Abstract
The chloritization of biotite and stable isotopes of silicate have been studied for the Zafarghand porphyry copper deposit, Ardestan, Iran. The studied area, in the central part of the Urumieh–Dokhtar magmatic belt, contains porphyry-style Cu mineralization and associated hydrothermal alteration within the Miocene (19–26 Ma, Zircon U-Pb age) granodioritc stock and adjacent andesitic to rhyodacitic volcanic rocks (ca. 56 Ma, zircon U-Pb age). The primary and secondary biotite that formed during potassic alteration in this porphyry and these volcanic host rocks are variably chloritized. Chloritization of biotite pseudomorphically is characterized by an increase in MgO, FeOt, and MnO, with decreasing in SiO2, K2O, and TiO2. Based on the Ti-in-biotite geothermometer of Henry et al. (Am Mineral 90:316–328, 2005) and Al-in-chlorite geothermometer of Cathelineau (Clay Miner 23:417–485, 1988), crystallization temperatures of primary biotite representative of magmatic conditions and later chloritization temperature range from 617° to 675 °C ± 24 °C and 177° to 346 °C, respectively. Calculated isotopic compositions of fluids that chloritized primary and secondary biotite display isotopic compositions of 1.1 to 1.7 per mil for δ18O and −19.9 to −20.5 per mil for δD consistent with meteoric water. Sericite, barren, and A-type-quartz veins from phyllic alteration were produced by mixed magmatic and meteoric water with δ18O values from −2.8 to 2.5 and δD values of ∼ −23 per mil; the narrow range of δD values of the propylitic epidote may be due to a meteoric water with δ18O values from 0.8 to 1.6 and δD values from −14.6 to −16.9 per mil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding the source and physicochemical conditions under which hydrothermal fluids originate and evolve is important to obtaining clear insight into sources of the ore-forming materials and mineralization processes. Although sulfide ± oxide equilibria assemblages are normally used to estimate temperature, pressure, fugacities of volatile species, and source of fluids, coexisting silicates may provide valuable information on conditions of mineralization and alteration (Jacobs and Parry 1979). Among the rock-forming silicates, quartz, chlorite, biotite, and epidote-group minerals are commonly used to decipher source and physicochemical attributes of the hydrothermal systems (Bird and Helgeson 1980; Walshe 1986). For example, Beane (1982) considered that chloritic alteration of biotite was associated with chalcopyrite deposition from a hydrothermal solution in porphyry copper environments.
Numerous porphyry copper deposits have been discovered within the Alpine–Himalayan orogenic and metallogenic belt, including several giant deposits in Iran (Sungun (cf. Calagari 2004; Hezarkhani 2006a), Sar-Cheshmeh (cf. Hezarkhani and Williams-Jones 1998; Shahabpour 2000), Miduk (Taghipour et al. 2008; Boomeri et al. 2009), Darrehzar (cf. Shafiei and Shahabpour 2008)). The Urumieh–Dokhtar Magmatic Arc (UDMA) belongs to a part of the Alpine-Himalayan orogenic belt. Calc-alkaline volcano-plutonic rocks in this magmatic arc (UDMA) in Iran host several porphyry-, disseminated-, and vein-type copper deposits. Ore deposits associated with porphyry copper systems in the UDMA are generally restricted to Miocene granitoids with dominant rock compositions of granodiorite and quartz diorite. Although the majority of the well-known porphyry Cu deposits in Iran have been studied in terms of base-metal contents, fluid inclusion characteristics, stable isotopes, and alteration geochemistry (Brimhall 1980; Hezarkhani and Williams-Jones 1998; Zarasvandi et al. 2005; Hezarkhani 2006a, b; Atapour and Aftabi 2007; Taghipour et al. 2008; Boomeri et al. 2009; Afshooni et al. 2011), some of them are still not well known, such as the Zafarghand deposit, and many are yet to be discovered.
This area has recently been discovered through remote sensing images followed by geochemical and geophysical studies and is still under exploration. Alteration and mineralization in this area are superimposed onto the associated porphyritic body and the surrounding country rock. The aim of this work was to reconstruct the conditions of alteration of primary biotite, biotitization associated with potassic alteration, and finally the alteration of those biotite assemblages to chlorite, associated with decreasing fluid temperature, and evolving oxygen and hydrogen isotope analyses on hydrothermal silicate mineral was also done to further test the hypothesis of the magmatic to meteoric transition during the hydrothermal evolution.
Geological setting
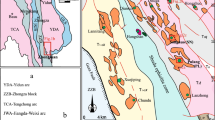
The Zafarghand area is located within the NW–SE trending UDMA (Fig. 1), one of the main subdivisions of Zagros orogenic belt (Alavi 1994). The UDMA volcanism was most active and widespread during the Eocene and Oligocene (55–25 Ma), lasting much longer than previously thought (Chiu et al. 2013). The UDMA magmatism ceased progressively from northwest to southeast, with magmatic activity ending in the Early Miocene (ca. 22 Ma) in Meghri, the Middle Miocene (ca. 16 Ma) in Kashan, and the Late Miocene (ca. 10–6 Ma) in Anar, respectively (Chiu et al. 2013). The Eocene magmatic activity in the UDMA is coeval with widespread magmatic activity throughout most of the Iranian plateau. All the large Iranian porphyry Cu deposits (Sarcheshmeh, Sungun, Meiduk, Darehzar etc.; Fig. 1) are located within this arc (Shahabpour 2005).
