Abstract
Element mobility and fluid-rock interaction related to the formation of late-metamorphic quartz veins have been studied by combination of mineral chemistry, whole-rock geochemistry, mass balance analysis and fluid-mineral equilibria modeling. The quartz veins are hosted by very low-grade metasedimentary rocks of the fold-and-thrust belt of the Rhenish Massif (Germany). The veins record two stages of evolution, a massive vein filling assemblage with elongate-blocky quartz, chlorite, apatite and albite, and a later open space filling assemblage with euhedral crystals of quartz, ankerite-dolomite and minor calcite and sulfides. Detailed mass balance analysis of an alteration profile adjacent to a representative quartz vein demonstrates that element mobility is restricted to the proximal zone. The most important element changes are gain of Ca, Fe, Mg, Mn, P and CO2, and loss of Si, K and Na. The data demonstrate that wall-rock carbonation is one of the main alteration features, whereas mobility of Si, K and Na are related to dissolution of quartz and destruction of detrital feldspar and muscovite. The whole-rock geochemical data, in conjunction with fluid composition data and pressure-temperature estimates, were used as input for fluid-mineral equilibria modeling in the system Si-Al-Fe-Mg-Ca-Na-K-C-S-O-H-B-F-Cl. Modeling involved calculation of rock-buffered fluid compositions over the temperature interval 100–500 °C, and reaction-path simulations where a rock-buffered high-temperature fluid reacts with fresh host-rocks at temperatures of 400, 300 and 200 °C. Calculated rock-buffered fluid compositions demonstrate that retrograde silica solubility is a strong driving force for quartz leaching in the temperature-pressure window of 380–450 °C and 0.5 kbar. These conditions overlap with the estimated temperatures for the initial stage of vein formation. Reaction-path models show that high-temperature alteration can produce the observed silica leaching, suggesting that fast advection of external hot fluids from deeper crustal levels was essential for the early stage of vein formation. Fluid advection must have occurred as multiple pulses, which allowed for periods of influx of fluids that leached quartz, alternating with periods of cooling and quartz precipitation in the veins. Reaction-path models at high temperatures (300–400 °C) do not produce carbonate alteration, whereas fluid-rock reaction at 200 °C produces carbonate alteration, consistent with the temperature estimates for the late-stage vein carbonate assemblage. Comparison between modeling results and geochemical data suggests that the observed alteration features are the product of fluid-rock reaction under conditions where the external fluid gradually cooled down and evolved with time. The results of this study highlight the importance of late-orogenic fluid migration for the formation of quartz vein arrays in fold-and-thrust belts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metamorphic veins in orogenic belts are hosted by a wide range of rock types ranging from metasediments to metavolcanics and they are one of the most important indicators of mass transfer during deformation and metamorphism (Ague 1994a, b, 2011). Quartz vein arrays provide clear evidence for small- to large-scale crustal fluid flow, and yield key information about the nature and composition of fluids that migrate through the upper and middle crust (Bickle and Mckenzie 1987; Ague 2003; Cox 2007; Penniston-Dorland and Ferry 2008; Van Noten et al. 2011; Jacques et al. 2013). Fluid flow and mass transfer play a significant role in the mineralogical, chemical, and structural evolution of the continental crust (Etheridge et al. 1983; Mullis et al. 1994; Nesbitt and Muehlenbachs 1995; Oliver 1996). Continental collision transports entire crustal terrains to deeper levels where rocks are subjected to higher temperature-pressure conditions, where hydrous minerals, carbonates and sulfides become increasingly unstable and release large volumes of metamorphic fluids (Cartwright and Buick 2000). Development of major shear zones and faults during deformation facilitates drainage of fluids from large rock volumes and successive migration from deeper to shallower levels of the crust (Yardley 1983; Dipple and Ferry 1992; Yardley 1997; Connolly 1997; Jamtveit and Yardley 1997; Bons 2001). Focused fluid flow results in redistribution of heat, isotopes, and chemical species (Yardley 1983, 1997; Bickle and Mckenzie 1987; Chamberlain and Rumble 1988; Connolly and Thompson 1989; Connolly 1997; Ague 2003, 2011), and the presence of fluids impacts on mineral reactions and rock deformation behavior (Etheridge et al. 1983, 1984; Cox et al. 1987; Baumgartner and Ferry 1991; Ferry and Dipple 1991; Oliver 1996; Ague 1997; Cox 2007). Interaction of deep crustal fluids with shallow-crustal rocks typically results in strong chemical potential gradients, which drives the development of alteration haloes and selvages around veins and fault zones. If time-averaged fluid fluxes are sufficiently high, the alteration reactions can lead to major changes in the bulk composition of the wall-rocks (Oliver and Bons 2001).
Detailed studies of alteration haloes using whole-rock geochemistry and stable and radiogenic isotopes allows to quantitatively reconstruct fluid-rock interaction and mass transfer (Cox 2007; Penniston-Dorland and Ferry 2008; Wagner et al. 2010). Small-scale geochemical analysis of cross sections through alteration zones makes it possible to constrain the scale of mass transfer, to calculate the amount of mass that has been exchanged between rock and fluid, and to estimate the volume of fluid involved (Ague 1994a, b, 2011; Mountain and Williams-Jones 1996; Halter et al. 1996). Mass balance analysis allows to discriminate among elements that have behaved rather immobile during fluid-rock interaction and those that were highly mobile, and to quantify gains and losses of mobile elements (Gresens 1967; Grant 1986, 2005). This information constrains whether metamorphic veins have formed in response to crustal-scale fluid advection along temperature-pressure gradients or whether local derivation of material driven by chemical potential gradients was dominant (Jamtveit and Yardley 1997; Oliver and Bons 2001; Masters and Ague 2005). In particular, the presence of large volumes of silica that fill metamorphic quartz veins leads to the question whether they represent fossilized large-scale fluid flow pathways in metamorphic terrains, the product of local mobilization of material from the surrounding host-rocks, or a combination of both (Yardley and Bottrell 1992; Penniston-Dorland and Ferry 2008; Wagner et al. 2010). Resolving external and local contributions to vein-wall-rock systems in metamorphic belts requires to combine geochemical analysis of alteration zones, mass balance calculations, chemical microanalysis of fluid inclusions (Miron et al. 2013; Marsala et al. 2013) and geochemical modeling of fluid-rock interaction (Cleverley and Oliver 2005; Mernagh and Bierlein 2008).
This study reconstructs the fluid-rock interaction processes and fluid evolution of late-metamorphic veins in the low-grade metasedimentary rocks of the fold-and-thrust belt of the Rhenish Massif (Germany). Previous work has addressed the field relations, mineral assemblages, mineral chemistry, vein textures (Wagner and Cook 2000), stable isotope geochemistry (Wagner et al. 2010), pressure-temperature evolution and fluid inclusion compositions (Marsala et al. 2013). The data demonstrate that the veins formed from advecting external fluids that were substantially hotter than the metasedimentary rocks hosting the veins, and that fluid-rock disequilibrium caused wall-rock alteration and mass transfer. Building on this work, the present study combines small-scale whole-rock geochemical analysis of alteration envelopes with fluid-mineral equilibria modeling. The spatially-resolved geochemical dataset is used for quantitative mass balance analysis and evaluation of the scale of element mobility. The whole-rock geochemical data are combined with fluid inclusion compositions to model progressive fluid-rock interaction using a reaction-path Gibbs energy minimization code.
Geological setting
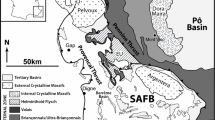
The investigated quartz veins are hosted in very low-grade metasedimetary rocks (metapelites, metasiltstones and metasandstones) of the Hunsrück nappe complex, a major tectono-stratigraphic unit of the fold-and-thrust belt of the Rhenish Massif, SW Germany (Fig. 1). The Rhenish Massif belongs to the external Rhenohercynian zone of the Central European Variscan orogenic belt (Oncken et al. 1999), and is mainly composed of metamorphosed sedimentary and volcano-sedimentary rock units. The rocks formed between Early Devonian and Late Carboniferous, and were deposited during basin development in a passive continental margin setting (Matte 1991; Oncken et al. 1999). The Lower Devonian rocks are sandstones, siltstones and pelites with few intercalations of tuffitic horizons (Franke et al. 1978). Middle-Upper Devonian to Lower Carboniferous units are lithologically more heterogeneous and comprise shallow-marine platform carbonates, volcano-sedimentary rocks, submarine volcanics, pelites and black shales (Franke et al. 1978).
