage (t s) between two encoding periods. The CPMG echo train with a variable number of echoes, up to 128, was used to encode the signal amplitude in the indirect dimension. The magnetization decay was recorded in the direct dimension via a CPMG pulse sequence of 300 echoes. The echo time was 4 ms and the storage time was t s = 20 ms (see Fig. 1b).
The decay of transverse magnetization during the CPMG echoes train was considered to be multi-exponential described by,
where \(\tau = nt_{\text{E}}\) is the time of apparition of the nth echo, T 2 is the transverse relaxation time of each particular statistical sub-ensemble (pools) of protons and f(T 2) is the distribution function which has to be determined. To this purpose the experimental data was analyzed using Laplace inversion procedure [10–12, 33–45]. We use the Prospa numeric software [36]. The experimental two-dimensional relaxation decays was inverted to obtain the 2D T 2-relaxation spectrum using an algorithm based on the relationship,
where, the i and d indices represent the indirect and direct dimensions.
3 Results
3.1 One-Dimensional T 2 Laplace Distributions
Examples of CPMG echoes train decays are presented in Fig. 2a for an ovariectomized rat measured at 2 weeks after ovariectomy on proximal part of femoris, diaphysis and distal epiphysis. The corresponding transverse relaxation distributions are presented in Fig. 2b for the ovariectomized Rat 2. In Fig. 2a the continuous, dashed and doted lines correspond to the best fit of experimental data as resulted from Laplace inversion (Eq. 1).
a CPMG echoes train decays measured for samples of proximal part of femoris (open circle and continuous line), diaphysis (open square and dashed line) and distal epiphysis (open up triangle and dotted line) rat femurs for the ovariectomized rat 2. For all CPMG decays, 3000 echoes were measured but only 5 % evenly distributed are shown. In insert the same CPMG decay is shown in the initial time regime. The lines represent the best fits of the experimental data. b Transverse relaxation time distributions obtained from CPMG decays obtained by 1D Laplace inversion
In all distributions one can observe four peaks. Three peaks are in the domain from several milliseconds to several hundreds of milliseconds, range observed also in the T 2-distributions measured by Fantazzini et al. for rat lumbar vertebral bone [13]. A larger number of peaks are observed in the T 2-distributions measured for human cortical bone [17, 18, 20]. Horch et al. [26] observe a number of five peaks in the domain 0.1 ms to 1 s for rat optical nerve with a distribution similar with those presented in Fig. 2b for rat femur. Compared with our Laplace distributions, Horch et al. observe an additional peak located at several milliseconds, but in both cases the main peak is located ~60–100 ms. Usually, the peaks with T 2 values smallest than 1 ms were associated to collagen bound water [18, 20, 21] and peaks with T 2 values than 0.1 ms were associated with collagen hydrogen [20, 21]. The peaks located at T 2 values larger than 1 ms can be associated with pore water and lipids [18, 20] and are of interest for the study of osteoporosis.
3.2 The Peaks Assignments
The peak assignment in the T 2-distributions (Fig. 2b) corresponding to T 2 values larger than 1 ms can be made if we treat the bone as a porous media [42]. In trabecular bone it is easy to see differences in dimensions of intertrabecular spaces, but for that, the bone has to be de-fatted and saturated with water [11, 12, 42]. This is not our case, where NMR was used as a non-destructive method which preserves the samples to be used for additional analysis (i.e., demineralization for histological images) where the bone integrity is compromised.
In literature are described three levels of porosity in bone [2], which are hierarchically embedded one inside another [46]. The largest pores are associated with the vascular porosity (VP), which forms all the tunnels in bone that contain blood vessels and includes all the primary and secondary osteonal canals as well as transverse (Volkmann) canals. The second largest pores are associated with lacunar–canalicular porosity (LCP) and contain the osteocyte lacunae and canaliculi channels. The space between the osteocyte and lacunar–canalicular wall is filled by the osteocyte’s glycocalyx and interstitial fluid. The smallest pores are found in the collagen-apatite porosity (CAP). The pores are spaced between the collagen and the crystallites of the mineral apatite. At this level, most of the water is bound water [47, 48].