Magmatism in the Zafarghand arc segment of the UDMA occurred predominantly during Eocene times (ca. 56 Ma; Chiu et al. 2013), but resumed after a dormant period during the Middle Miocene (26–19 Ma; Chiu et al. 2013). Age constraints for the UDMA volcano-plutonic units are mostly inferred from zircon U–Pb ages of 50 igneous rock samples in the Urumieh–Dokhtar and Sanandaj–Sirjan magmatic arcs and Zafarghand neighboring area as granodiorite from Natanz (from 21.2 ± 0.3 to 19.6 ± 0.3 Ma) and volcanic rocks from this area (56.5 ± 2.6 Ma) (Chiu et al. 2013). Consequently, magmatism in the Zafarghand copper district is linked to a microgranular I-type granodiorite that intruded into effusive volcanic and pyroclastic rocks, including andesites, dacites, and rhyodacites associated with ignimbrites and pyroclastic tuff breccias (Fig. 1). The entire igneous complex is intersected by NW–SE to E–W-trending andesitic and dacitic dykes and N–S-trending andesitic basalt dykes (Aminoroayaei Yamini 2016). The geochemical data of these formations (Aminoroayaei Yamini et al. 2016) are consistent with (i) the modal mineralogy derived from petrographic analysis of the samples and (ii) the regional tectonic setting of the samples having been emplaced in an active subduction-related continental volcanic arc setting during the Zagros Orogeny. Both the alteration and mineralization patterns associated with fractures and faulting (Aminoroayaei Yamini 2016; Chelevy 2015) indicate potential for porphyry deposits with epithermal vein mineralization. Copper mineralization in the porphyry deposit shows an average grade of 0.4% Cu, 200 ppm Mo, 0.2 ppm Ag, and 0.01 g/t Au and a maximum age of 21 Ma, which implies that copper mineralization, was synorogenic, coeval with the Alpine–Himalayan orogeny in this region (Aminoroayaei Yamini et al. 2016). This deposit has experienced different magmatic activities that have led to the formation of several main groups of veins, including M-magnetite veins, A-quartz veins, B-quartz-molybdenite veins associated with numerous barren quartz, anhydrite, calcite, epidote, and chlorite (Chelevy 2015). Some mineralization from the Zafarghand deposit is shown in Fig. 2.
Photographs of a hydrothermal breccia zone trending NW-SE and 20 to 70 m wide within a groundmass of potassic altered granodiorite, b barren quartz vein trending NE-SW and 0.001 to 0.007 m wide has been crosscut by A-type quartz vein trending NW-SE and 0.002 to 0.015 m wide in phyllic altered granodiorite, c A-type quartz vein trending NW-SE and 0.10 m width within a phyllic altered granodiorite, d copper mineralization from potassic zone, with malachite and azurite on altered granodiorite in the Zafarghand Copper Exploration District (ZCED)
Petrography of hydrothermally altered rocks and mineralization
Macro- and microscopic observations of the magmatic rocks of the Zafarghand district show that they are affected by pronounced hydrothermal alteration (Aminoroayaei Yamini et al. 2016). This alteration is primary minerals replaced pervasively by secondary alteration minerals or vein type (development of hydrothermal minerals in veins). Several styles of alterations were observed, and in chronological (paragenetic) order are potassic alteration, phyllic alteration, argillic alteration, and propylitic alteration.
Potassic alteration is the earliest alteration in the form of a patch that is visible in the surface (Aminoroayaei Yamini et al. 2016). This zone was produced by fluxes of alkali metasomatic activity that caused the development of hydrothermal quartz in veins and veinlets, amphiboles replaced by biotite and pyrite along cleavage planes (Fig. 3a) and plagioclase converted to K-feldspar and biotite into chlorite (Fig. 4b) in the granodiorite intrusion; and in the dacitic and rhyodacitic rocks: development of hydrothermal K-feldspar, hydrothermal biotite, and quartz as groundmass minerals.
Series of photomicrographs obtained in CPL (cross-polarized light), PPL (plane-polarized light) and RL (reflected plane-polarized light), respectively. a–c Mafic phenocryst replaced by biotite and pyrite hosted by granular quartz and plagioclase, and K-feldspar (sample 82ZA). d–e Magmatic biotite pervasively replaced by bornite (Sample 96ZA)
Photomicrographs of different biotite types from the Zafarghand copper mineralization. a Primary biotite in granodiorite (sample 90ZA). b Primary biotite completely replaced by chlorite in granodiorite porphyry (sample 72ZA). c Oxidized biotite in dacite porphyry (sample 60ZA). d Oxidized and secondary biotites in rhyodacite (sample 106) (a, c, and d are PPL and b is CPL)
The zone of phyllic alteration overprints the earlier formed potassic zone. Corresponding to the hydrolysis reactions of mafic and intermediate minerals in the granodiorite, plagioclase and mafic minerals transformed into sericite and in the andesitic rocks replacement of plagioclase by sericite and mafic minerals by chlorite. Argillic alteration is recognized by the transformation of both granodiorite and rhyodacite into clay minerals. The affected rocks are soft and white colored. Kaolinite, sericite, illite, and quartz characterize this alteration assemblage (Aminoroayaei Yamini et al. 2016). The argillic alteration is highly destructive, completely replacing the original mineral phases.