Geological map of the fold-and-thrust belt of the Rhenish Massif (Germany), modified and redrawn after Oncken et al. (1999). The black star indicates the sampling location (Kaub). The insert shows the large-scale Variscan geodynamic framework of central Europe and the location of the Alpine front. Abbreviations: S.T. Siegen Thrust, B.T. Boppard Thrust, T.T. Taunus Thrust, RH Rhenohercynian zone, ST Saxothuringian zone, MO Moldanubian zone
Progressive NW-SE oriented compression affected the Rhenish Massif during the Late Carboniferous, resulting in a SW-NE strike of the first-order structural features such as the major cleavage, fold axes and imbricated nappes, which are separated by major thrust zones (Fig. 1). The main tectono-stratigraphic units are separated by the Siegen, Boppard and Taunus thrust systems, with the Boppard thrust system and the Taunus thrust system (Fig. 1) bounding the Hunsrück nappe complex to the north and south (Oncken et al. 1999). Progressive deformation resulted in development of the first pervasive cleavage (S1) related to the major folding and thermal peak, which was partially overprinted by two subsequent generations of incipient crenulation cleavage (S2 and S3) (Oncken 1984, 1988). The deformation ages are constrained by K-Ar and Ar-Ar dating of syn-deformational phyllosilicates, showing a systematic decrease from 325 to 300 Ma across the belt (Ahrendt et al. 1983; Plesch and Oncken 1999).
The peak metamorphic conditions were estimated from deformation microtextures, mineral assemblages, coal rank and illite crystallinity data, and generally do not exceed sub-greenschist facies conditions (Oncken 1991; Oncken et al. 1995). The southernmost tectono-stratigraphic unit is the only one that recorded slightly higher metamorphic conditions, up to lower greenschist facies (Oncken 1991; Dittmar et al. 1994), with temperatures and pressures estimated at 320–350 °C and 4–6 kbar (Massone 1995; Oncken et al. 1995). The metamorphic peak conditions in the Hunsrück nappe complex have been estimated from mineral chemistry, vitrinite reflectance and illite crystallinity data, and are in the range of 270–300 °C and 2–4 kbar (Wolf 1978; Oncken 1991). Following essentially orthogonal compressional deformation, the Late-Paleozoic strike-slip regime in Central Europe (Arthaud and Matte 1977; Henk 1997) resulted in rotation of the regional stress field from NW-SE towards E-W (Oncken et al. 1999). As a consequence, the pervasive S1 cleavage was partially overprinted, resulting in back-rotation of structures and re-activation of fault zones (Oncken 1991). The formation of late-metamorphic quartz vein systems in the Hunsrück nappe complex is related to late-orogenic brittle overprinting structures. The temperature-pressure conditions during the initial stage of vein formation were estimated as 370–420 °C and 0.2–0.7 kbar from chlorite thermometry, oxygen isotope geothermometry and fluid inclusion data (Wagner and Cook 2000; Wagner et al. 2010). Detailed fluid inclusion studies show a systematic decrease in temperature down to 130–240 °C (Wagner and Cook 2000; Marsala et al. 2013). Stable isotope data from veins and related wall- and host-rocks demonstrate both internal and external contributions, and that veins formed in a continuum between rock- and fluid-buffered conditions (Wagner et al. 2010).
Characteristics of quartz veins and their wall- and host-rocks
Field documentation and sampling of quartz veins and their wall- and host-rocks was done in one of the large underground roofing slate mines in the Kaub area (Fig. 1). This allowed to map the distribution of vein systems on a km-scale and to obtain well-documented and fresh sample material of veins in their structural context. Detailed sampling focused on representative large extension veins (Fig. 2a), and on detailed cross sections of their associated alteration envelopes that extended into fresh host-rocks within the same lithological unit.
The host-rocks are mainly composed of a thick sequence of dark grey-brown monotonous slates (Fig. 2b), which are mainly metasiltstones with local intercalations of rather pure metapelites and few horizons of metasandstones. A pervasive slaty cleavage (S1) is always present in the host-rocks, striking SW-NE with almost subvertical dip. Rarely, a later incipient crenulation cleavage (S2) is visible, predominantly in the vicinity of fault zones. The slaty cleavage is narrow-spaced and rather continuous in fine-grained rock types (Fig. 3a), but is more widely-spaced and discontinuous in coarser-grained lithologies (Fig. 3b). The foliation in fine-grained rocks is defined by a dense layering of sheet silicate minerals, mainly chlorite and muscovite, in a very fine-grained quartz matrix with porphyroclasts of quartz (Fig. 3c and e), chlorite-mica stacks (Fig. 3c), pyrite aggregates (Fig. 3d), feldspar (Fig. 3e) and patchy carbonates (Fig. 3f). By contrast, the foliation in more coarse-grained rocks shows an alternation of anastomosing thin layers of chlorite + muscovite surrounding lithons composed of quartz and carbonate, with some irregularly distributed porphyroclasts of chlorite, muscovite and quartz. The wall-rocks adjacent to the quartz veins have typically a slightly darker color than the fresh host-rocks, but there are no obvious mineralogical differences between the altered slates and their unaltered precursors.
Microphotographs in transmitted light illustrating key textures of host-rock slates. a Continuous foliation marked by dense layers of subparallel-oriented phyllosilicates (chlorite and white mica). b Coarsely-spaced foliation with alternation of phyllosilicate layers and quartz-carbonate lithons. c Porphyroclast composed of chlorite (Chl) and muscovite (Mus), surrounded by fine-grained quartz (Qtz) and phyllosilicates that are arranged along the main foliation. d Pyrite porphyroclast composed of recrystallized framboids. e Albite (Ab) porphyroclast with sigmoidal shape. Crossed polarizers. f Partly replaced carbonate (Carb) porphyroclast. Crossed polarizers
The quartz veins occur isolated or in small groups (two to five) and they are always surrounded by a network of smaller veinlets and fractures with similar structure and mineral assemblage. The shape is tabular or lenticular with thicknesses between 15 and 80 cm, and the contacts with the wall-rocks are sharp (Fig. 2a). The veins strike NW-SE with subvertical dip, and they crosscut the main foliation of the host-rocks (S1) at high angles. Two different successive stages in the development of the veins were recognized, which are the massive vein filling assemblage (elongate-blocky quartz, chlorite, apatite and albite) and the late-stage open vein filling assemblage (euhedral quartz, ankerite-dolomite, calcite, chalcopyrite, and pyrite).
The massive vein filling assemblage represents 90–95 % of the vein volume and is mainly composed of fibrous to elongate-blocky quartz (Bons and Jessell 1997). The quartz typically grows perpendicular to vein-wall-rock contacts and rims slate fragments. These fragments are frequently chloritized and partly replaced by vein minerals (Fig. 4a and b). Crack-seal textures (Ramsay 1980) parallel to the vein-wall-rock contacts are sometimes visible in the elongate-blocky quartz. They are restricted to the early stages of quartz growth and were not found in the central part of the veins (Fig. 4c). Chlorite is always present as inclusions within elongate-blocky quartz, and is the first mineral crystallizing from the fluid or replacing wall-rock fragments. The chlorite shows a characteristic vermicular shape (Fig. 4e), composed of numerous minute crystals (50–150 μm). Euhedral albite crystals are rare and restricted to contacts between vein quartz and wall-rock fragments (Fig. 4d).
Microphotographs in transmitted light illustrating quartz vein textures. a Elongate-blocky quartz (Qtz) enclosing wall-rock fragments that are altered and partly replaced by quartz and chlorite. b The same image with crossed polarizers. Note the considerable grain-size variation of the elongate-blocky quartz crystals. c Crack-seal texture inside coarse-grained elongate-blocky quartz. The crack-seal texture is defined by multiple bands of solid inclusions. Crossed polarizers. d Albite (Ab) crystals growing at the contact to a wall-rock fragment. Crossed polarizers. e Vermicular chlorite (Chl) intergrown with elongate-blocky quartz. f Euhedral crystals of ankerite-dolomite (Ank) and quartz present as late-stage open-space filling of an open cavity. Crossed polarizers
In the late open-space filling stage, cavities and vugs in the center of the veins were filled by euhedral crystal assemblages. Quartz is the first and most abundant mineral, showing prismatic shapes with rhombohedral terminations. Euhedral to subhedral ankerite-dolomite is intergrown with quartz crystals or has grown on top of them (Fig. 4f). Rare calcite, chalcopyrite and pyrite crystals have grown on the crystal faces of euhedral quartz and ankerite-dolomite. The transition between the massive vein filling and the subsequent open space filling assemblage is rather continuous, as demonstrated by elongate-blocky quartz crystals that continue to grow euhedrally into open fissures and cavities.