For the measured T 2-distribution we will make the following assignments: (1) the peaks observed at smallest T 2 values (of the order of several hundreds of microseconds) will be associated with the NMR signal arising from protons from collagen or bounded water to collagen [18, 19, 21]; (2) the peaks observed at several milliseconds will be associated with the protons located in pores which form the space between the osteocyte and lacunar–canalicular wall (see the lacunae in Fig. 3a); (3) the peaks located at several tens of milliseconds will be associated with protons located in secondary osteonal canals (see the Haversian channels in Fig. 3a) and transverse Volkmann canals and (4) the peaks located at several hundreds of milliseconds will be associated with protons located in primary pores (see the cavities from Fig. 3b). But, these cavities are not empty as in ordinary porous materials and parts of these are filled with bone marrow (see Fig. 3c). Therefore, any associations must be based on some experimental tests which are present below.
a Optical image with magnification ×20 of a transverse section of a long bone diaphysis showing multiple Haversian systems, the relative dimensions of Haversian channel, lamellae and interlamellar lacunas; b optical image with no magnification of a bone transverse section showing numerous cavities in trabelcular bone; c histological image (magnification ×10) with hematoxylin–eosine staining of a transverse section in rat diaphysis recorded for a non-ovariectomized Wistar rat with evidence of bone pores, trabelculas and bone marrow
3.3 Experimental Tests to Support the Peak Assignments
The first test is based on the interpretation of a 2D T 2–T 2 molecular exchange Laplace–Laplace spectrum (Fig. 4) obtained for non-ovariectomized rat femurs. Three peaks are located on the main diagonal. Three extra-diagonal peaks are observed as an indication of the existence of molecular exchange between protons from different pools. The main peak (T 2 ~ 70 ms) is connected via two exchange peaks to the position located at T 2 ~ 400 ms. This exchange phenomena must takes place between two locations corresponding to two adjacent levels in the bone’s hierarchy. The third exchange peak connects the main peak (T 2 ~ 70 ms) to the peak located at T 2 ~ 11 ms. The peak located at T 2 ~ 70 ms correspond to protons species which can be directly exchanged with protons characterized by higher and lower relaxation times. The protons with highest T 2 values are not connected directly to the protons with lowest T 2. In conclusion, most probably the protons associated with the main peak are located spatially in the medium cavities of rat bone femurs, like Haversian channels, which are anatomically connected with large cavities and communicate also with spaces between the osteocyte and lacunar–canalicular wall (see Fig. 3a).
The second test is based on the observation of the dehydration process where the mobile fractions from femoral bone are extracted. For that, two fresh harvested rat femoral bones belonging to the 25th witness rat, right and left, were measured during drying process [27]. The 1D T 2-distributions measured separately for proximal part of femoris, diaphysis and distal epiphysis are presented in Fig. 5. Initially, the distribution presents four wide peaks. No significant differences are to be mentioned between the distributions obtained for different sections. A rapid extraction of mobile tissue fractions was obtained once the right rat femur was exposed during 4 h at 105 °C into an ECOCELL oven with hot air. The corresponding T 2-distributions are presented in Fig. 4a–c. Dramatic changes are observed for all three parts of the rat right femurs. All distributions have much smaller intensities. The T 2-distributions observed for proximal part of femoris and distal epiphysis are characterized by: (1) three wider peaks; (2) an insignificant peak located at several microseconds; (3) two peaks with the largest T 2-values which merged and (4) the peak with the smallest T 2-value which present some components with a lower T 2-value than 100 µs.
One-dimensional distribution of transverse relaxation times measured for three sections of a rat right femur: a proximal part of femoris; b diaphysis and c distal epiphysis and for the left femur; d proximal part of femoris; e diaphysis and f distal epiphysis. The T 2 distribution were shown for fresh, harvested 1 day before, after 4 h exposure at 105 °C into an oven and then dried naturally at room temperature and measured after 24 days (dots) and 64 days (continuous line). The left femur was not treated in oven
After thermal treatment, the right rat femur was stored into a plastic box with a cap to dry naturally at room temperature. The T 2-distributions were measured again after 24 and 64 days. In all cases, the T 2-distributions become extremely narrow, indicating for each hierarchical level a homogeneous distribution. The T 2-distributions are characterized by four peaks, with the exception of those recorded for proximal part of femoris at 64 days (see Fig. 5a). In the case of T 2-distributions measured for proximal part of femoris after 64 days of natural drying the peaks corresponding to large T 2-values have an extremely low integral area. The majority of protons are characterized by a low T 2-value which can be associated with bounded water, and then we can consider that the mobile tissue fractions were dried out from rat bone. Therefore, the pores from proximal part of femoris present more connections, fact which explains the increased incidence of osteoporosis in proximal part of femoris compared with the rest of femur bone.