The later propylitic alteration is the most abundant alteration type in the study region. There is a relatively sharp boundary between the propylitic and phyllic alteration zones. The propylitic zone occurs in the peripheral parts of the system. Propylitic alteration is irregular in intensity and generally diffuses, except for some epidote and chlorite veins and veinlets. These types of rocks are generally pale green in color, which is due to the abundance of epidote and chlorite. The propylitic alteration is characterized by chlorite, epidote, carbonate, albite, clay minerals, and quartz developed in these rocks. Also microscopic observations of the propylitic zone show that some biotites are replaced by bornite (Fig. 3d–f). Figure 5 summarizes the mineral assemblages within these alteration zones.
The copper mineralization in the Zafarghand deposit shows the typical supergene enrichment blanket profile of copper deposits within the region with a deeper zone of hypogene sulfides preserved that consists of pyrite, chalcopyrite, molybdenite, and bornite within quartz veins or as fine disseminations in all rock units. The hypogene mineralization is genetically linked to the hydrothermal system related to Zafarghand granodioritic porphyry. In the supergene zone, bornite occurs in association with biotite as a replacement phase among magmatic biotite. Covellite occurs as a substitute on the margin of chalcopyrite. In some parts, supergene leaching of hypogene ore led to remnants of primary sulfides (mainly pyrite) and traces of copper oxides such as cuprite. In this zone pyrite and biotite formed along amphibole cleavages during hydrothermal alteration (Aminoroayaei Yamini et al. 2016).
Sampling and analytical methods
A total of 200 samples were collected from various lithologies of volcanic to plutonic units around Zafarghand. About 60 polished thin sections were made and petrographic observations with special emphasis on biotite and chlorite were carried out using the polarizing microscope. Two sample suites were selected for determination of the biotite and chlorite compositions by electron probe microanalyser (EPMA) at the Laboratory of Naruto University, Japan and the University of Oklahoma, America. Suite 1 consists of magmatic biotite (5 biotite grains) and suite 2 reflects the various chloritized biotite samples (15chlorite grains). Standard operating conditions included an accelerating potential of 15 kV, a beam current of 20 nA and 20s count time.
Chlorite and biotite formulae were recalculated on an anhydrous 28 O-atom and 22 O-atom per-formula-unit bases. The chlorite recalculation scheme together with the end-member names, mole fractions, ferric and ferrous stimation on the basis of stoichiometric constraints. Biotite formula was determined using the Mica + program (Yavuz 2003). The XPh, XAnn, XPDO, and XSid are mole fractions of phlogopite, annite, proton-deficient oxyannite and sidrophylite respectinely that determined on basis of octahedral ions (calculations from Jacobs and Parry 1979). The XMg and XFe values of biotites are determined from cation fractions and are defined as Mg/(Fe + Mg) and (Fe + AlVI)/(Fe + Mg + AlVI), respectively (Zhu and Sverjensky 1992).
Eight samples of minerals were also analyzed for stable isotopes (hydrogen and oxygen), including two chloritic biotite from potassic alteration zone of granodiorite and rhyodacite porphyry, four samples of sericite from rhyodacite, quartz from barren and A-type veins in phyllic zone, two epidotes in propylitic alteration (see Table 3). The samples were purified by crushing and hand picking to a purity of about 99%. The gases were extracted in the Laboratory for Stable Isotope Geochemistry, Institute of Geology and Geophysics, Chinese Academy of Sciences. Standard operating conditions included V-SMOW for H and O isotope. Error of oxygen and hydrogen isotope analyses is better than 0.2‰.
Results
Chloritization of biotite
Biotite in host volcano-plutonic rocks from the Zafarghand copper mineralization fall into two populations based on petrography and composition. The population that constitutes less than 10% consists of magmatic biotite that typically occurs in granodiorite as euhedral to subhedral phenocrysts and microphenocrysts (Fig. 4a). Whereas secondary biotite commonly occurs as subhedral to anhedral crystals, which are frayed, ragged, splintery, and kinked. The term “secondary” describes biotite inferred to have precipitated from the hydrothermal fluid responsible for potassic alteration. Secondary biotite is petrographically distinct from magmatic biotite, and occurs as aggregates of fine-grained flakes (Fig. 4d). Secondary biotite partially replaces magmatic hornblende, and occurs throughout the granodiorite (Fig. 3a–c).
Compositions of biotite are presented in Table 1. In the chemical classification plot of Nachit et al. (1985), magmatic biotite occupies the primary biotite fields (Fig. 6a). The term “primary” indicates the biotite is inferred to have crystallized directly from that silicate melt. Biotite phenocrysts and secondary biotites in granodiorite and dacite locally exhibit strong chloritization. Chemical analyses of chlorite together with the calculated unit cell formulae are presented in Table 2. Si value ranges from 6.3 to 6.6 per formulae unit. The Fe/(Fe + Mg) values in these chlorite phases vary from 0.5 to 0.6 (Table 2). The compositions of these chlorite analyses plot in the field of brunsvigite and pycnochlorite fields of Hey’s classification diagram (1954) and in the fields of magnesian chamosite and iron clinochlore of Bayliss classification (Bayliss 1975; Table 2).