Pressure-temperature conditions of vein formation
The formation conditions and pressure-temperature evolution of the quartz veins are well-constrained from fluid inclusion data and other geothermobarometric information. Estimated pressure-temperature conditions for the early vein stage (elongate-blocky quartz, chlorite, albite and apatite) are in the range of 370–420 °C and 0.2–0.7 kbar, based on chlorite thermometry, quartz-apatite oxygen isotope thermometry and fluid inclusion isochores (Wagner and Cook 2000; Wagner et al. 2010). The fluid inclusion data demonstrate fluid cooling down to temperatures of 130–240 °C (pressure corrected homogenization temperatures) for the late-stage open-space filling assemblage (euhedral quartz cystals and ankerite-dolomite in open cavities). Detailed fluid inclusion studies of euhedral crystals from open cavities show that three successive fluid inclusion generations record systematically lower temperatures of 210–225, 148–164 and 124–139 °C. The temperature decrease is paralleled by a decrease in fluid salinity from 4.7–5.3 to 3.1–3.9 and then to 1.4–1.7 wt.% equivalent NaCl (Marsala et al. 2013). Microanalysis of fluid inclusions using laser-ablation inductively-coupled plasma-mass spectrometry (LA-ICPMS) shows that the changes in temperature and bulk salinity are correlated with a decrease in the concentration of many elements that include the alkalis, but also fluid-volatile elements such as B, As and Sb (Marsala et al. 2013). The fluid evolution is interpreted in terms of fluid mixing between a hot metamorphic fluid of moderate salinity and a cooler dilute fluid that was of meteoric origin. The fluid evolution is consistent with vein formation in a shallow crustal environment and records ingression of meteoric water during late-orogenic uplift (Marsala et al. 2013).
Methodology
Mineral chemistry
The major and minor element composition of mineral phases (chlorite, albite, carbonates) in the veins and in the wall- and host-rocks was determined by electron probe micro-analysis (EPMA) using a JEOL-8200 instrument with 5 wavelength-dispersive spectrometers. The operating conditions were 15 kV acceleration voltages and 20 nA beam current for silicates and 2 nA beam current for carbonates. Standards used for silicates were: wollastonite (Si-Kα and Ca-Kα) clinopyroxene (Na-Kα), K-feldspar (K-Kα), hematite (Fe-Kα), corundum (Al-Kα), periclase (Mg-Kα), rutile (Ti-Kα) and pyrolusite (Mn-Kα). Standards used for carbonate analysis were: wollastonite (Ca-Kα), magnesite (Mg-Kα), fluoro-apatite (P-Kα and F-Kα), barite (Ba-Lα), siderite (Fe-Kα), scapolite (Cl-Kα), strontianite (Sr-Lα) and rhodochrosite (Mn-Kα). The trace element composition of chlorite and carbonate phases was determined by LA-ICPMS microanalysis. Analysis used an excimer ArF laser-ablation system (ETH GeoLas prototype), operating at 193 nm wavelength, coupled to a Perkin Elmer Elan 6100 DRC quadrupole ICP mass spectrometer (Günther et al. 1997). Data processing was done with the SILLS software package (Guillong et al. 2008), using NIST-610 as external standard. The CaO concentration obtained from EPMA was used as internal standard for carbonates, whereas the SiO2 concentration was used for chlorites.
Whole-rock geochemistry
Selected major and trace element concentrations in wall- and host-rock samples were determined with wavelength-dispersive X-ray fluorescence (XRF) analysis using a PANalytical Axios instrument. Major and selected trace elements (Rb, Sr, Ba, Nb, Zr, Hf, Ga, Zn, Cu, Ni, Co, Cr, V, Sc, Pb, Th, U) were measured on fused glass beads obtained from standard lithium-tetraborate assay. Pressed rock powder pills were used to analyze the concentration of S, F, W, Sn, Sb and Cs. In addition, the FeO content was independently determined at the University of Lausanne using wet chemistry and colorimetric methods (Wilson 1960; Johnson and Maxwell 1981), which permitted to calculate the Fe2O3/FeO ratio using total Fe obtained from XRF analysis. The carbon content was measured with a UIC Inc. CM 5012 carbon dioxide analyzer using coulometric titration. Total carbon content is analyzed by furnace combustion (CM 5200 Autosampler) and subsequent coulometric determination, whereas the inorganic carbon content is determined by acid digestion (CM 5130). Organic carbon is then calculated as the difference between total carbon and inorganic carbon content. The water content of the samples was not independently measured, but calculated by mass balance from the loss on ignition, the volatile concentrations (CO2, Corg, S) and the gain on ignition obtained from the FeO and Fe2O3 content. LA-ICPMS analysis on the fused glass beads were performed to determine As and rare earth elements concentration, and the same type of analysis was done on the pressed powder pills to obtain Li and B concentration. Instrumentation, analytical procedures and data processing techniques were the same as applied for trace element analysis in minerals.
Illite and chlorite crystallinity
Textured, air dried, < 2 μm fractions of host-rock samples were analyzed to determine the illite and chlorite crystallinity, i.e., the Kübler index (KI) and the Árkai index (AI). The KI represents the full width at half maximum (FWHM) in Δ°2θ for (001) basal reflection of illite/muscovite and the AI the FWHM in Δ°2θ for (001) and (002) basal reflections of chlorite. Sample preparation and instrument settings were the same as described previously (Kisch 1991; Árkai et al. 2000). X-ray diffraction (XRD) patterns were recorded with an automated Bruker (AXS D8 Advance) X-ray powder diffractometer equipped with a Lynxeye superspeed detector. The program EVA 3.0 (Bruker AXS) was used for identifying the mineral phases in the XRD patterns. Data quantification was done with a Rietveld method using the AutoQuan software package.
Geochemical modeling
Fluid-mineral equilibria modeling was performed in the model system Si-Al-Fe-Mg-Ca-Na-K-C-S-O-H-B-F-Cl. The calculations were done with the GEM-Selektor Gibbs free energy minimization package (Wagner et al. 2012; Kulik et al. 2013), which allows to simulate complex multicomponent-multiphase equilibria that simultaneously account for aqueous speciation, mineral solubility, mineral stability and mineral solid-solution behavior. The GEM-Selektor code makes it possible to calculate simple static isothermal-isobaric equilibria, but has also a provision for reaction path simulations such as titration and flow-through models and simple 1D reactive-transport models (Reed 1998).
The thermodynamic dataset covers all main aqueous species, rock-forming minerals and their solid-solutions in the model system. It is based on the model used by Dolejs and Wagner (2008), with additions for aqueous carbon, sulfur, boron and fluorine species and the respective minerals (carbonates, graphite, sulfides, anhydrite, fluorite). Thermodynamic properties of aqueous species were calculated with the Helgeson-Kirkham-Flowers (HKF) equation of state (Helgeson and Kirkham 1976; Helgeson et al. 1981; Tanger and Helgeson 1988). Data for charged and neutral aqueous species (Shock and Helgeson 1988; Shock et al. 1989, 1997; Johnson et al. 1992; Sverjensky et al. 1997) were augmented by critically evaluated data for aluminum (Tagirov and Schott 2001) and boron (Akinfiev et al. 2006) species. The end-member data for rock-forming minerals were taken from the Holland and Powell (1998) database, and the data for sulfur minerals from Evans et al. (2010). Data for fluorite came from Robie and Hemingway (1995).
Activity-composition relationships for mineral solid-solutions were described by ideal, non-ideal symmetric and asymmetric models (Powell and Holland 1993, 1999; Holland and Powell 1996, 1998, 2003; Holland et al. 1998; Coggon and Holland 2002; Wei and Powell 2003; Dale et al. 2005; Warren and Waters 2006; Evans et al. 2010). Aqueous activity coefficients were calculated with the extended Debye-Hückel equation, and the activity coefficient of water solvent from the osmotic coefficient (Helgeson et al. 1981; Walther 1997, 2001). The extended Debye-Hückel model is not strictly valid for saline brines, but thermodynamic analysis of experimental data in moderately concentrated NaCl and KCl solutions (to 4.5 mol kg−1) at temperatures and pressures up to 600 °C and 3 kbar has shown that it represents the data within experimental uncertainty (Sharygin et al. 2002).
Mineral chemistry data
Combined EPMA and LA-ICPMS analysis was performed on representative samples of carbonates (Table 1) and chlorite (Table 2) from the quartz veins. In addition, the major element composition of carbonates, chlorite, white mica and albite (Table 3) from host- and wall-rocks was determined by EPMA, but trace elements could not be analyzed due to the small grain size.