If the natural drying process starts with protons located in the largest pores (the cavities) and continues hierarchically with tissues located in pores with one level smaller then the smaller T 2-values could correspond to bounded protons and the larger T 2-values could correspond to protons located in the largest cavities of rat femoral bone.
To ensure that the thermal treatment was not destroying the bone’s internal structure, a similar experiment was performed on the left femurs harvested from the same rat but untreated. The corresponding normalized T 2-distributions are presented in Fig. 4d–f. The main features presented by the T 2-distributions measured for left rat femur sections are similar with those observed in the case of right femur and the small differences which are not significant and will not change the general observations.
The third test is based on the comparison of T 2-distributions measured for the bone with marrow and defatted bone with pores partial or fully filled with formalin (see Fig. 6). In Fig. 6a the T 2-distributions of freshly harvested femur from the 25th rat (shown in Fig. 5a) are compared with the T 2-distribution measured for the same section defatted and saturated with water. One can observe that: (1) the fifth peaks which appear for saturated pores is located at T 2 larger than 1 s which corresponds to free water probably from medullar channel, therefore, will be neglected; (2) at saturation the peaks become broaden and the amount of water decrease with the increase of T 2-values; (3) the amount of collagen protons is similar for the bone with marrow (39.5 %) and water saturated bone (35.9 %); (4) the presence of bone marrow affect mostly the peaks integral areas and less the peaks position. The last observation is supported also by the measurements of T 2-distributions in the case of partial saturated pores (see Fig. 6b).
In conclusion, from the 1D T 2-distributions measured for the rat femurs during drying process the water located in pores and bone marrow are characterized by peaks in the NMR T 2-distributions in the domain from several milliseconds to several hundred of milliseconds. This result is in agreement with the result obtained by Fantazzini et al. on rat lumbar vertebrae [13] and with the study on human cortical bone [15–20]. Moreover, the mobility of these components was evidenced in the 2D T 2–T 2 exchange map presented in Fig. 4. The 1D T 2-distributions, measured for saturated and partial saturated pores in bone, show that the marrow affects less the peaks position. These three tests demonstrate that our initial assumption of pores hierarchy associated with particular T 2 values is valid. In the following our focus will be oriented to the characterization of bone osteoporosis from 1D 1H NMR T 2-distributions. In this sense the peak associated with bound water to collagen will be excluded from discussion.
The morphological changes of dynamic tissues like the bones are related to multiple mechanisms like the rat genetic material, physical activity, food and/or medical treatment [10, 12]. Two apparent identical rats can present diverse features of the measured T 2-distributions. Then, a unique transverse relaxation time distribution measured for a selected rat cannot be relevant to characterize the specific features for each study group. To avoid this problem, a relatively large number of rats must to be studied. The choice of a large number of rats, which has to be scarified, is in contradiction with the research ethic. In this sense, we consider that a number of three rats per group of study is a reasonable compromise. In fact, in our extended investigation (which assumes also the study of the effect of simvastatin and fenofibrate [10–12]) less than 5 % of the measured T 2-distributions present a certain deviation from the average distribution. Therefore, in our case three rats per group of study is statistically relevant.
4 Discussions
4.1 The Effects of Induced Osteoporosis
The volume average T 2-distributions were measured and compared for all femur parts for ovariectomized and non-ovariectomized rats. In each category we included four groups according to the number of weeks of observation before sacrifice 2, 4, 6 and 8. Figure 7 shown the average normalized T 2-distributions measured for rat femurs belonging to all six categories. On the left side (Fig. 6a–c) are presented the distributions belong to the NOVX rats while the average T 2-distributions for OVX rats are presented on the right side (Fig. 6d–f).