a Primary nature of biotite from the mineralized Zafarghand Igneous rocks as plotted in terms of the TiO2–FeOtotal–MgO ternary plot of Nachit et al. (1985). b, c Plots of MgO–FeO*–Al2O3 (Rossi and Chevremont 1987; Abdel-Rahman 1994) of primary biotite composition (FeO* = FeO + Fe2O3). d Variations in Fe3+–Fe2+–Mg2+; diagram after Wones and Eugster (1965). e Variations in the (FeO)/ (FeO + MgO) ratio vs. MgO in biotites from the Zafarghand granodiorite; diagram after Zhou (1986)
Oxygen and hydrogen isotope systematics
Biotite from the potassic altered samples yields oxygen and hydrogen isotope compositions varying from 4.0 to - 4.6 and −59.8 to −60.4‰, respectively (Table 3). The most probable factor controlling the observed variation in isotopic composition of oxygen and hydrogen was the interaction of the host rock with hydrothermal fluids. Many authors postulate that during chloritization of biotite, isotopic exchange occurs between minerals and fluids present in the system (Criss and Taylor 1983; Nabelek et al. 1983; Brigham and O’Neil 1985). Other factors controlling the variability of isotopic composition of H and O, like lack of magma homogenization, assimilation of country rocks, and kinetic processes, e.g., differential degassing of the pluton (Rayleigh effect, see Nabelek et al. 1983).
Isotopic analyses of phyllic alteration were conducted on hydrothermal sericite and quartz from the barren and A-type quartz veins. The oxygen and hydrogen isotopic compositions of sericite for sample Se24ZA of phyllic alteration are 3.4 and −34.7‰, respectively (Table 3). The oxygen isotope compositions of two samples of quartz barren veins are 8.3 and 7.8‰. These samples are δ18O-enriched relative to those of A-type quartz vein. The sample Qz45ZA from the A-type quartz vein indicates oxygen isotope compositions of 5.7‰ (Table 3). Propylitic alteration in two samples from epidote veins have been analyzed and yield similar oxygen and hydrogen isotope compositions and varying from 2.7 to 3.5 and −39.7 to −42‰, respectively (Table 3).
Discussion
Biotite replacement by chlorite
Primary biotites that have been analyzed are Mg-rich with ferroan phlogopite compositions. The green color of biotite from arc-related granites reflects a high Mg and Fe3+ with low Al indicating relatively more oxidizing conditions (Lalonde and Bernard 1993). The AlVI abundances of biotite permit discrimination between I- and S-type granites, as suggested by Whalen and Chappell (1988) that I-type granites are associated with biotites with low AlVI abundances (0.144–0.224), whereas S-type granites are associated with biotites with higher AlVI abundances (0.353–0.561). The AlVI abundances in biotites from the Zafarghand granodiorite ranged from 0.0 to 0.03 (Table 1), indicating that the Zafarghand granodiorite is an I-type granite. Abdel-Rahman (1994) also found that biotites in I-type granites are relatively enriched in magnesium, whereas S-type granite biotites are relatively enriched in aluminum. In addition, Zafarghand granodiorite biotites are magnesian, which also supports an I-type granite classification for this intrusion. Biotite compositions can be used to discriminate tectonic settings of granitoids. When plotted on the Al2O3–FeOtot–MgO ternary diagrams (Rossi and Chevremont 1987; Abdel-Rahman 1994), all analyzed biotites fall in the field of calc-alkaline orogenic suites (Fig. 6b, c). Zhou (1986) suggested that a (ΣFeO)/(ΣFeO + MgO) vs. (MgO) diagram using biotite compositions could be used to discriminate granites of differing origins. Using this diagram, biotites from the Zafarghand granodiorite plot within the mixed mantle–crust source (MC) area (Fig. 6e). These results are consistent with isotopic analyses in the Miocene Kuh Panj porphyries showing the 206Pb/204Pb, 207Pb/204Pb,and 208Pb/204Pb ratios ranged between 18.52–18.61, 15.58–15.65, and 38.55–38.80, respectively, and had a mixed mantle–crust source (Shafiei 2010; Asadi et al. 2014).
The structural formula indicate that the biotite is essentially free of octahedral Al, and that the tetrahedral sheets of biotite and chlorite have a similar Si:Al ratio. The six paired analyses of Ferry (1979) show the same similarity between tetrahedral sheets. Parry and Downey (1982) noticed that chlorite from more altered biotite had a higher Mg content than chlorite from less altered biotite. Major differences between biotite and its chlorite reaction product are the high Al content of the chlorite octahedral sheet. Thus, although the tetrahedral sheets appear to be inherited intact to there is free element exchange in the octahedral site. Alternatively chlorites contain minimal original Ti content of biotite. TiO2 values vary from 3.13 to 1.39 wt.%, which is in general not an important constituent of chlorite (Fig. 4b). Ca, K, and Na typically occur as impurities in chlorite (Albee 1962; Deer et al. 1962). Czamanske et al. (1981) suggested that these elements are, occasionally, absorbed or occur as interlayer cations in chlorite. The depletion of K2O and decrease in SiO2 are related to the formation of K-feldspar accompanying the breakdown of biotite to chlorite.
The stability of mica during physical-chemical changes of the environmental conditions depends not only on its cationic content but also on the composition of its anionic network and layer charges. By comparing the structural formulae of magmatic biotite with those of ideal mica, it is likely to be affected by the process of decomposition of the hydroxyl group during oxidation and dehydration. The decomposition of the hydroxyl group takes place either as a result of oxidation of iron or by loss of water (and/or fluorine; Rimsaite 1970). The process of decomposition thus depends on the quantity of ferrous iron in the mica, composition of the hydroxyl group, and available oxygen, or environmental conditions (Rimsaite 1970). Biotite phenocrysts in Zafarghand are provided for naturally dehydrated and oxidized micas to illustrate the decomposition of the hydroxyl group following predominantly the process of oxidation at the expense of meteoric water.