All analyzed chlorite compositions including vein and wall-rock chlorites are very similar. The Fe/(Fe + Mg) ratios of vein chlorites lie in a very narrow range of 0.62–0.64, comparable to wall- and host-rock chlorites that have Fe/(Fe + Mg) ratios of 0.60–0.66. Chlorites from veins have low but detectable concentrations of MnO in the range of 0.15–0.22 wt.%, very similar to those of chlorites from the wall- and host-rock samples which are in the range 0.21–0.28 wt.%. Vein-hosted chlorites contain detectable concentrations of Li, As, P, Ti, V, Cr, Co, Ni, and Zn (Table 2). The vein hosted carbonates are essentially ankerite-dolomite, with the Fe/(Fe + Mg) ratio in the range of 0.37–0.53 and low to moderate MnO concentrations of 1.1–2.5 wt.% (Table 1). Carbonates from the host-rocks have similar major element compositions (Table 3), with Fe/(Fe + Mg) ratios of 0.40–0.46 and MnO concentrations of 1.5–2.1 wt.% (Table 2). The vein ankerite-dolomite contains detectable concentrations of Na, Al, Sr, B, Cr and Zn (Table 1). The white mica in the wall-rocks conforms to almost pure muscovite and the feldspars are essentially pure albite.
Alteration geochemistry and mass balance analysis
Analysis of alteration geochemistry has been performed on a representative cross section that extends from the vein-wall-rock contact into the fresh host-rock slate. The cross section has a length of about 30 cm and is associated with a 10–20 cm thick vein. The sample has been cut into 18 slabs in intervals of 1 cm for the first 10 cm, 2 cm for the next 10 cm, and 3 cm for the last 10 cm (Fig. 5). The spatial resolution is finer in the most proximal part of the alteration envelope, because we expected to see more chemical changes in this part of the cross section. Each slab has been analyzed for the full suite of geochemical data, including standard major and trace elements, Fe2+/Fe3+ ratio, loss on ignition (LOI), CO2, Corg, S and the REE (Table 4).
Pictures illustrating the geometry of the studied alteration zone and the sampling pattern. a Image of the wall-rock sample indicating the location of the 18 slabs cut out for whole-rock analysis. b Sketch of the vein-wall-rock geometry illustrating the location of the samples in relation to the quartz vein. S1 is the main foliation of the host-rocks
Plots of the element and oxide concentrations as a function of the distance from the vein contact (Fig. 6) give a first-order indication of element mobility and the spatial extent of the alteration zone. Especially the profiles of SiO2, (FeO + Fe2O3), MgO, CaO, P2O5, Li, Rb, Ni, and Zn show concentration changes, and these appear to be restricted to the first 12–14 cm next to the vein-wall-rock contact (Fig. 6). Beyond this point, no significant gains or losses of elements are visible and the concentrations of most elements remain constant within analytical error. For a vein of 10–20 cm thickness (average about 15 cm), the scale of major mass changes is therefore restricted to approximately 15 cm distance from the vein contact. An interesting feature of the alteration profile is the appearance of a sharp peak in the concentrations of a group of elements between 7 and 10 cm distance from the vein contact (Fig. 6). In this interval, the concentrations of Ca, Fe, Mg, Mn, CO2, P, Li, Sr and Zn show a sharp increase above the curve of the alteration profile.
Mass balance calculations were performed along the alteration profile using an immobile element reference frame, following the methods of Grant (1986) and Mountain and Williams-Jones (1996) (Table 5). We selected Zr as the most immobile element, because it shows no systematic changes along the alteration profile, whereas Al that is commonly considered as a suitable immobile element reference frame, clearly shows changes in the cross section (Fig. 6). Calculations using the isocon method (Grant 1986) made it possible to identify additional immobile elements and to obtain the relative mass changes for all the mobile elements. When the average concentrations of elements (or oxides) in the altered wall-rocks (1–15 cm distance from vein) are plotted as function of concentrations in the least altered precursor host-rock (16–29 cm distance from vein), clear trends are visible (Fig. 7). Isocon analysis indicates considerable gain of Fe, Mg, Mn, Ca, P and CO2, and loss of Si, Na and K. The trace elements gained are Li, Sr, Co, Ni, Zn, Sn, Cu, F, Sb and the REE, while B, Rb, As, Ba, Cr, V, Nb and Sc have been lost.
Isocon diagram comparing the average composition of proximal samples from the alteration envelope (1–15 cm distance from the vein) with the average composition of the least altered slates (16–29 cm distance). Major element oxides are plotted as wt.%. and trace elements as ppm. The isocon line based on the assumption of immobile Zr is given for reference
REE geochemistry
The REE concentrations were normalized to Post-Archean Australian Sediments (PAAS) (McLennan 1989), in order to relate them to a typical crustal sedimentary source. PAAS-normalized REE patterns are essentially flat for the fresh and least altered slate samples (14–29 cm distance from the vein), indicating no REE fractionation in the distal part of the alteration profile (Fig. 8). By contrast, the PAAS-normalized REE patterns of the proximal samples (first 12 cm) show a pronounced bell-shaped enrichment in the middle REE (MREE). These patterns demonstrate significant REE mobility in the proximal part of the alteration profile, and the scale of REE mobility conforms to the scale where mobility of other major and trace elements is observed (Fig. 6). The REE patterns of the proximally altered wall-rocks can be compared to REE patterns of the vein carbonates (Fig. 9) and apatite (Wagner et al. 2010), with vein carbonates (ankerite-dolomite) and apatite showing a similar bell-shaped enrichment in the MREE. The absolute concentrations of all REE are higher in proximally altered wall-rocks compared to the least-altered host-rocks, which can be explained by passive REE enrichment due to silica leaching from altered wall-rocks. The combination of wall-rock carbonation, apatite enrichment and passive REE enrichment is able to explain the strong MREE enrichment and the higher REE concentrations in altered wall-rocks. This interpretation is consistent with the increase in Ca, Fe, Mg, Mn and CO2 in samples close to the vein-wall-rock contact.
Illite and chlorite crystallinity
The average value of illite crystallinity (KI) for all samples from the investigated cross section is 0.23 ± 0.01, whereas the average chlorite crystallinity (AI) is 0.17 ± 0.01. When plotted as function of the distance from the vein contact, both the KI and the AI show no systematic change that could be related to effects of fluid-rock interaction (Fig. 10). The average value of the illite crystallinity value of 0.23 would indicate metamorphic conditions of approximately 300 °C, corresponding to the epizone (Kübler and Jaboyedoff 2000).
Geochemical modeling of fluid-mineral equilibria
The quantitative analysis of alteration geochemistry provides important insight into element mobility and mass transfer during fluid-rock interaction. Combination of the alteration geochemistry data with vein mineralogy, textural characterization, pressure-temperature evolution (Wagner and Cook 2000; Wagner et al. 2010; Marsala et al. 2013) and chemical composition of fluid inclusions (Marsala et al. 2013) results in an unique set of constraints to test geochemical-thermodynamic models for fluid-rock reaction. In turn, thermodynamic modeling of fluid-mineral equilibria makes it possible to link the observed fluid compositions and mineral assemblages with important system parameters such as pressure-temperature conditions, oxidation state and pH, and the fluid/rock ratio. We used simulations of rock-buffered equilibria (at constant low fluid/rock ratio) to evaluate how the fluid composition changes as function of temperature and pressure. We then used the results from the rock-buffered simulations to test if the fluid inclusions were trapped under conditions of local fluid-rock equilibrium or if they would represent an externally derived fluid that had equilibrated with similar host-rocks at higher pressure-temperature conditions. Subsequently, we performed reaction-path simulations at variable fluid/rock ratios (Heinrich et al. 1996) to investigate which alteration features are produced via reaction of the fresh host-rocks with a fluid that has equilibrated with similar rocks at higher pressure-temperature conditions.
Model design and input data
The migration path of aqueous metamorphic fluids in low-grade metasedimentary rocks, from fluid production via mineral dehydration at deeper levels to vein formation in shallower parts of the metamorphic nappe pile, extends over a considerable range in temperatures and pressures. We have therefore modeled the fluid evolution by performing simulations of rock-buffered fluid-mineral equilibria (at a constant mass-based fluid/rock value of 0.1) at several isobars (2.5, 1.0 and 0.5 kbar) and at temperatures of 200–500 °C. The chosen temperature-pressure conditions cover the metamorphic peak conditions estimated for the Hunsrück nappe complex (270–300 °C and 2–4 kbar; Wolf 1978; Oncken 1991) and tectono-stratigraphic units of the Rhenish Massif metamorphosed at higher metamorphic grades (Oncken 1991; Dittmar et al. 1994; Massone 1995; Oncken et al. 1995), as well as the conditions estimated for the late-metamorphic quartz veins (130–400 °C at 0.4–0.6 kbar; Wagner and Cook 2000; Marsala et al. 2013). Reaction-path simulations were performed at variable fluid/rock ratios (ranging from 0.01 to 1000) for temperatures of 200, 300 and 400 °C and a fixed pressure of 0.5 kbar. The reaction-path simulations were run at several temperatures to evaluate the effect of fluid-rock reaction under conditions of a progressively cooling fluid flow system, consistent with the fluid inclusion record.
