Abstract
Electron paramagnetic resonance (EPR) was used as a method to record nitric oxide (NO) production in the tissues of the brain, heart and liver of healthy rats, and rats after modeling of ischemic stroke. Direct measurement of the dynamics of NO production by EPR spectroscopy in our experiments showed that after the emergence of signs of ischemic stroke, 5 h after the start of ischemia, the content of NO in the hippocampus decreased two- to threefold and this decrease was maintained at 24 and 72 h. Deserving special attention is the data demonstrating that there is a greater decrease of NO production in the tissues of the heart and liver than in the brain. Consequently, the change in intensity of NO production in the modeling of ischemic events in the brain has a systemic, not a local character.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hypoxia is a pathological process that occurs when there is an insufficient supply of oxygen, or disruption of its utilization in biological oxidation; this is an important component of the pathogenesis of many diseases [1, 2]. Brain ischemia can be caused by a decrease in the oxygen content, or disruptions of the cerebral blood flow can lead to a lack of oxygen supply to parts of the brain, which can culminate in ischemic insult defined as acute damage of brain tissue, with a disruption of its functions due to the difficulty or cessation of blood flow to brain areas [3–5]. In this regard, the study of pathogenesis, treatment and the mechanisms of stroke are important from both the theoretical and practical points of view [6, 7].
The functioning of neurotransmitter systems, including the nitric monoxide system, is disrupted during hypoxia and brain ischemia. Nitric oxide (NO) is known as one of the most important signaling molecules regulating the physiological functions of the organism and the metabolism of cells [8, 9]. There is interest in the participation of NO in the mechanisms of development of various pathological processes [10–14]. NO participates in the regulation of intracellular Ca2+ ion concentration, through activation of hem-containing soluble guanylyl cyclase and ADP-ribosyltransferase, and is involved in pH regulation during cerebral ischemia [15–19]. At present there is no consensus about the role of endogenous NO in the processes involved in the damage of the nervous system [20]. In addition to vasodilatation, neurotransmitter and stress limiting properties, the involvement of NO in reactions of oxidative stress, the glutamate-calcium cascade and in inflammation has been demonstrated [8, 9, 13, 21]. NO is synthesized by constitutive isoforms of NO synthases (NOS) [22], provides adequate blood flow to the brain regions, influences the activity of neurons, and regulates cell metabolism. In animals, NO has a particularly important role in the functioning of the cardiovascular [12] and nervous systems [13].
There are many methods of measuring NO production in biological systems. Precise measurement of both the steady concentration of NO and the speed of NO generation in biological systems is a difficult task due to the low activity of NOS, and its short half-life [23–26].
A large number of studies have been carried out to measure the activity of NOS [9, 27, 28]. There are a number of techniques that allow direct measurement of the NO content in different tissues. These include electrochemical techniques, with a sensitivity of 0.3–10 nM, which allow real-time direct measurements, but are very susceptible to temperature and electrical noise and difficult to calibrate [26, 29], and fluorometry, with a sensitivity of 0.6–8 nM, allowing direct measurement, but with the disadvantage of a lack of sensitivity [26, 30, 31]. The spectrophotometric method, based on the reaction of oxyhemoglobin with NO also has a relatively high specificity and sensitivity to NO [32].
In the last few years, one of the most effective methods for the detection and quantification of nitric oxide in biological tissues has been electronic paramagnetic resonance (EPR) [15, 24, 33–37]. This is due to the method developed by Vanin et al. [38], in which they used a technique known as spin trapping. Spin trapping is based on the reaction of a radical (in this case NO) with the spin trap. In the subsequent reaction an adduct is formed with a characteristic EPR spectrum. The complex Fe2+ with diethyldithiocarbamate (DETC) was used to capture NO and to form a stable ternary complex (DETC)2–Fe2+–NO in tissues of animals. These complexes are characterized by an easily recognizable EPR spectrum with g factor g = 2.035–2.040 and a triplet hyperfine structure [15, 36, 37]. The method has a sensitivity of 0.04–0.4 nM, allows direct measurement, and is highly sensitive due to the use of spin traps. The disadvantages include semiquantitativity and complicated evolution of the complex of NO with the spin trap [26, 34].