Normalized distribution of transverse relaxation times T 2 measured after 2, 4, 6, and 8 weeks of observation for non-ovariectomiezed rats on the a proximal part of femoris, b diaphysis, and c distal epiphysis for non-ovariectomized rats. The same distributions are shown for d proximal part of femoris, e diaphysis, and f distal epiphysis for ovariectomized rats
In general, the volume average normalized T 2-distributions for NOVX rats, compared at different weeks of observation before measurements, are not identical but the differences are small. All three peaks of interest (with T 2 in the range of several milliseconds to one second) are well resolved. The amplitude of the main peak, T 2 ~ 65 ms associated with the protons located in secondary pores like Haversian canals, decreases slightly from week two to week eight, but the center of peak is not affected. For normalized distributions, the decrease of amplitude is associated with an increase of the distribution widths. For a bone, this effect could be a consequence of a wider distribution of pores sizes.
The peak corresponding to small pores with T 2 ~ 10 ms (protons from lacunae), recorded for the rats group scarified at 2 weeks, presents some shoulders (see Fig. 7a) but these can be rather attributed to the averaging procedure than to real distributions of pores. With the increase of the observation time, the plateau (with zero probability) between the peaks centered at T 2 ~ 10 ms and the peaks centered at T 2 ~ 65 ms is reduced, then one can assume that the parts of lacunae will increase in size and parts of secondary canals will have a reduced dimension. A μCT study showed that the average lacunar volume increases in osteoporosis [49], supporting our observation. The peak associated with bone cavities localized between 200 and 300 ms at 2 weeks, in time becomes more broaden extending a tail towards 800–900 ms at 8 weeks. This is not an unexpected process for the non-ovariectomized rats, since with time the rats become older.
Large changes can be found when the average normalized T 2-distributions of proximal part of femoris measured for the OVX rats are compared at different times (Fig. 7d). The major difference is observed for the average normalized T 2-distribution obtained at 8 weeks after ovariectomy, where the main peak (located at T 2 between ~ 40 and ~100 ms) presents a right shoulder, which is an indication of major changes at the level of secondary pores structure. The peaks appear to be broad compared with the case of non-ovariectomized rats (Fig. 7a). A histomorphometric analysis has shown that, for ovariectomized rats, the volumetric fraction of trabecular bone in the proximal part of the femoris is increased [50]. This could explain the high frequency of femoral neck fractures in patients with osteoporosis. The peaks located at several milliseconds seem to be less affected especially at 4 and 6 weeks. Finally, one can conclude that the lacunar pores located in proximal part of femoris are less affected by the induced osteoporosis than the larger pores.
The porous structure of diaphysis for both NOVX and OVX rats (Fig. 6b, e) seems to be more organized compared to proximal region of femoris and distal epiphysis. The average normalized T 2-distribution recorded for the NOVX rats’ diaphysis after 2 weeks of observation present the narrowest peaks (Fig. 7b). In the T 2-distributions, we may observe that: (1) the main peak (located at T 2 ~ 60 ms) become broader, indicating an increase of secondary pores size distribution; (2) the peaks corresponding to the largest cavities migrate from T 2 < 200 ms at 2 weeks towards T 2 ~ 400 ms at 6 and 8 weeks but start to collapse (having small amplitude and large width) even at 4 weeks (Fig. 7b); (3) the same effect of broadening and migration from smaller to larger T 2-values can be observed also for the peak located at several milliseconds associated with inter-lamellar/lacunar pores. The time evolution of average normalized T 2-distribution recorded for the ovariectomized rats’ diaphysis is not so spectacular (Fig. 7e). The major indication of bone diaphysis time evolution is the slight broadening of the main peak (T 2 ~ 60 ms).
The evolution in time of the volume average normalized T 2-distributions recorded for rats’ distal epiphysis (Fig. 6c, f) is similar with those of the proximal part of femoris. The main peaks, with T 2 ~ 60 ms associated with protons from secondary canals, and middle peaks, with T 2 ~ 10 ms associated with protons from lacunar pores, recorded for non-ovariectomized rats (Fig. 7c) in time are less affected. Contrary, major changes in peak position and shape can be observed for peaks located at large T 2-values which migrate from T 2 ~ 200 ms towards T 2 ~ 400 ms. We concluded that the increased sizes of cavities from distal epiphysis are most probably due to a natural process of bone growing. Broaden peaks in the average normalized T 2-distributions can be observed for distal epiphysis belonging to ovariectomized rats (Fig. 7f). The evolution in time is noticeable only for the peaks associated with large cavities in distal epiphysis. Narrow and resolved peaks, with large zero probability between them, can be observed at 8 weeks for both ovariectomized and non-ovariectomized rats.