Rock-buffered and reaction-path simulations were run for contrasting end-member scenarios of reducing and oxidizing fluid conditions, because the oxidation state of the fluid largely depends on the amount of reactive organic matter and pyrite in the host-rocks which is difficult to evaluate. Reducing fluid conditions were produced by including the measured concentration of organic carbon (about 0.3 wt.%) into the bulk rock composition, whereas oxidizing fluids were produced by decreasing the amount of organic carbon by an order of magnitude. These end-member compositions reflect the range in reducing organic carbon measured in the lithologically variable metasedimentary rocks (comprising metapelites, metasiltstones and metasandstones) of the Hunsrück nappe complex. Because of the unusually elevated Al concentrations in the fluid, demonstrated by fluid inclusion studies using LA-ICPMS microanalysis (Marsala et al. 2013), we have also investigated the effect of adding fluorine to the chemical system. Experimental studies have shown that Al solubility is greatly enhanced at temperatures of 400–450 °C in the presence of fluorine, due to the formation of very stable Al and Na-Al hydroxyfluoride complexes (Tagirov et al. 2002a, b). Reflecting the variations in bulk rock input data (reducing or oxidizing conditions) and the chemical system (presence or absence of fluorine), four calculation series of rock-buffered and four calculation series of reaction-path simulations were performed; a summary of the parameters and conditions for each series is given in Tables 6, 7, and 8.
The bulk rock composition used in the rock-buffered simulations was taken from the whole-rock geochemical data of a fresh host-rock sample that is representative for average metasediments of the Hunsrück nappe (Wagner and Cook 2000; Wagner and Jochum 2002). The bulk rock composition used in the reaction-path simulations was taken from the most distant rock sample of the alteration profile. The analyzed bulk rock compositions were converted into the SiO2-Al2O3-Fe2O3-FeO-MgO-CaO-Na2O-K2O-CO2-C-H2O-FeS2(−CaF2) composition space and normalized to 100 wt.% (Table 7). This was done by first subtracting TiO2, MnO and stoichiometric amounts of CaO (to account for modal apatite) from the bulk-rock composition. Subsequently, the analyzed S concentration was converted into a stoichiometric amount of FeS2, and an equivalent amount of FeO was subtracted. For simulations that considered F, the measured F was converted into CaF2 and a stoichiometrically equivalent amount of CaO was subtracted. The model fluid input composition for rock-buffered simulations was constructed from the measured average salinity, sulfur and boron concentrations, which are the essential fluid-volatile elements. The fluid composition was recalculated from the microthermometric and LA-ICPMS data for fluid inclusions of the first generation, and expressed as molalities of NaCl, H2S and H3BO3 (Table 6). The input fluid composition for reaction-path simulations was taken from the results of rock-buffered simulations, and represents a multicomponent equilibrium fluid. We selected the fluid composition obtained at 450 °C and 0.5 kbar as starting fluid for the reaction-path simulations, because these conditions reflect the uppermost limit for formation of the quartz veins. Reaction-path simulations were run over a range in initial mass-based fluid/rock ratio from 0.01 to 1000. Due to mineral dissolution and precipitation, the equilibrium fluid/rock ratio is different from the initial fluid/rock ratio, and all diagrams that illustrate the results of reaction-path calculations use the equilibrium fluid/rock ratio.
Results of rock-buffered simulations
Figures 11, 12 and 13 summarize the modeling results obtained at 2.5, 1.0 and 0.5 kbar for the fluorine-free system, including bulk fluid composition, selected aqueous species abundance, pH, oxidation state and mineral stability. The total element solubility curves and the other fluid parameters have essentially similar temperature trends at all three pressures, indicating that pressure effects are minor compared to temperature effects. The only notable exception is shown by Si, which shows a retrograde solubility behavior above 400 °C at 0.5 kbar (Fig. 14), whereas solubility is prograde at all other pressure-temperature conditions investigated. Elements that are affected by variations in the oxidation state are mainly C, Ca and S. The higher C solubility above 340–360 °C in reducing fluids is essentially caused by higher concentrations of CH4(aq) compared to carbonate species (compare Fig. 11c and d), which impacts on the stability of carbonate minerals in the host-rock (compare Fig. 11k and l). Higher Ca solubility below 300 °C in the oxidizing fluid is due to CaSO4 being present in higher concentrations at lower temperatures (Fig. 11c), which results in anhydrite saturation (Fig. 11k). As expected, sulfur shows different solubility curves for reducing and oxidizing fluids. Under reducing fluid conditions, the S solubility curve increases smoothly with temperature (Fig. 11b). By contrast, the solubility curve for the oxidizing fluid shows a concave shape with a minimum between 360 and 320 °C and higher values at lower and higher temperatures (Fig. 11a). The high S concentration below 300 °C is caused by high concentrations of sulfate species, mainly CaSO4 and SO4 2− (Fig. 11c and e).
Calculated rock-buffered fluid composition and whole-rock mineralogy as function of temperature at 2.5 kbar. Left column illustrates results for oxidizing conditions (0.03 wt.% Corg in the rock) and right column those for reducing conditions (0.3 wt.% Corg in the rock). a and b Total molalities of elements in the fluid. c and d Distribution of the main Ca and C species. e and f Distribution of the main S species. g and h Calculated pH compared to the neutral pH value. i and j Oxidation state (logarithm of oxygen fugacity) compared to the fayalite-quartz-magnetite (FQM) and hematite-magnetite (HM) mineral buffers. k and l Stable mineral phases
Calculated rock-buffered fluid composition and whole-rock mineralogy as function of temperature at 1.0 kbar. Left column illustrates results for oxidizing conditions (0.03 wt.% Corg in the rock) and right column those for reducing conditions (0.3 wt.% Corg in the rock). a and b Total molalities of elements in the fluid. c and d Distribution of the main Ca and C species. e and f Distribution of the main S species. g and h Calculated pH compared to the neutral pH value. i and j Oxidation state (logarithm of oxygen fugacity) compared to the FQM and HM mineral buffers. k and l Stable mineral phases
Calculated rock-buffered fluid composition and whole-rock mineralogy as function of temperature at 0.5 kbar. Left column illustrates results for oxidizing conditions (0.03 wt.% Corg in the rock) and right column those for reducing conditions (0.3 wt.% Corg in the rock). The curves terminate at 450 °C, because at higher temperatures the water density would be below 0.33 g cm−3, outside the permissible range of the HKF equation of state for aqueous electrolytes (Johnson et al. 1992). a and b Total molalities of elements in the fluid. c and d Distribution of the main Ca and C species. e and f Distribution of the main S species. g and h Calculated pH compared to the neutral pH value. i and j Oxidation state (logarithm of oxygen fugacity) compared to the FQM and HM mineral buffers. k and l Stable mineral phases
The pH is not affected by oxidation state and pressure variations, but essentially controlled by buffering silicate and carbonate mineral assemblages in the host-rock (i.e., chlorite, muscovite, albite, ankerite-dolomite, calcite). The pH values are close to neutral at high temperatures and increase to more alkaline with decreasing temperature, ranging between 5 and 7 (Fig. 11g and h). The fluid oxidation state is not significantly affected by pressure, but as expected the oxygen fugacity values are several orders of magnitude higher in the oxidizing fluid (Fig. 11i and j). At high temperatures, the oxidation state for both simulations is more reduced relative to the FQM (Fayalite-Quartz-Magnetite) and HM (Hematite-Magnetite) mineral buffers, and increasingly shifts to more oxidizing conditions with decreasing temperature.
In Fig. 15, selected calculated element ratios are directly compared to the measured values from LA-ICPMS analysis of fluid inclusions. The element ratios and pressure-corrected homogenization temperatures of the fluid inclusions are plotted as average values for each main fluid inclusion generation along with error bars that bracket the minimum and maximum values. The average fluid inclusion temperatures are around 240 °C for fluid generation 1 and decrease to 130 °C for fluid generation 3. As noticed earlier, the Ca solubility behaves differently under oxidizing and reducing conditions. Within the temperature range that is defined by the pressure-corrected homogenization temperatures, the calculated Ca/Na ratios for the oxidizing fluid are slightly higher than the measured ones (Fig. 15a), whereas the Ca/Na ratios for the reducing fluid are close to the measured ones (Fig. 15b). The calculated K/Na and Mg/Na ratios are not affected by the fluid oxidation state. The K/Na ratios are generally in good agreement with the measured fluid inclusion data (Fig. 15c and d). By contrast, the analyzed Mg/Na ratios and fluid inclusion temperatures are not in agreement with the calculated values (Fig. 15e and f). The calculated curves would match with the measured Mg/Na ratios at temperatures of 350–400 °C.