The role of NO in the development of ischemia has attracted the attention of researchers for a long time. Samdani et al. using the method of measuring NOS activity found that 10 min after the beginning of brain ischemia there is an increase in neuronal NOS (nNOS) activity, with its maximum at 3 h [28]. In another study, it has been shown that after focal ischemia, the expression of iNOS (the selective block of which may be a neuroprotective factor in ischemia [39]) began between 24 and 48 h after ischemia and reached a peak after 96 h [27]. Using the EPR spectroscopy method it was found that NO production increased after 5 min of ischemia and continued for 60 min [40, 41]. Using also the EPR spectroscopy method in combination with spin trap it was found increase of the relative concentration of free NO on 132 % after 15 min of ischemia induced by occlusion of the middle cerebral artery [42]. However, this effect is reversible –the level of NO is gradually reduced and reaches baseline values after 60 min [43]. The twofold increase in NO production in the cerebral hemispheres has been shown in a rat model of global ischemia [44]. Overproduction of NO after acute hypoxia has also been demonstrated [10].
There have also been results published which contradict the point of view of a neurotoxic role of NO generated by ischemia. It has been shown that inhibitors of NO-synthase L-NNA and L-NAME do not reduce the value (volume) of infarct in a model of focal cerebral ischemia in rats [45, 46], and, conversely, increases focal ischemic stroke [47]. There are numerous studies on the use of NO donors as neuroprotective agents after transient and permanent ischemic damages [5, 48–50]. There are also results showing a reduction of the NO content in ischemic parts of the left hemisphere of rats after simulation of ischemic stroke [51]. Therefore, based on the time course of ischemia-induced changes in NO levels and NOS regulation in the brain and cerebral blood vessels, several strategies have been suggested for the manipulation the NO system for the treatment of stroke [52].
Thus, the dynamics of the NO content (and production) in brain tissues during cerebral ischemia still remain unclear, despite the recognition of the fact that the main contribution of NO production are made by nNOS and iNOS [13, 39, 53].
The aim of our study was to measure the effects of experimental diffuse ischemic stroke on the dynamics of the intensity of NO production by EPR using spin trapping in the hippocampus, heart and liver of rats 5, 24 and 72 h after ischemic stroke.
2 Materials and Methods
2.1 Experimental Protocol: Simulation of Ischemic Stroke in Rats and Application of Trapping for Nitric Oxide
Modeling of ischemic stroke was produced on rats in the Institute of Physiology of NAS of Belarus, Minsk. Animals were kept in standard vivarium conditions (12/12 light and dark rhythm, air temperature of 23 ± 1 °C and stable supply and exhaust ventilation) with free access to water and food (ad libitum) and a diet in accordance with the standards for keeping laboratory animals. For the modeling of ischemic stroke, animals were subjected to 5-min hypoxia (conditional rise to a height of 4500 m above sea level, which corresponded to a pressure of 432 mmHg and a decrease of oxygen partial pressure pO2 from 159 to 90 mmHg on average) [54, 55]. Then the animals were divided into 4 groups: 3 experimental and 1 control of 10 animals each—with extraction of the hippocampus, heart and liver tissue 5, 24 and 72 h after presentation of the hypoxic stimulus. A similar extraction of tissue samples were also produced from control animals. In both series, on the day of the experiment, rats were anesthetized by intraperitoneal injection of a mixture of ketamine–chloralose–acepromazine (55.6, 5.5 and 1.1 mg/kg, respectively). 1.5 × 1.5 mm brain fragments were isolated immediately after decapitation. After opening the chest and abdomen, 2.0 × 2.0 mm fragments of heart and liver were selected and removed. The selected areas of the brain, heart, and liver were placed in the freezer at a temperature of 77 K. The tissue fragments were kept and transported in plastic containers at the temperature of liquid nitrogen (77 K) for measurement by EPR spectroscopy.