In addition, Fig. 3a–c shows amphibole replaced by biotite and pyrite along cleavage planes. As suggested by Brimhall et al. (1985), biotitization of primary igneous hornblende occurred at temperatures of 300 to 500 °C at 1 kb. The main factors controlling the completeness of the reaction are time and the volume ratio of fluid to hornblende. Biotitization of hornblende is similar to other biotite-forming reactions in porphyry copper deposits. The chemical effect of the pseudomorphic replacement of hornblende by biotite is to fix Mg2+ (with Fe2+ and Fe3+), K+, and SO4 2− (H2S) components from the fluid in the mineral assemblage as a mixture of biotite, anhydrite, and iron oxides and sulfides locally. The reacting fluid, in contrast, may become enriched in Fe2+ and Ca2+. Silica, released by the destruction of hornblende, precipitates as quartz (if oversaturated), and the iron saturates as pyrite or magnetite implying a link with the mineralizing process (Brimhall et al. 1985).
The concentration of Ti in biotite is very sensitive to temperature and fO2, making it possible to use biotite to obtain reliable temperature estimates for igneous and metamorphic rocks (PatinoDouce 1993). Here, we use the empirical Ti-in-biotite geothermometer of Henry et al. (2005) to estimate the primary biotite crystallization temperatures (Table 1). Biotite compositions from the Zafarghand samples fall within the calibrated compositional and temperature ranges specified (XMg = 0.275–1.000, Ti = 0.04–0.60 apfu, T = 480–800 °C); however, they likely formed at slightly lower pressure than the calibration range (400–600 MPa). Mercer and Reed (2013) suggest that errors arising from application of the biotite geothermometer relate to microprobe analytical uncertainties and the precision of the empirical fit reported by Henry et al. (2005). Precision of the geothermometer is estimated to be ±24 °C in the 480 to 700 °C range and ±12 °C in the 700° to 800 °C range. These data indicate, as would be expected, that primary biotites are typically characterized by high temperatures (617–675 °C) (Table 1). Given that Ti is apparently highly mobile in biotite (Henry et al. 2005).Mercer and Reed (2013) suggest that these temperatures represent conditions prevailing near the end of the granite cooling history. In addition, Ti activity may not be accurately predicted as well. In the Fe3+–Fe2+–Mg biotite diagram shown in Fig. 6d, all of the biotites that were analyzed are the same or slightly higher than the Fe2O3–Fe3O4 buffer, indicating that they crystallized under conditions of high oxygen fugacity (Wones 1989).
The empirical chlorite geothermometer has been employed to constrain the temperature of the chlorite formation replacing biotite in these deposits. The calculated temperatures from the geothermometer of Cathelineau (1988) represent the conditions of chloritization of biotite in the Zafarghand area. The sample of the potassic alteration with chlorite shows temperatures between about 177 and 346 °C (Table 2).
Evolution of isotopic composition of hydrothermal fluids
The isotopic compositions of fluid that was in equilibrium with chloritized biotite at temperature of 337 °C (based on microprobe analyses data) were calculated using published oxygen and hydrogen isotope fractionation factors by Cole and Ripley (1999) and Graham et al. (1984b) (Table 3). The projection points of the water involved in the chloritization of biotite are plotted in Fig. 7. H2O isotopic data for the chloritization of biotite plot distinctly outside the magmatic water field. This shift is towards the meteoric water line.
δ18O vs δD for calculated water of fluids responsible for chloritized biotite in potassic zone (210 °C; Table 2) and epidote in propylitic zone (230 °C) formation from Zafarghand. The meteoric water line is taken from Craig (1961), and the range of magmatic waters and seawater composition from Sheppard (1977)
Isotopic analyses of phyllic alteration were conducted on hydrothermal sericite and quartz from the barren and A-type quartz veins. On the basis of fluid inclusion data of quartz (Chelevy 2015), the estimated temperatures for quartz formation are around 350 °C. In addition, paragenetic relations show that sericite formed following to quartz thus, we used the estimated temperature of 300 °C to calculate δ18O and δD for fluids involved in sericite formation. The equations of Clayton et al. (1972), Sheppard and Gilg (1996) and Suzuoki and Epstein (1976) were used to calculate the isotopic composition of water responsible for phyllic alteration. The results indicate that the phyllic altered rocks may have been altered by mixed meteoric and magmatic water. Whereas the calculated δ18O values of the phyllic fluids from sericite and quartz veins vary between −2.8 and 2.5‰, the δD values estimated from sericite in this alteration is −23.2‰ (Table 3). The propylitic alteration could be interpreted on the basis of microprobe data and using the chlorite geothermometer (Aminoroayaei Yamini et al. 2016), the estimated temperatures for chlorite formation from propylitic alteration are around 230 °C. Furthermore, paragenetic relations show that epidote formed subsequent to chlorite thus, we used the estimated temperature of 230 °C to calculate δD for fluids involved in epidote formation. Also the equations of Chacko et al. (1999) were used to calculate the isotopic composition of water responsible for propylitic alteration. The results show a single progressive reaction path of meteoric water with granodiorite, dacite, and rhyodacite of Zafarghand with δ18O from 0.8 to 1.6‰ and δD from −14.6 to −16.9‰ (Fig. 7, Table 3).