We show that the most affected rat’s femur part by the ovariectomy induced osteoporosis is the proximal part of femoris and the less affect part is the diaphysis [51]. The trabecular cavities are the pores most susceptible to undergo the effects. Figure 8 show the evolution of T 2-center of gravity (Fig. 8a) and log-width (Fig. 8b) calculated for peaks associated with water pools from trabecular cavities located at the level of proximal part of femoris. For a non-symmetric peak the peak maximum may not be a valid quantity, therefore, the T 2-center of gravity can be defined as T 2,CG = ∑{f(T 2)·T 2}/∑{f(T 2)} [11]. The width in logarithmic scale was calculated as the width at half-height intensity of probability peaks. For NOVX rats one may observe an increase of T 2,CG and log-width values with the increase of observation time and this is associated with natural growth of bone. With a single exception (of log-width at 8 weeks; Fig. 8b) the values obtained for ovariectomized rats are larger than the values of T 2,CG and log-width obtained for NOVX rats, which is a clear indication of the induced osteoporosis measured by NMR.
4.2 The Trabecular Bone Content
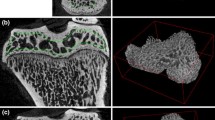
The observation of induced osteoporosis by ovariectomy is partly masked by the effect of natural growing of rat’s femurs. A direct observation of trabecular cavities can be achieved from histologic images as those presented in Fig. 3c. For that, such images are numerically cleaned of bone marrow. Examples of images with and without bone marrow are presented in Fig. 9 two for NOVX and two for OVX rats at 2 and 8 weeks. The narrowing of trabecular walls for osteoporotic bone is immediately observed from histological images as those presented in the bottom of Fig. 9. In Fig. 10 we present the trabecular bone content function of observation time from 2 to 8 weeks. The values were estimated for NOVX and OVX rats from cleaned histological images. For non-ovariectomized rats, we observe a general tendency of bone grooving from ~37 % at 2 weeks to ~59 % at 8 weeks. For the ovariectomized rats the content of trabecular bone decreases from ~40 % at 2 weeks to ~16 %. This can be understood as a clear result of the growing process discussed in the case of non-ovariectomized rats and a clear osteoporosis status for the ovariectomized rats.
Dependence of trabecular bone content during 8 weeks of observation time for non-ovariectomized and ovariectomized rats estimated from histological images of rat’s diaphysis transverse section as those presented in Fig. 8. The errors are of the order of 5 %
5 Conclusions
The femoral bone of ovariectomized and non-ovariectomized rats was treated as a quasi-porous media and the pores distributions were estimated by 1H NMR relaxometry function of observation time or evolution after ovariectomy. The 1D T 2-distributions obtained by Laplace inversion analysis of CPMG decays presents four peaks which were associated with protons located in four major pools: fluids and soft mater from primary pores like cavities, secondary pores like osteonal canals, osteocyte lacunae and canaliculi channels and finally bound water. This assignment was supported by three dedicated experiments. The studied sections of rat femurs present different structural characteristics as observed from NMR measurement and are supported by our additional histological images. In this sense, as resulted from dehydration experiment and from observation of all average normalized T 2-distributions measured ovariectomized and non-ovariectomized rats during 8 weeks of observation, we can conclude that the proximal part of femoris section of femoral rat’s bone is the region most sensitive to osteoporosis, while the diaphysis is the least sensitive region.