2.2 Formation of the Complex of NO with the Spin Trap in Rat Tissue
During EPR sample preparation the spin trap method was used [56]. DETC–Na was introduced into rats’ bodies intraperitoneally at a concentration of 500 mg/kg in 2.5 ml water in each animal [57, 58]. The mixture of solutions, of 37.5 mg/kg iron sulfate (FeSO4·7H2O, Sigma, USA) and 187.5 mg/kg sodium citrate (all in 1 ml water to one animal), that was prepared before injection, was injected under the skin at three points—left and right legs and in the rostral part of the interscapular region. When mixed, iron sulfate and sodium citrate produce iron citrate. DETC–Na and iron citrate distribute in the organism and their interaction generates the water insoluble hydrophobic DETC–Fe complex, which can interact with NO to form the paramagnetic mononitrosyl iron complex (DETC)2–Fe2+–NO which can be measured by EPR spectroscopy. The complex of the spin trap with NO (DETC)2–Fe2+–NO is characterized by an easily recognizable EPR spectrum with g factor g = 2.038 and triplet hyperfine structure. Furthermore, the spin trap interacts with Cu, forming the complex Cu(DETC)2, which can also be recorded by EPR spectroscopy. The experiments with detection of (DETC)2–Fe2+–NO complexes were conducted on an ER 200 SRC EPR spectrometer (Bruker), which measure in X-band area, with the following parameters: modulation 100 kHz, amplitude modulation 2 Gs, microwave power 30 mW, time constant 200 ms, temperature 77 K. All experiments were performed in a flack duar and were conducted without microwave saturation and over modulation. The sample was weighed before the experiments. The sample mass was about 100 mg. The EPR spectra amplitude was normalized to the mass of sample and to the EPR signal amplitude of a standard sample (the details of the EPR signal measurement procedure have been described previously [51]).
2.3 Statistical Processing of Experimental Results
Results are presented as M ± m (average value ± standard deviation). Statistic processing of data was performed using the Student’s t test. The differences were significant if p < 0.05.
3 Results
3.1 EPR Spectra of the Cerebellum, Hippocampus and Heart of Healthy Rats and Rats After Modeling of Ischemic Stroke
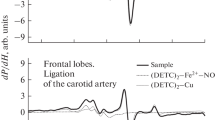
Figure 1 shows the EPR spectra from tissues of the cerebellum of healthy rats. We see the characteristic triple EPR spectra from the complex (DETC)2–Fe2+–NO with a g factor of 2.038 [56], the signal from R-conformers of hemoglobin associated with NO [12, 59], and the signal from the complex (DETC)2–Cu is present in the same field. Figures 2 and 3 show the EPR spectra of hippocampal and heart tissue from healthy rats and from rats 5 h after the modeling of ischemic stroke. The solid line shows the spectra of the sample, and the dotted line shows the signal from nitric oxide, associated with the spin trap in the composition of the spectra of the complex ((DETC)2–Fe2+–NO). The relative change in the number of NO-containing complexes was evaluated by the integral of the signal intensity of the spin trap, (DETC)2–Fe2+–NO.
3.2 Dynamics of NO Content in the Hippocampus, Heart and Liver After Modeling of Ischemic Stroke
Figures 4, 5 and 6 present statistical data on the integral intensities of the signal from (DETC)2–Fe2+–NO in the spectra of the samples of biological tissue, demonstrating the effects of ischemic stroke on the production of nitric oxide in the brain tissue (the hippocampus), heart and liver at different times after stroke. The results show a significant reduction in NO production after modeling of ischemic stroke. The results show that after 5 h there is a two- to threefold decrease of the NO content in the hippocampus (Fig. 4a). This decrease in NO production remains at 24 and 72 h after stroke. It should be noted that there is a more significant decrease (3–4 fold) in NO production in the heart (Fig. 5) and liver (Fig. 6) tissues after ischemic events in the brain. This effect demonstrated the regulatory role of the nervous system on NO distribution in tissues and organs of the body. It is also shown that the copper content in the hippocampus does not change 72 h after modeling of ischemic stroke; however, after 5 h there was a decrease in copper content (Fig. 4b).
4 Discussion
4.1 Discussion of the Design of the Experiment
The problem of ischemia is topical worldwide [4, 5, 7, 60]. Every year around the world and in the Russian Federation a large number of diseases of the cardiovascular system and the brain are recorded, among which myocardial infarction and ischemic stroke take a leading place. Brain stroke is the second leading cause of death and most common cause of disability worldwide, mortality from stroke in Russia is one of the highest in the world—more than 180 people per 100 thousand [3]. As a rule, a stroke develops due to a vessel being blocked by a thrombus which leads to the formation of a risk area (which has practically no blood supply) and surrounding penumbra (with a partial blood admission due to the blood flow from other vascular regions) [60, 61]. It is known that hypoxia is accompanied by disturbances in the oxygen supply to the brain, which can cause cerebral ischemia, which may culminate in ischemic stroke [4, 5]. In the conducted experiments, we modeled ischemic stroke by subjecting animals to 5-min hypobaric hypoxia (conditional rise to a height of 4500 m above sea level, which corresponded to a pressure of 432 mmHg, and decrease in oxygen partial pressure pO2 from 159 to 90 mmHg on average) [54, 55].