Based on the isotopic data on the phyllic and propylitic alteration from this study and the main stage data from Sheppard and Taylor (1974), the mineralization event at Zafarghand may have taken place as the following processes. The biotite and orthoclase of potassic alteration may have formed initially by magmatic water (Chelevy 2015). Chemical composition of chlorite coexisting with biotite and orthoclase suggests a high temperature (471 to 361 °C) fluids caused main stage alteration (Aminoroayaei Yamini et al. 2016). Then this fluid has mixed with shallow meteoric water within average of 2.39 per mil for δ18O, which was circulating from volcano plutonic rocks and formed barren quartz veins. Afterward, this fluid has remobilized metals, such as Cu, Pb, Zn into the ore fluid (Brimhall 1979, 1980). This ore-bearing fluid mixed with meteoric water with δ18O = 0.39‰, which were responsible for A-type-quartz veins. The sericites of phyllic alteration may have formed subsequently by meteoric water with isotopic compositions of −2.76‰ for δ18O and −23‰ for δD. Fluid inclusion data suggest a temperature range of 263 °C to 510 °C for these main stage alterations. The significant variation of oxygen isotopic composition and the D-depletion in the phyllic altered samples resulted from isotopic exchange of sericite with the younger, low-temperature, meteoric-dominated phyllic fluids, as initially proposed by Sheppard and Taylor (1974). The phyllic fluids could have altered the earlier potassic altered rock, oxidized the primary biotites and chloritized the primary and secondary biotites from the potassic alteration by meteoric water with isotopic compositions of ∼1.44 per mil for δ18O and −20.2 per mil for δD. The decomposition of the hydroxyl group takes place as a result of the oxidation of iron at the expense of meteoric water. Subsequently, the epidote of propylitic alteration may possibly have formed by meteoric water with isotopic compositions of 0.8 to 1.6‰ for δ18O and −14.65 to −16.9‰ for δD. Chemical composition of chlorite coexisting with epidote suggests a temperature range of 196 to 266 °C for the late stage alterations (Aminoroayaei Yamini 2016). Paragenetic (Fig. 5), isotopic compositions, and temperature of fluid from this zone were responsible for the late main stage mineralization and propylitic alteration.
Conclusions
Mineralization and alteration in the Zafarghand area are superimposed onto the associated porphyritic body and the surrounding country rock. The crystallization temperatures of biotites from the Zafarghand granodiorite ranged from 617 °C to 675 °C ± 24 °C. These crystallization conditions also indicate that the Zafarghand granodiorite formed at high temperatures, and under conditions of very high oxygen fugacity suggesting that the Zafarghand granodiorite is highly prospective for mineral exploration and genetically associated with the Zafarghand porphyry copper deposit. Based on the mineral-chemistry and isotopic data on the phyllic and propylitic alteration, magmatic fluid from potassic alteration has mixed with shallow meteoric water within average of 2.39 per mil for δ18O, which was circulating from volcano plutonic rocks at Zafarghand and formed barren quartz veins. Afterward, this fluid has remobilized metals, such as Cu, Pb, Zn into the ore fluid. This ore-bearing fluid mixed with meteoric water with δ18O = 0.39‰, which was circulating from east to west at Zafarghand that were responsible for A-type-quartz veins. The sericites of phyllic alteration may have formed subsequently by meteoric water with isotopic compositions of −2.76‰ for δ18O and −23‰ for δD. The phyllic fluids could have altered the earlier potassic altered rock, oxidized the primary biotites and chloritized the primary and secondary biotites from the potassic alteration by meteoric water with isotopic compositions of ∼1.44 per mil for δ18O and −20.2 per mil for δD. The decomposition of the hydroxyl group takes place as a result of the oxidation of iron at the expense of meteoric water. The composition of chlorite, mainly bronsvigite and pycnochlorite, is closely related to composition of its host biotite. Breakdown of biotite into chlorite was accompanied by the following chemical changes: decrease in SiO2, K2O, and TiO2; increase in MgO, FeO total and MnO and slight increase in Al2O3. The data indicate that the chloritization temperatures of biotite from potassic alterations are in the range between 177 and 346 °C. Subsequently, the epidote of propylitic alteration may possibly have formed by meteoric water with isotopic compositions of 0.8 to 1.6‰ for δ18O and −14.65 to −16.9‰ for δD and fluids that were responsible for the late main stage mineralization and propylitic alteration.