Calculated element ratios in rock-buffered fluids as function of temperature, compared to average measured fluid inclusion (FI) data (Marsala et al. 2013). Error bars represent maximum and minimum values for all individual fluid inclusions from FI generations 1 to 3. Left column illustrates results for oxidizing conditions (0.03 wt.% Corg in the rock) and right column those for reducing conditions (0.3 wt.% Corg in the rock). a and b Log(Ca/Na). c and d Log(K/Na). e and f Log(Mg/Na)
Adding fluorine to the system results in a substantial increase in Al solubility by one to three orders of magnitude (Fig. 16), whereas the oxidation state of the fluid has no significant impact on Al solubility. The effect of F becomes more important with increasing temperature, and particularly above 350–400 °C the Al concentrations increase dramatically to values that are more than 3 orders of magnitude higher than in the F-free system (Fig. 16a and b). The measured Al concentrations in the fluid inclusions match with the calculated solubilities in the F-bearing system at temperatures of 390–440 °C (Fig. 16a and b). The F solubility curves between 400 and 450 °C show a similar trend as does Al (Fig. 16c and d), suggesting a chemical coupling between the two elements. Inspection of the speciation results shows that Al and Na-Al hydroxyfluoride species, i.e., Al(OH)F2, NaAl(OH)3F and NaAl(OH)2F2, become increasingly stable as temperature increases, and they dominate Al speciation above about 350 °C (Fig. 16e and f), in agreement with experimental results in the Na-Al-F-O-H system (Tagirov et al. 2002a, b).
Effect of fluorine addition on calculated rock-buffered fluid compositions, plotted as function of temperature at 0.5 kbar. Left column illustrates results for oxidizing conditions (0.03 wt.% Corg in the rock) and right column those for reducing conditions (0.3 wt.% Corg in the rock). a and b Al concentration in fluorine-bearing system (black curves) compared to the F-free system (grey curves). c and d Total Ca and F concentrations in the fluid. e and f Distribution of the main Al species. Note that solubility enhancement in the F-bearing system is mainly due to the very stable NaAl(OH)2F2 complex. g and h Distribution of the main Ca and F species. i and j Stable mineral phases
Results of reaction-path simulations
The main results of the reaction-path simulations are summarized in Figs. 17, 18 and 19. The plots illustrate mineral abundance, relative mass changes, total element solubilities in the fluid, fluid pH and oxidation state as function of increasing fluid/rock ratio for simulations at 400, 300 and 200 °C. The initial mineral assemblage at 400 °C at low fluid/rock ratios comprises quartz, albite, chlorite and muscovite, for both oxidizing and reducing conditions (Fig. 17a and b). In the calculations at 300 and 200 °C, ankerite-dolomite is stable instead of albite (Figs. 18a, b and 19a, b). The main difference between calculations with the reducing fluid compared to those with the oxidizing fluid is the precipitation of large amounts of graphite from the fluid at high fluid/rock ratios. The amount of precipitated graphite increases at lower temperatures, resulting in graphite making up most of the rock mineralogy (Figs. 18b and 19b). In addition, increasing the fluid/rock ratio results in formation of iron sulfides, either pyrrhotite + pyrite at 400 °C (Fig. 17a) or pyrite alone at lower temperatures (Figs. 18a, b and 19a, b). Albite, or ankerite-dolomite at lower temperatures, always reacts out around fluid/rock ratios of about 10, and chlorite disappears after albite or ankerite-dolomite at higher fluid/rock ratios at 300 and 400 °C. In the runs at 200 °C, chlorite disappears at very low fluid/rock ratios, which is related to increasing carbonation (formation of siderite-magnesite) at intermediate fluid/rock ratios (Fig. 19a, b). The formation of iron sulfides usually results in gain of FeO and S to the rock at high fluid/rock ratios, especially under oxidizing conditions that suppress the precipitation of graphite (Figs. 17c, 18c and 19c). The precipitation of graphite in the simulations with the reducing starting fluid adds considerable amounts of C to the rock (Figs. 17d, 18d and 19d). The mass gain of C becomes dominant at the highest fluid/rock ratios, which are probably not attained in the metasediments hosting the vein-wall-rock system because this style of alteration is not observed.
Calculated fluid composition, mineral assemblage and mass changes as function of fluid/rock ratio at 400 °C and 0.5 kbar. The fluid-rock interaction model assumes that rock-buffered fluid (450 °C) reacts with the fresh host-rock. Left column illustrates results for oxidizing conditions (0.03 wt.% Corg in the rock) and right column those for reducing conditions (0.3 wt.% Corg in the rock). a and b Mineral abundance (wt.%). c and d Percentage mass changes relative to the whole-rock composition of the fresh host-rock. e and f Total molalities of elements in the fluid. g and h Calculated pH compared to the neutral pH value. i and j Oxidation state (logarithm of oxygen fugacity) compared to the FQM and HM mineral buffers
Calculated fluid composition, mineral assemblage and mass changes as function of fluid/rock ratio at 300 °C and 0.5 kbar. Left column illustrates results for oxidizing conditions (0.03 wt.% Corg in the rock) and right column those for reducing conditions (0.3 wt.% Corg in the rock). a and b Mineral abundance (wt.%). c and d Percentage mass changes relative to the whole-rock composition of the fresh host-rock. e and f Total molalities of elements in the fluid. g and h Calculated pH compared to the neutral pH value. i and j Oxidation state (logarithm of oxygen fugacity) compared to the FQM and HM mineral buffers
Calculated fluid composition, mineral assemblage and mass changes as function of fluid/rock ratio at 200 °C and 0.5 kbar. Left column illustrates results for oxidizing conditions (0.03 wt.% Corg in the rock) and right column those for reducing conditions (0.3 wt.% Corg in the rock). a and b Mineral abundance (wt.%). c and d Percentage mass changes relative to the whole-rock composition of the fresh host-rock. e and f Total molalities of elements in the fluid. g and h Calculated pH compared to the neutral pH value. i and j Oxidation state (logarithm of oxygen fugacity) compared to the FQM and HM mineral buffers
Mineral transformations produce sharp changes in the solubility curves of some elements. They are more evident in the low-temperature calculations due to the increasing temperature difference between the reacting fluid and the rock (Figs. 18e, f and 19e, f). The concentration of all elements in the fluid is usually constant up to a fluid/rock ratio of 10, when albite or ankerite-dolomite have completely reacted out. Beyond this point, the S solubility always increases and Ca solubility decreases (Fig. 18e and f). The solubility of Mg increases as well, but the trend reverses when chlorite (or siderite-magnesite at 200 °C) disappears (Fig. 18e and f). The pH values remain close to neutral as long as the initial rock-buffer mineral assemblage is stable. Successive mineral transformations shift the pH to more acidic values (Fig. 18g and h). The oxidation state does not appear to be strongly affected by variations in fluid/rock ratio, and the values lie always between the FQM and HM mineral buffers (Fig. 18i and j). Compared to the simulations in the F-free system, the calculation series with F show only small differences, with the most notable one being the precipitation of fluorite at higher fluid/rock ratios.
Discussion and conclusions
The results of our combined alteration geochemistry and modeling study complement the existing datasets on vein mineralogy, pressure-temperature evolution, stable isotope geochemistry and fluid composition, and provide important constraints on the driving forces for fluid-rock reaction and vein formation. The insight gained from the approach adopted in our work results in a multi-faceted picture of the vein-wall-rock relations, and provides useful insight into vein formation processes in very low-grade metamorphic rocks in other fold-and-thrust belts.
Controls on alteration geochemistry
Although there is no macroscopically visible alteration envelope surrounding the veins and no major change in the wall-rock mineralogy, the detailed geochemical mass balance analysis demonstrates element mobility and defines the scale of mass transfer as the first 12–14 cm adjacent to the vein-wall-rock contact (Fig. 6). The essential mass changes are loss of Si, K and Na and gain of Fe, Ca, Mg, CO2 and P. The element suite that is gained in the alteration halo conforms to the elements that make up the Fe-Mg vein carbonates (ankerite-dolomite) and apatite. This is supported by the REE patterns of vein carbonates and apatite, compared to those of wall-rocks from the proximal part of the alteration envelope which show a similar enrichment in the middle REE. Therefore, carbonation is one of the main alteration reactions in the proximal wall-rock zone. The reaction-path modeling using an oxidizing input fluid produces carbonate alteration at moderate fluid/rock ratios at 200 °C, whereas the simulations at higher temperatures do not produce such carbonate alteration. The temperature window for the carbonation reaction around 200 °C is in good agreement with the temperatures of 120–230 °C estimated for deposition of vein carbonates inside open cavities along with euhedral quartz crystals (Wagner and Cook 2000; Marsala et al. 2013).