Since the spin trap technique we used imposes some restrictions on the protocol of the experiment, for example, the 30 min waiting time for the accumulation of a signal, it does not make sense to study short time intervals (up to 3 h) from the beginning of ischemia. Taking into account the dynamics of the NOS activation and the limitations of the experimental technique, at 5, 24 and 72 h after modeling the stroke in anesthetized animals euthanasia was performed and samples for measuring the NO content were taken 30 min before samples were taken, the spin trap for NO was injected.
4.2 Discussion of the Experimental Results
At present the development of brain ischemia and the subsequent occurrence of stroke is associated with disorders of cerebral blood flow and impairment of its regulation by NO [4, 6, 39, 61–63]. In pathological conditions, the brain cells, and the cells of the immune system are capable of expressing the inducible isoform of the enzyme (iNOS), the activity of which is 50–100 times higher than constitutive isoforms and does not depend on the calcium content in the cell [11, 25, 27, 64, 65].
The direct measurement of the dynamics of NO content in the hippocampus in our experiments after the ischemic stroke caused by 5 min hypoxia of animals (conditional rise to a height of 4500 m above sea level) has shown that the NO concentration in a complex with a spin trap in the hippocampus significantly decreases within 5 h after the start of ischemia and this decrease is maintained at 24 and 72 h. The results of our study differ from data obtained by the group of S.T. Ohnishi who measured NO dynamics by EPR spectroscopy. They found that after 5–15 min of ischemia, the production of NO was potentiated within 5 min (increased several times), which continued during the 60 min of ischemia [40, 41]. The similar results were obtained by other researchers [42]. However, the authors did not carry out measurements of NO in the later stages of ischemia. The reasons for the difference in results are not clear to us, although it must be emphasized that we measured the dynamics of NO in the later stages of ischemia. Interestingly, our data demonstrate that the reduction of NO production in the heart and liver is greater than in the brain. Such a result may be a confirmation of the significant influence that the central nervous system has on the regulation of NO production in the body, and may also be a reflection of the nonlocality of NO regulation in the body.
It is known that the main damaging factor in the development of apoptosis is the peroxynitrite ion (ONOO−), which is formed during the interaction of NO with superoxide (O2−). Dismutation of superoxide via the cytosolic enzyme Cu, Zn-COD (superoxide dismutase) is the primary and basic protection from free radical oxidation; however, peroxynitrite generated during excessive production of NO itself can inactivate the COD and speed up the processes of free radical oxidation [11, 13]. We have shown that the copper content in the hippocampus was a decrease 5 h after the modeling of ischemic stroke; however, after 72 h there does not change in copper content. The use of L-NAME reduced the concentration of this element twofold [66]. Thus, it can be concluded that 72 h after stroke, the antioxidant system may counteract hypoxic factors.
Earlier, using EPR spectroscopy we found that in the ischemic part of the left hemisphere 5 h after ischemic stroke caused by coagulation in the left middle brain artery, the NO content in the spin trap and R-conformer compositions decreases by 500 and 30 %, respectively. This decrease in the NO content remains at 9 and 24 h after the stroke [51]. Other authors using the method of measuring NOS activity found that 10 min after the beginning of the brain ischemia there is an increase in neuronal NOS (nNOS) activity, with its maximum at 3 h. This response is associated with activation of the glutamate system and is thought to cause the early damage of neurons. Activation of iNOS appears in the affected region after 12 h after ischemia with a peak response within 48 h. The inducible isoform of the enzyme accompanies the development of inflammatory responses and causes delayed damage in the brain and in this case, the peroxynitrite is the basic damaging compound derived from NO. An hour after ischemic attack, endothelial NOS (eNOS) activity increases and is maintained for one day [28]. The enzyme action leads to vasodilatation of blood vessels and reduces the damage region. In another study, it has been shown that after focal ischemia, the expression of iNOS began between 24 and 48 h after ischemia and reached a peak after 96 h [27]. Also it was found that an excess of NO can act as a neurotoxin in brain damage during cerebral ischemia [60]. At the same time, selective blocking of iNOS may be a neuroprotective factor in ischemia [39, 64].