References
Abdel-Rahman AM (1994) Nature of biotites from alkaline, calc-alkaline, and per aluminous magmas. J Petrol 35:525–541
Afshooni SZ, Asadi Harooni H, Esmaili D (2011) The microthermometry study of fluid inclusions in quartz veins of Kahang deposit (north eastern of Isfahan). 2nd National Symposium of Iranian Society of Economic Geology. Lorestan University (in Persian with English abstract)
Alavi M (1994) Tectonic of the Zagros orogenic belt of Iran: new data and interpretations. Tectonophysics 229:211–238
Albee AL (1962) Relationships between the mineral association, chemical composition and physical properties of the chlorite series. Am Mineral 47:851–870
Aminoroayaei Yamini M (2016) Mineralogy, geochemistry, alteration and mineralization of volcano-plutonic rocks in Zafarghand, south of Ardestan. Ph.D. thesis, University of Tehran, 267 pp. (in Persian with English abstract)
Aminoroayaei Yamini M, Futti F, Haschke M, Ahmadiam J, Murata M (2016) Synorogenic copper mineralization during the Alpine-Himalayan orogeny in the Zafarghand copper exploration district, Central Iran: petrography, geochemistry and alteration thermometry. Geol J. doi:10.1002/gj.2755
Asadi S, Moore F, Zarasvandi A (2014) Discriminating productive and barren porphyry copper deposits in the southeastern part of the central Iranian volcano-plutonic belt, Kerman region, Iran: a review. Earth Sci Rev 138:25–46
Atapour H, Aftabi A (2007) The geochemistry of gossans associated with sarcheshmeh porphyry copper deposit, Rafsanjan, Kerman, Iran: implications for exploration and the environment. J Geochem Explor 93:47–65
Bayliss P (1975) Nomenclature of the trioctahedral chlorites. Can Mineral 13:178–180
Beane R (1982) Hydrothermal alteration in silicate rocks. In: Titley SR (ed) Advances in the geology of the porphyry copper deposits: southwestern North America. The University of Arizona Press, Tucson, p 117–137
Bird DK, Helgeson HC (1980) Chemical interaction of aqueous solutions with epidote-feldspar mineral assemblages in geologic systems. I: thermodynamic analysis of phase relations in the system CaO–FeO–Fe2O3–Al2O3–SiO2–H2O–CO2. Am J Sci 280:907–941
Boomeri M, Nakashima K, Lentz DR (2009) The Miduk porphyry Cu deposit, Kerman, Iran: a petrologic analysis of the potassic zone including halogen element systematics related to Cu mineralization processes. J Geochem Explor 103:17–29
Brigham RH, O’Neil JR (1985) Genesis and evolution of water in a two-mica pluton: a hydrogen isotope study. Chem Geol 49:159–177
Brimhall GH Jr (1979) Lithologic determination of mass-transfer mechanism of multiple-stage porphyry copper mineralization at Butte, Montana: vein formation by hypogene leaching and enrichment of potassium silicate protore. Econ Geol 74:556–589
Brimhall GH (1980) Deep hypogene oxidation of porphyry copper potassium silicate protore at Butte, Montana: a theoretical evaluation of the copper remobilization hypothesis. Econ Geol 75:384–409
Brimhall GH, Agee C, Stoffregen R (1985) The hydrothermal conversion of hornblende to biotite. Can Mineral 23:369–379
Calagari AA (2004) Geology and fracture-related hypogene hydrothermal alteration and mineralization of porphyry copper deposit at Sungun. J Geol Soc India 64:595–618
Cathelineau M (1988) Cation site occupancy in chlorites and illites as a function of temperature. Clay Miner 23:417–485
Chacko T, Riciputi LR, Cole DR, Horita J (1999) A new technique for determining equilibrium hydrogen isotope fractionation factors using the ion microprobe: application to the epidote-water system. Geochim Cosmochim Acta 63:1–10
Chelevy M (2015) The study of mineralization and origin of the hydrothermal vein in igneous rocks of Zafarghand. M.Sc. thesis, University of Tehran, 164 pp. (in Persian with English abstract)
Chiu HY, Chung SL, Zarrinkoub MH, Mohammadi SS, Katib MM, Izuka Y (2013) Zircon U–Pb age constraints from Iran on the magmatic evolution related to Neotethyan subduction and Zagros orogeny. Lithos 162–163:70–87
Clayton RN, O’Neil JR, Mayeda TK (1972) Oxygen isotope exchange between quartz and water. J Geophys Res 77:3057–3067
Cole DR, Ripley EM (1999) Oxygen isotope fractionation between chlorite and water from 170 to 350 °C: a preliminary assessment based on partial exchange and fluid rock experiments. Geochim Cosmochim Acta 63:449–457
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Criss RE, Taylor HP Jr (1983) An18O/16O and D/H study of tertiary hydrothermal systems in the southern half of the Idaho batholith. Geol Soc Am Bull 94:640–663
Czamanske GK, Ishihara S, Atkin SA (1981) Chemistry of rock-forming minerals of the Cretaceous-Paleocene batholith in southwestern Japan and implications for magma genesis. J Geophys Res 86(B11):10431–10469
Deer WA, Howie RA, Zussman J (1962) Rock-forming minerals. John Wiley and Sons, New York
Ferry JM (1979) Reaction mechanisms, physical conditions and mass transfer during hydrothermal alteration of mica and feldspar in granitic rocks from south-central Maine, U.S.A. Contrib Mineral Petrol 68:125–139
Graham CM, Atkinson J, Harmon RS (1984b) Hydrogen isotope fractionation in the system chlorite-water. Progress in Experimental Petrology 6,139-140 Natural Environment Research Council of U.K. Publication Series D, 25, London
Henry DJ, Guidotti CV, Thomson JA (2005) The Ti-saturation surface for low to medium pressure metapelitic biotite: implications for geothermometry and Ti-substitution mechanisms. Am Mineral 90:316–328