Silica leaching is mainly related to quartz dissolution, and decrease in K and Na is likely related to reactions involving detrital feldspars and muscovite-illite. The average amount of silica that was leached from the wall-rocks is on the order of about 5 g per 100 g of precursor host-rock (Table 5). If this amount is compared with the vein thickness of 10–20 cm, leaching from the wall-rock alteration zone is not sufficient to explain the formation of the quartz veins, and an additional external fluid source for silica is clearly required. This finding is in agreement with earlier conclusions based on oxygen isotope systematics suggesting that the vein quartz is partly derived from leaching of the altered wall-rocks and partly from advecting external fluids (Wagner et al. 2010).
The retrograde silica solubility in the temperature window 400–450 °C at 0.5 kbar (Fig. 14) appears to be the essential driving force for silica leaching in the alteration halo, in good agreement with the pressure-temperature estimates for the early stage of vein formation and incipient alteration (Wagner and Cook 2000; Wagner et al. 2010). Taken together, the data are best interpreted in such a way that the early stage of vein formation during late-orogenic uplift is related to advection of external fluids that were supplied from a deeper and substantially hotter source. The external fluids were produced by metamorphic devolatilization at conditions that clearly exceed the peak metamorphic conditions inferred for the rock units of the Hunsrück nappe complex currently exposed at the surface. Considering previous estimates for the initial stage of vein formation (i.e., early elongate-blocky quartz and chlorite), which are around 400 °C and 0.5 kbar (Wagner and Cook 2000), fluid advection from the hot zone of fluid production must have been rather fast and close to adiabatic, preventing considerable heat loss to the surrounding host-rocks. Interaction between the hotter fluid and the colder wall-rocks resulted in quartz leaching, because the pressure-temperature path would intersect the window of retrograde quartz solubility. Because the fluids also deposited large amounts of quartz inside the veins, fluid advection must have occurred as multiple pulses, which allowed for periods of influx of hot external fluids that leached quartz alternating with periods of fluid cooling and quartz precipitation.
Comparison between calculated element ratios and the measured fluid inclusion data gives somewhat inconclusive results. While the analyzed Ca/Na and K/Na ratios would suggest that the fluids are compositionally close to rock-buffered fluids that equilibrated with typical metasedimentary rocks of the Hunsrück nappe complex around 200 °C, the Mg/Na ratios and the Al concentrations are in better agreement with fluid-rock equilibrium having been attained at temperatures of at least 400 °C. These results explain why application of empirical solute geothermometers to element ratios obtained by LA-ICPMS microanalysis of fluid inclusions was not successful (Marsala et al. 2013). The mixed messages obtained from comparison between modeled rock-buffered fluid compositions and analyzed fluid inclusion data might indicate that the fluids have been produced by devolatilization of different source rocks at high temperatures, or that fluid production occurred during a transient stage that did not allow for attainment of full fluid-rock equilibrium. Discrete and transient pulses of fluid production that could result in disequilibrium fluid compositions could relate to prograde metamorphic reactions that involve successive breakdown and dehydration of clay and hydrated mica phases. Experimental and thermodynamic modeling studies of the dehydration behavior of clays and hydrated micas have shown that water loss occurs discontinuously via a series of reactions that result in breakdown of discrete water layers (Vidal and Dubacq 2009; Dubacq et al. 2010). The discontinuous water release results in a sequence of discrete pulses of aqueous fluid production as clay-rich rocks are subjected to a prograde metamorphic gradient.
Controls on Al solubility
The unusually high Al concentrations that were analyzed by LA-ICPMS in the fluid inclusions from quartz veins in the Kaub area, but also in fluid inclusions hosted by late-stage euhedral quartz crystals from other similar veins in the Hunsrück nappe complex (Marsala et al. 2013), can be explained by the strong Al solubility enhancement that is caused by fluorine complexation. The host-rocks contain sufficient F (500–700 ppm) and the low Ca concentrations (below 0.5–1 wt.% CaO) that control fluorine concentrations in the fluid (coupled through the fluorite solubility product) allow for building up sufficiently high fluorine levels in rock-buffered fluids. The substantial increase in Al solubility above 350–400 °C is caused by the high stability of Al and Na-Al hydroxyfluoride complexes, in particular NaAl(OH)3F and NaAl(OH)2F2 (Fig. 16e). The thermodynamic data for these complexes are well-constrained by carefully conducted solubility experiments at 400 and 450 °C and 0.5 and 1.0 kbar (Tagirov and Schott 2001; Tagirov et al. 2002a, b), which clearly demonstrated the combined effect of Na and F on increased Al solubility. The experiments cover exactly the pressure-temperature range that is relevant for of the late-metamorphic quartz veins of our study (Wagner and Cook 2000; Marsala et al. 2013).
By comparison, measured Al concentrations in fluid inclusions from low-grade metasedimentary rocks in the accretionary-wedge of the Swiss Alps (the so-called Bündnerschiefer) are considerably lower (Miron et al. 2013) than those analyzed in fluid inclusions of the quartz veins of the Rhenish Massif (Marsala et al. 2013). The metasedimentary rocks of the Bündnerschiefer are lithologically different from the rocks of the Hunsrück nappe complex and contain large volumes of calc-schists along with metapelites and metasiltstones. Therefore, Ca concentrations in the rocks are much higher (typically 2–4 wt.% CaO), which will result in depressing F concentrations in rock-buffered fluids. This, in turn, will result in lower concentrations of Na-Al hydroxyfluoride species that control the Al solubility budget in fluorine-bearing systems at temperatures above 350–400 °C and low pressures. At higher pressures above 4–5 kbar, Al solubility might be enhanced by Al-Si complexing (Manning 2007; Beitter et al. 2008; Newton and Manning 2008) even in fluorine-poor systems.
Evolution of the vein-wall-rock system
The main features of the alteration envelope are silica leaching and addition of elements related to Fe-Mg carbonate deposition in the wall-rocks. Silica leaching can be essentially explained by the pressure-temperature path of the advecting external fluids intersecting the window of retrograde quartz solubility between 400 and 450 °C (and extending down to 350–360 °C if the fluids were quartz-saturated at 450 °C), and is clearly a feature of high-temperature alteration. In contrast to the Si alteration, the carbonate alteration cannot be explained by high-temperature fluid-rock interaction, regardless whether the fluid is oxidizing or reducing. The reaction-path simulations at 400 and 300 °C do not produce wall-rock carbonation, but rather result in progressive dissolution of ankerite-dolomite that is stable at low fluid/rock ratios. Only low-temperature fluid-rock reaction around 200 °C is able to produce carbonate alteration. This is consistent with the field observations and fluid inclusion data that demonstrate that vein hosted ankerite-dolomite (and younger calcite) precipitated during the late-stage in open vugs and cavities at temperatures of 120–230 °C (Wagner and Cook 2000; Marsala et al. 2013).
Taken together, the field observations, fluid inclusion data and modeling results suggest that alteration and vein formation cannot be explained by a single-stage high-temperature fluid-rock interaction process, but require at least two sequential stages of fluid-rock reaction, with the first stage causing silica leaching at high temperatures and the second stage causing wall-rock carbonation at much lower temperatures. This suggests that quartz vein formation and the associated hydrothermal alteration are the product of multiple fluid pulses reacting with the host-rocks under conditions where the fluid system gradually cooled down with time. The late-metamorphic quartz veins in the fold-and-thrust belt of the Rhenish Massif therefore record a continuum of evolving fluid composition that reflects decreasing metamorphic fluid production, temperature and pressure decrease in response to orogenic uplift, and the additional cooling effect of ingression of meteoric water at shallower depths (Marsala et al. 2013). The key role of an externally derived fluid for formation of the late-metamorphic quartz vein arrays highlights the importance of late-orogenic fluid migration for the evolution of fold-and-thrust-belts.