The precise determination of the NO content in tissues in different functional states, including pathological, it is not only crucial for experimental studies, but also relevant for practitioners. The idea is that donors, NO precursors and modulators of the functional activity of the NO receptors are widely used in clinical practice. Especially important is the role of drugs, the main active component of which is NO, in the therapy of such socially significant diseases as cardiovascular disease.
5 Conclusions
Thus, the analysis of data from the literature and our results shows the ambiguity of the obtained data, reflecting the known fact of dose-dependent effects of nitric oxide in the brain. In our work, we demonstrated an integrated approach and the application of precise methods of NO measurement to get data on the dynamics of NO levels in nervous tissue. There is a real prevention of NO adverse effects, when the disruption of the control of its level becomes a cause of the formation of excessive amounts of peroxynitrite. One of the mechanisms of cytotoxicity of peroxynitrite is its interaction with superoxide dismutase. Reacting with metal ions that are part of superoxide dismutase, peroxynitrite causes the formation of highly toxic NO2+, which reinforces toxicity and is fatal to living cells processes.
References
E.B. Manukhina, I.Y. Malyshev, B.V. Smirin, S.Y. Mashina, V.A. Saltykova, A.F. Vanin, Nitric Oxide 3, 393–401 (1999)
V.B. Koshelev, in Selected Lectures on Modern Physiology (Art-cafe, Kazan, 2010), pp. 178–194 (in Russian)
G.A. Donnan, M. Fisher, M. Macleod, S.M. Davis, Stroke. Lancet 371, 1612–1623 (2008)
S.E. Lakhan, A. Kirchgessner, M. Hofer, J. Transl. Med. 7, 97 (2009)
M. Godinez-Rubi, A.E. Rojas-Mayorquin, D. Ortuno-Sahagun, Oxidat. Med. Cell. Longevity 2013, 1–16 (2013)
K.P. Doyle, R.P. Simon, M.P. Stenzel-Poore, Neurophrmacology 55, 310–318 (2008)
K.L. Lambertsen, K. Biber, B. Finsen, J. Cereb. Blood Flow Metab. 32, 1677–1698 (2012)
A.F. Vanin, Biokhimiya 63, 924–938 (1998) (in Russian)
D. Boehning, S.H. Snyder, Annu. Rev. Neurosci. 26, 105–131 (2003)
I.Y. Malyshev, T.A. Zenina, L.Y. Golubeva, V.A. Saltykova, E.B. Manukhina, V.D. Mikoyan, L.N. Kubrina, A.F. Vanin, Nitric Oxide 3, 105–113 (1999)
P. Pacher, J.S. Beckman, L. Liaudet, Physiol. Rev. 87, 315–427 (2007)
V.P. Reutov, V.E. Okhotin, A.V. Shuklin, E.G. Sorokina, N.S. Kosicin, V.N. Gurin, Usp. Fiziol. Nauk 38, 39–58 (2007) (in Russian)
J.R. Steinert, T. Chernova, I.D. Forsythe, Neuroscientist 16, 435–452 (2010)
A.A. Timoshin, O.I. Pisarenko, O.V. Tskitishvili, L.I. Serebriakova, I.M. Studneva, D. Drobotova, E.K. Ruuge, A.F. Vanin, Biofizika 55, 1099–1107 (2010) (in Russian)
A.F. Vanin, A. Huisman, E.E. Van Faassen, Methods Enzymol. 359, 27–42 (2003)
G.F. Sitdikova, A.L. Zefirov, Ross. Fiziol. Zhurn. im. I. M. Sechenova 92, 872–882 (2006) (in Russian)
G. Tomomi, M. Masataka, Arterioscler. Thromb. Vasc. Biol. 26, 14–39 (2006)
S. Erusalimsky, S. Moncada, Arterioscler. Thromb. Vasc. Biol. 27, 2524–2531 (2007)