Hey MH (1954) A new review of the chlorites. Mineral Mag 30:277–292
Hezarkhani A (2006a) Hydrothermal evolution of the Sar-Cheshmeh porphyry Cu–Mo deposit, Iran: evidence from fluid inclusion. J Asian Earth Sci 28:409–422
Hezarkhani A (2006b) Fluid inclusion investigations of the Raigan porphyry copper system, Kerman-Bam, Iran. Int Geol Rev 48:255–270
Hezarkhani A, Williams-Jones AE (1998) Controls of alteration and mineralization in the Sungun porphyry copper deposit, Iran: evidence from fluid inclusion and stable isotopes. Econ Geol 93:651–670
Jacobs DC, Parry WT (1979) Geochemistry of biotite in the Santa Rita porphyry copper deposit, New Mexico. Econ Geol 74:860–887
Lalonde AE, Bernard P (1993) Composition and color of biotite from granites: two useful properties in the characterization of plutonic suites from the Hepburn internal zone Wopmayorogen, Northwest Territories. Can Mineral 31:203–217
Mercer C, Reed M (2013) Porphyry Cu-Mo stock work formation by dynamic, transient hydrothermal pulses: mineralogic insights from the deposit at Butte, Montana. Econ Geol 108:1347–1377
Nabelek PI, O’Neil JR, Papike JJ (1983) Vapor phase exsolution as a controlling factor in hydrogen isotope variation in granitic rocks: the Notch Peak granite stock, Utah. Earth Planet Sci Lett 66:137–150
Nachit H, Razafimahefa N, Stussi JM, Carron JP (1985) Composition chimique des biotites et typologie magmatique des granitoides. CR Hebd Acad Sci 301–11:813–818
Parry WT, Downey LM (1982) Geochemistry of hydrothermall chlorite replacing igneous biotite. Clay Clay Miner 30:81–90
PatinoDouce AE (1993) Titanium substitution in biotite: an empirical model with applications to thermometry, O2 and H2O barometers, and consequence for biotite stability. Chem Geol 108:132–162
Rimsaite J (1970) Structural formulae of oxidized and hydroxyl-deficient micas and decomposition of the hydroxyl group. Contrib Mineral Petrol 25:225–240
Rossi P, Chevremont P (1987) Classification des associations magmatiques granitoides. Géochronique 21:14–18
Shafiei B (2010) Lead isotope signatures of the igneous rocks and porphyry copper deposits from the Kerman Cenozoic magmatic arc (SE Iran), and their magmatic–metallogenetic implications. Ore Geol Rev 38:27–36
Shafiei B, Shahabpour J (2008) Gold distribution in porphyry copper deposit of Kerman region, southeastern Iran. J Sci I R Iran 19:247–260
Shahabpour J (2000) Behavior of Cu deposit, Kerman, Iran. CIM Bull 93:44–51
Shahabpour J (2005) Tectonic evolution of the orogenic belt in the region located between Kerman and Neyriz. J Asian Earth Sci 24:405–417
Sheppard SMF (1977) Identification of the origin of ore forming solutions by the use of stable isotopes. Geol Soc Spec Publ 7:25–41
Sheppard SMF, Gilg HA (1996) Stable isotope geochemistry of clay minerals. Clay Miner 31:1–24
Sheppard SMF, Taylor HP Jr (1974) Hydrogen and oxygen isotope evidence for the origin of water in the Boulder Batholith and the Butte ore deposits, Montana. Econ Geol 69:926–946
Suzuoki T, Epstein S (1976) Hydrogen isotope fractionation between OH bearing minerals and waters. Geochim Cosmochim Acta 40:1229–1240
Taghipour N, Aftabi A, Mathur R (2008) Geology and Re-Os geochronology of mineralization of the Miduk porphyry copper deposits, Iran. Resour Geol 58:1–18
Walshe JL (1986) A six component chlorite solid solution model and the conditions of chlorite formation in hydrothermal and geothermal systems. Econ Geol 81:681–703
Whalen JB, Chappell BW (1988) Opaque mineralogy and mafic mineral chemistry of I- and S- type granites of Lachlan fold belt, southeast Australia. Am Mineral 73–3:281–296
Wones DR (1989) Significance of the assemblage titanite + magnetite + quartz in granitic rocks. Am Mineral 74:744–749
Wones DP, Eugster HP (1965) Stability of biotite: experiment, theory, and application. Am Mineral 50:1228–1272
Yavuz F (2003) Evaluating micas in petrologic and metallogenic aspect: I–definitions and structure of the computer program Mica+. Comput Geosci 29:1203–1213
Zarasvandi A, Liaghat S, Zentilli M (2005) Geology of the Darreh-Zerreshk and Ali-Abad porphyry copper deposits, Central Iran. Int Geol Rev 47:620–646
Zhou ZX (1986) The origin of intrusive mass in Fengshandong, Hubei province. Acta Petrol Sin 2–2:59–70
Zhu C, Sverjensky DA (1992) F–Cl–OH partitioning between biotite and apatite. Geochim Cosmochim Acta 56:3435–3467
Acknowledgements
This paper presents the PhD thesis work of lead author Maryam Aminoroayaei. We thank David Lentz, Xueming Yang, Hassan Heidarian, and an anonymous expert for helpful reviews. The isotopic analysis was supported by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB03010801) and NSFC (41672085).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: L. Nasdala
Rights and permissions
About this article
Cite this article
Aminroayaei Yamini, M., Tutti, F., Aminoroayaei Yamini, M. et al. Examination of chloritization of biotite as a tool for reconstructing the physicochemical parameters of mineralization and associated alteration in the Zafarghand porphyry copper system, Ardestan, Central Iran: mineral-chemistry and stable isotope analyses. Miner Petrol 111, 747–759 (2017). https://doi.org/10.1007/s00710-016-0486-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-016-0486-7