References
W. Koopman, M. Moreland, Arthritis and Allied Conditions: A Textbook of Rheumatology, 15th edn. (Lipincott Williams & Wilkins, Philadelphia, 2004), pp. 78–96
L. Cardoso, S.P. Fritton, G. Gailani, M. Benalla, S.C. Cowin, J. Biomech. 46, 253–265 (2013)
E.A. Martin, E.L. Ritman, R.T. Turner, Bone 32, 261–267 (2003)
C.C. Netto, V.C. Vieira, L.P. Marinheiro, S. Agellon, H. Weiler, M.R. Marostica Jr., Arq. Bras. Endocrinol. Metab. 56, 259–264 (2012)
W.S.S. Jee, W. Yao, J. Musculoskel. Neuron. Interact. 1, 193–207 (2001)
D. Sharma, C. Ciani, P.A. Ramirez Marin, J.D. Levy, S.B. Doty, S.P. Fritton, Bone 51, 488–497 (2012)
J.E.M. Brouwers, B. van Rietbergen, R. Huiskes, K. Ito, Osteoporos. Int. 20, 1823–1835 (2009)
H.A. Tanriverdi, A. Barut, S. Sarikaya, Eur. J. Obstet. Gynecol. Reprod. Biol. 120, 63–68 (2005)
M. Jablonski, V.M. Gun’ko, A.P. Golovan, R. Leboda, J. Skubiszewska-Zieba, R. Pluta, V.V. Turov, J. Colloid Interface Sci. 392, 446–462 (2013)
R.I. Chelcea, R.S. Şipos, R. Fechete, D. Moldovan, I. Şuş, Z. Pávai, D.E. Demco, Sudia UBB Chemia LX (1), 57–70 (2015)
R.S. Şipos, R. Fechete, D. Moldovan, I. Şuş, S. Szasz, Z. Pávai, Open. Life Sci. 10, 379–387 (2015)
R.S. Şipoş, R. Fechete, R.I. Chelcea, D. Moldovan, Z. Pap, Z. Pávai, D.E. Demco, Rom. J. Morphol. Embryol. 56(2), 743–752 (2015)
P. Fantazzini, C. Garavaglia, M. Palombarini, R.J.S. Brown, G. Giavaresi, R. Giardino, Magn. Reson. Imaging 22, 689–695 (2004)
T.M. Link, S. Majumdar, P. Augat, J.C. Lin, D. Newitt, Y. Lu, N.E. Lane, H.K. Genant, J. Bone Miner. Res. 13, 1175–1182 (1998)
S. Majumdar, H.K. Genant, Osteoporosis Int. 5, 79–92 (1995)
S. Anumula, S.L. Wehrli, J. Magland, A.C. Wright, F.W. Wehrli, Bone 46, 1391–1399 (2010)
R.A. Horch, J.S. Nyman, D.F. Gochberg, R.D. Dortch, M.D. Does, Magn. Reson. Med. 64, 680–687 (2010)
R.A. Horch, D.F. Gochberg, J.S. Nyman, M.D. Does, Magn. Reson. Med. 68, 1774–1784 (2012)
Q. Ni, A. De Los Santos, H. Lam, Y.X. Qin, Adv. Sp. Res. 40, 1703–1710 (2007)
R.A. Horch, D.F. Gochberg, J.S. Nyman, M.D. Does, PLoS One 6(e16359), 1–5 (2011)
J.S. Nyman, Q. Ni, D.P. Nicolella, X. Wang, Bone 42, 193–199 (2008)
F.W. Wehrli, J. Magn. Reson. 229, 35–48 (2013)
J.S. Nyman, A. Roy, X. Shen, R.L. Acuna, J.H. Tyler, X. Wang, J. Biomech. 39, 931–938 (2006)
W.C. Bae, P.C. Chen, C.B. Chung, K. Masuda, D. D’Lima, J. Du, J. Bone Miner. Res. 27, 848–857 (2012)
R. Biswas, W. Bae, E. Diaz, K. Masuda, C.B. Chung, G.M. Bydder, J. Du, Bone 50, 749–755 (2012)
R.A. Horch, J.C. Gore, M.D. Does, Magn. Reson. Med. 66, 24–31 (2011)
J.S. Nyman, L.E. Gorochow, R.A. Horch, S. Uppuganti, A. Zein-Sabatto, M.K. Manhard, M.D. Does, J. Mech. Behav. Biomed. Mater. 22, 136–145 (2013)
P.A. Timmins, J.C. Wall, Bone Water Calcif. Tissue Res. 23, 1–5 (1977)