R.R. Borodulin, L.N. Kubrina, V.D. Mikoyan, A.P. Poltorakov, V.O. Shvydkiy, D.Sh. Burbaev, V.A. Serezhenkov, E.R. Yakhontova, A.F. Vanin, Nitric Oxide 29, 4–16 (2013)
V. Calabrese, C. Cornelius, E. Rizzarelli, J.B. Owen, A.T. Dinkova-Kostova, D.A. Butterfield, Antioxid. Redox Signal. 11, 2717–2739 (2009)
E.B. Manukhina, I.Y. Malyshev, Ross. Fiziol. Zhurn. im. I. M. Sechenova 86, 1283–1292 (2000) (in Russian)
U. Forstermann, W.C. Sessa, Eur. Heart J. 33, 829–837 (2012)
N.V. Voevodskaya, A.F. Vanin, Biochem. Biophys. Res. Commun. 186, 1423–1428 (1992)
V.V. Khramtsov, L.B. Volodarsky, Biol. Magn. Reson. 14, 109–180 (1998)
D.D. Thomas, L.A. Ridnour, J.S. Isenberg, W. Flores-Santana, C.H. Switzer, S. Donzelli, P. Hussain, C. Vecoli, N. Paolocci, S. Ambs, C.A. Colton, C.C. Harris, D.D. Roberts, D.A. Wink, Free Radical Biol. Med. 45, 18–31 (2008)
C. Csonka, T. Pali, P. Bencsik, A. Gorbe, P. Ferdinandy, T. Csont, Brit. J. Pharmacol. 172, 1620–1632 (2015)
C. Iadecola, F. Zhang, R. Casey, M. Nagayama, M.E. Ross, J. Neurosci. 17, 9157–9164 (1997)
A.F. Samdani, T.M. Dawson, V.L. Dawson, Stroke 28, 1283–1288 (1997)
S. Griveau, F. Bedioui, Analyt. Bioanalyt. Chem. 405, 3475–3488 (2013)
H.X. Zhang, J.B. Chen, X.F. Guo, H. Wang, H.S. Zhang, Analyt. Chem. 86, 3115–3123 (2014)
C.C. Winterbourn, Biochim. Biophys. Acta 1840, 730–738 (2014)
V.V. Zinchuk, Usp. Fiziol. Nauk 34, 33–35 (2003) (in Russian)
L.N. Kubrina, W.S. Caldwell, P.I. Mordvintcev, I.V. Malenkova, A.F. Vanin, Biochim. Biophys. Acta 1099, 233–237 (1992)
A. Mulsch, P.I. Mordvintcev, A.F. Vanin, R. Busse, Biochem. Biophys. Res. Commun. 196, 1303–1308 (1993)
P. Kleschyov, T. Wenzel, J. Munzel, Chromatogr. B 851, 12–20 (2007)
N. Hogg, Free Radical Biol. Med. 49, 122–129 (2010)
C.L. Hawkins, M.J. Davies, Biochim. Biophys. Acta 1840, 708–721 (2014)
A.F. Vanin, P.I. Mordvintcev, A.L. Kleschyov, Studia Biophys. 102, 135–143 (1984)
J.P. Bolanos, A. Almeida, Biochim. Biophys. Acta 1411, 415–436 (1999)
S. Sato, T. Tominaga, T. Ohnishi, S.T. Ohnishi, Brain Res. 647, 91–96 (1994)
T. Tominaga, S. Sato, T. Ohnishi, S.T. Ohnishi, J. Cereb. Blood Flow Metab. 14, 715–722 (1994)
S.H. Chen, P.C. Fung, R.T. Cheung, Free Radical Biol. Med. 32, 776–784 (2002)
Z. Yuan, W. Liu, B. Liu, A. Schnell, K.J. Liu, Brain Res. 1352, 248–254 (2010)
O.E. Fadiukova, A.A. Alekseev, V.G. Bashkatova, I.A. Tolordava, V.S. Kuzenkov, V.D. Mikoian, A.F. Vanin, V.B. Koshelev, K.S. Raevskiĭ, Eksper. Klinic. Farmakol. 64, 31–34 (2001)
D.A. Dawson, K. Kusumoto, D.I. Graham, J. McCulloch, I.M. Macrae, Neurosci. Lett. 142, 151–154 (1992)
G. Sancesario, M. Iannone, M. Morello, G. Nistico, G. Bernardi, Stroke 25, 436–443 (1994)
S. Yamamoto, E.V. Golanov, S.B. Berger, D.J. Reis, J. Cereb. Blood Flow Metab. 12, 717–726 (1992)
M. Willmot, L. Gray, C. Gibson, S. Murphy, P.M. Bath, Nitric Oxide 12, 141–149 (2005)
K.H. Jung, K. Chu, S.Y. Ko, S.T. Lee, D.I. Sinn, D.K. Park, J.M. Kim, E.C. Song, M. Kim, J.K. Roh, Stroke 37, 2744–2750 (2006)
O.V. Evgenov, P. Pacher, P.M. Schmidt, G. Haskó, H.H.H.W. Schmidt, J.-P. Stasch, Nat. Rev. Drug Discov. 5, 755–768 (2006)
KhL Gainutdinov, S.A. Gavrilova, V.S. Iyudin, A.V. Golubeva, M.P. Davydova, G.G. Jafarova, V.V. Andrianov, V.B. Koshelev, Appl. Magn. Reson. 40, 267–278 (2011)
N.A. Terpolilli, M.A. Moskowitz, N. Plesnila, J. Cereb. Blood Flow Metab. 32, 1332–1346 (2012)
V. Calabrese, C. Mancuso, M. Calvani, E. Rizzarelli, D.A. Butterfield, A.M.G. Stella, Nat. Rev. Neurosci. 8, 767–775 (2007)
M. Coupe, E. Tomilovskaya, F. Larcher, B. Diquet, L.K. Pastushkova, I.B. Kozlovskaya, I.M. Larina, G. Gauquelin-Koch, V.A. Kulchitsky, M.-A. Custaud, N.M. Navasiolava, Open J. Nephrol. 3, 13–24 (2013)
V. Kulchitsky, T. Semenik, Z. Kaliadzich, T. Andrianova, K. Tsishkevich, Clin. Neurophysiol. 125, 330–331 (2014)
V.D. Mikoyan, L.N. Kubrina, V.A. Serezhenkov, R.A. Stukan, A.F. Vanin, Biochim. Biophys. Acta 1336, 225–234 (1997)
A.I. Ismailova, O.I. Gnezdilov, L.N. Muranova, A.A. Obynochny, V.V. Andrianov, KhL Gainutdinov, A.G. Nasyrova, R.R. Nigmatullina, F.F. Rahmatullina, A.L. Zefirov, Appl. Magn. Reson. 28, 421–430 (2005)
KhL Gainutdinov, V.V. Andrianov, V.S. Iyudin, S.V. Yurtaeva, G.G. Jafarova, R.I. Faisullina, F.G. Sitdikov, Biophysics 58, 203–205 (2013)
V.V. Andrianov, F.G. Sitdikov, KhL Gainutdinov, S.V. Yurtaeva, L.N. Muranova, A.A. Obynochnyi, F.K. Karimov, V.M. Chiglintsev, V.S. Iyudin, Russ. J. Dev. Biol. 38, 352–356 (2008)
E.I. Gusev, V.I. Skvorceva, Ishemia of Brain (Medicina, Moscow, 2001) (in Russian)
L.X. Liu, Y.J. Yang, Y.J. Jia, Hunan Yike Daxue Xuebao 28, 133–136 (2003)
C. Griffiths, G. Garthwaite, D.A. Goodwin, J. Garthwaite, Eur. J. Neurosci. 15, 962–968 (2002)
E.B. Manukhina, V.U. Kalenchuk, S.A. Gavrilova, A.V. Goryacheva, I.Y. Malyshev, V.B. Koshelev, Ross. Fiziol. Zhurn. im. I. M. Sechenova 94, 198–205 (2008) (in Russian)
N. Tuteja, M. Chandra, R. Tuteja, M.K. Misra, J. Biomed. Biotechnol. 4, 227–237 (2004)
S. Cho, E.-M. Park, P. Zhou, K. Frys, M.E. Ross, C. Iadecola, J. Cereb. Blood Flow Metab. 25, 493–501 (2005)
V.V. Andrianov, G.G. Iafarova, A.A. Denisov, V.S. Iyudin, S.G. Pashkevich, M.O. Khotyanovich, T.Kh. Bogodvid, V.A. Kulchitchkii, Kh.L. Gainutdinov, in Proceedings of the Internat. conf. “Magnetic Resonance: Fundamental Research and Pioneering Applications (MR-70)”, Kazan. P05. 88 (2014)
Acknowledgments
This work was funded by the subsidy of the Russian Government to support the Program of Competitive Growth of Kazan Federal University among the World’s Leading Academic Centers (Agreement No. 02.A03.21.0002), by Russian Foundation for Basic Research (Grant No. 16-54-00098) and by Belarusian Republican Foundation for Fundamental Research (Grant B16R-166).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrianov, V.V., Pashkevich, S.G., Yafarova, G.G. et al. Changes of Nitric Oxide Content in the Rat Hippocampus, Heart and Liver in Acute Phase of Ischemia. Appl Magn Reson 47, 965–976 (2016). https://doi.org/10.1007/s00723-016-0815-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-016-0815-3