N.A. Andreollo, E.F. Santos, M.R. Araújo, L.R. Lopes, ABCD Arq. Bras. Cir. Dig. 25, 49–51 (2012)
P. Sengupta, Biomed. Int. 2, 81–89 (2011)
R.S. Sipos, Z. Pap, A.S. Szalai, I. Sus, A.V. Gabor, Z. Pavai, K. Branzaniuc, Acta Med. Marisiensis 56, 479–483 (2010)
L. Monteilhet, J.P. Korb, J. Mitchell, P.J. McDonald, Phys. Rev. E 74, 061404 (2006)
L. Venkataramanan, Y.Q. Song, M.D. Hurlimann, IEEE Trans. Sign. Proc. 50, 1017–1026 (2002)
Y.Q. Song, L. Venkataramanan, M.D. Hürlimann, M. Flaum, P. Frulla, C. Straley, Magn. Reson. 154, 261–268 (2002)
M.D. Hürlimann, M. Flaum, L. Venkataramanan, C. Flaum, R. Freedman, G.J. Hirasaki, Magn. Reson. Imag. 21, 305–310 (2003)
K.E. Washburn, P.T. Callaghan, Phys. Rev. Lett. 97, 175502 (2006)
D. Moldovan, R. Fechete, D.E. Demco, E. Culea, B. Blümich, Diffus. Fundam. 10, 20.1–20.3 (2009)
R. Fechete, D. Moldovan, D.E. Demco, B. Blümich, Diffus. Fundam. 10, 14.1–14.3 (2009)
K.E. Washburn, P.T. Callaghan, J. Magn. Reson. 186, 337–340 (2007)
E. Tonning, D. Polders, P.T. Callaghan, S.B. Engelsen, J. Magn. Reson. 188, 10–23 (2007)
G.C. Borgia, R.J.S. Brown, P. Fantazzini, J. Magn. Reson. 147, 273–285 (2000)
P. Fantazzini, R.J.S. Brown, G.C. Borgia, Magn. Reson. Imaging 21, 227–234 (2003)
D. Moldovan, R. Fechete, D.E. Demco, E. Culea, B. Blümich, V. Herrmann, M. Heinz, Macromol. Chem. Phys. 211, 1579–1594 (2010)
D. Moldovan, R. Fechete, D.E. Demco, E. Culea, B. Blümich, V. Herrmann, M. Heinz, J. Magn. Reson. 208, 156–162 (2011)
Z.F. Zhang, L.Z. Xiao, H.B. Liu, F. Deng, X. Li, T.L. An, V. Anferov, S. Anferova, Appl. Magn. Reson. 44, 849–857 (2013)
S.C. Cowin, G. Gailani, M. Benalla, Philos. Trans. R. Soc. A 367, 3401–3444 (2009)
W.F. Neuman, T.Y. Toribara, B.J. Mulryan, J. Am. Chem. Soc. 75, 4239–4242 (1953)
F.W. Wehrli, M.A. Fernandez-Seara, Ann. Biomed. Eng. 33, 79–86 (2005)
S.M. Tommasini, A. Trinward, A.S. Acerbo, F. Carlo, L.M. Miller, S. Judex, Bone 50, 596–604 (2012)
H. Fonseca, D. Moreira-Gonçalves, M. Vaz, M.H. Fernandes, R. Ferreira, F. Amado, M.P. Mota, J.A. Duarte, J. Bone Miner. Metab. 30, 281–292 (2012)
Y. Uyar, Y. Baytur, U. Inceboz, B.C. Demir, G. Gumuser, K. Ozbilgin, Maturitas 63, 261–267 (2009)
Acknowledgments
This work was supported by a grant of Romanian National Authority for Scientific Research, CNCS-UEFISCDI, Project Number PN-II-IDEI-307/2011. R. F. would like to acknowledge Prof. Bernhard Blümich for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Şipos, R.S., Fechete, R., Moldovan, D. et al. Ovariectomy-Induced Osteoporosis Evaluated by 1H One- and Two-Dimensional NMR Transverse Relaxometry. Appl Magn Reson 47, 1419–1437 (2016). https://doi.org/10.1007/s00723-016-0839-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-016-0839-8