References
Ague JJ (1994a) Mass transfer during Barrovian metamorphism of pelites, south-central Connecticut; I, evidence for changes in composition and volume. Am J Sci 294:989–1057
Ague JJ (1994b) Mass transfer during Barrovian metamorphism of pelites, south-central Connecticut; II, Channelized fluid flow and the growth of staurolite and kyanite. Am J Sci 294:1061–1134
Ague JJ (1997) Compositional variations in metamorphosed sediments of the Littleton Formation, New Hampshire; discussion. Am J Sci 297:440–449
Ague JJ (2003) Fluid flow in the deep crust. In: Heinrich DH, Karl KT (eds) Treatise on geochemistry. Pergamon, Oxford, pp 195–228
Ague JJ (2011) Extreme channelization of fluid and the problem of element mobility during Barrovian metamorphism. Am Mineral 96:333–352
Ahrendt H, Clauer N, Hunziker J, Weber K (1983) Migration of folding and metamorphism in the Rheinisches Schiefergebirge deduced from K-Ar and Rb-Sr age determinations. In: Martin H, Eder FW (eds) Intracontinental fold belts: case studies in the Variscan Belt of Europe and the Damara Belt in Namibia. Springer, Berlin, pp 323–339
Akinfiev NN, Voronin MV, Zotov AV, Prokofev VY (2006) Experimental investigation of the stability of a chloroborate complex and thermodynamic description of aqueous species in the B-Na-Cl-O-H system up to 350 °C. Geochem Int 44:867–878
Árkai P, Mata MP, Giorgetti G, Peacor DR, Toth M (2000) Comparison of diagenetic and low-grade metamorphic evolution of chlorite in associated metapelites and metabasites: an integrated TEM and XRD study. J Metamorph Geol 18:531–550
Arthaud F, Matte P (1977) Late paleozoic strike-slip faulting in southern europe and Northern Africa - result of a right-lateral shear zone between Appalachians and Urals. Geol Soc Am Bull 88:1305–1320
Baumgartner LP, Ferry JM (1991) A model for coupled fluid-flow and mixed-volatile mineral reactions with applications to regional metamorphism. Contrib Mineral Petr 106:273–285
Beitter T, Wagner T, Markl G (2008) Formation of kyanite-quartz veins of the Alpe Sponda, Central Alps, Switzerland: implications for Al transport during regional metamorphism. Contrib Mineral Petr 156:689–707
Bickle MJ, Mckenzie D (1987) The transport of heat and matter by fluids during metamorphism. Contrib Mineral Petr 95:384–392
Bons PD (2001) The formation of large quartz veins by rapid ascent of fluids in mobile hydrofractures. Tectonophysics 336:1–17
Bons PD, Jessell MW (1997) Experimental simulation of the formation of fibrous veins by localised dissolution-precipitation creep. Mineral Mag 61:53–63
Cartwright I, Buick IS (2000) Fluid generation, vein formation and the degree of fluid-rock interaction during decompression of high-pressure terranes: the Schistes Lustres, Alpine Corsica, France. J Metamorph Geol 18:607–624
Chamberlain CP, Rumble D (1988) Thermal anomalies in a regional metamorphic terrane: an isotopic study of the role of fluids. J Petrol 29:1215–1232
Cleverley JS, Oliver NHS (2005) Comparing closed system, flow‐through and fluid infiltration geochemical modelling: examples from K-alteration in the Ernest Henry Fe-oxide-Cu-Au system. Geofluids 5:289–307
Coggon R, Holland TJB (2002) Mixing properties of phengitic micas and revised garnet-phengite thermobarometers. J Metamorph Geol 20:683–696
Connolly JAD (1997) Devolatilization-generated fluid pressure and deformation-propagated fluid flow during prograde regional metamorphism. J Geophys Res B 102:18149–18173
Connolly JAD, Thompson AB (1989) Fluid and enthalpy production during regional metamorphism. Contrib Mineral Petr 102:347–366
Cox SF (2007) Structural and isotopic constraints on fluid flow regimes and fluid pathways during upper crustal deformation: An example from the Taemas area of the Lachlan Orogen, SE Australia. J Geophys Res B 112:B08208
Cox SF, Etheridge MA, Wall VJ (1987) The role of fluids in syntectonic mass transport, and the localization of metamorphic vein-type ore deposits. Ore Geol Rev 2:65–86
Dale J, Powell R, White RW, Elmer FL, Holland TJB (2005) A thermodynamic model for Ca-Na clinoamphiboles in Na2O-CaO-FeO-MgO-Al2O3-SiO2-H2O-O for petrological calculations. J Metamorph Geol 23:771–791
Dipple GM, Ferry JM (1992) Metasomatism and fluid flow in ductile fault zones. Contrib Mineral Petr 112:149–164
Dittmar U, Meyer W, Oncken O, Schievenbusch T, Walter R, von Winterfeld C (1994) Strain partitioning across a fold-and-thrust belt - the Rhenish Massif, Mid-European Variscides. J Struct Geol 16:1335–1352
Dolejs D, Wagner T (2008) Thermodynamic modeling of non-ideal mineral-fluid equilibria in the system Si-Al-Fe-Mg-Ca-Na-K-H-O-Cl at elevated temperatures and pressures: implications for hydrothermal mass transfer in granitic rocks. Geochim Cosmochim Ac 72:526–553
Dubacq B, Vidal O, De Andrade V (2010) Dehydration of dioctahedral aluminous phyllosilicates: thermodynamic modelling and implications for thermobarometric estimates. Contrib Mineral Petr 159:159–174
Etheridge MA, Wall VJ, Vernon RH (1983) The role of the fluid phase during regional metamorphism and deformation. J Metamorph Geol 1:205–226
Etheridge MA, Wall VJ, Cox SF, Vernon RH (1984) High fluid pressures during regional metamorphism and deformation - implications for mass-transport and deformation mechanisms. J Geophys Res 89:4344–4358
Evans KA, Powell R, Holland TJB (2010) Internally consistent data for sulphur-bearing phases and application to the construction of pseudosections for mafic greenschist facies rocks in Na2O-CaO-K2O-FeO-MgO-Al2O3-SiO2-CO2-O-S-H2O. J Metamorph Geol 28:667–687
Ferry JM, Dipple GM (1991) Fluid-flow, mineral reactions, and metasomatism. Geology 19:211–214
Franke W, Eder W, Engel W, Langenstrassen F (1978) Main aspects of geosynclinal sedimentation in the Rhenohercynian Zone. Z Dtsch Ges Gowiss 129:201–216
Grant JA (1986) The isocon diagram; a simple solution to Gresens’ equation for metasomatic alteration. Econ Geol 81:1976–1982
Grant JA (2005) Isocon analysis: a brief review of the method and applications. Phys Chem Earth 30:997–1004
Gresens RL (1967) Composition-volume relationships of metasomatism. Chem Geol 2:47–65
Guillong M, Meier D, Allan M, Heinrich C, Yardley BWD (2008) SILLS: A MATLAB-based program for the reduction of laser ablation ICP-MS data of homogeneous materials and inclusions. Mineral Assoc Canada Short Course Ser 40:328–333
Günther D, Frischknecht R, Heinrich CA, Kahlert HJ (1997) Capabilities of an Argon Fluoride 193 nm excimer laser for laser ablation inductively coupled plasma mass spectrometry microanalysis of geological materials. J Anal Atom Spectrom 12:939–944
Halter WE, Williams-Jones AE, Kontak DJ (1996) The role of greisenization in cassiterite precipitation at the East Kemptville tin deposit, Nova Scotia. Econ Geol 91:368–385
Heinrich CA, Walshe JL, Harrold BP (1996) Chemical mass transfer modelling of ore-forming hydrothermal systems: current practise and problems. Ore Geol Rev 10:319–338
Helgeson HC, Kirkham DH (1976) Theoretical prediction of the thermodynamic behavior of aqueous electrolytes at high pressures and temperatures. III. equation of state for aqueous species at infinite dilution. Am J Sci 276:97–240
Helgeson HC, Kirkham DH, Flowers GC (1981) Theoretical prediction of the thermodynamic behavior of aqueous electrolytes at high pressures and temperatures. IV. calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 600 °C and 5 KB. Am J Sci 281:1249–1516
Henk A (1997) Gravitational orogenic collapse vs plate boundary stresses: a numerical modelling approach to the Permo-Carboniferous evolution of Central Europe. Geol Rundsch 86:39–55
Holland T, Powell R (1996) Thermodynamics of order–disorder in minerals. II. symmetric formalism applied to solid solutions. Am Mineral 81:1425–1437
Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metamorph Geol 16:309–343
Holland TJB, Powell R (2003) Activity-composition relations for phases in petrological calculations: an asymmetric multicomponent formulation. Contrib Mineral Petr 145:492–501
Holland T, Baker J, Powell R (1998) Mixing properties and activity-composition relationships of chlorites in the system MgO-FeO-Al2O3-SiO2-H2O. Eur J Mineral 10:395–406
Jacques D, Derez T, Muchez P, Sintubin M (2013) Syn- to late-orogenic quartz veins marking a retrograde deformation path in a slate belt: Examples from the High-Ardenne slate belt (Belgium). J Struct Geol 58:43–58
Jamtveit B, Yardley BWD (1997) Fluid flow and transport in rocks: an overview. In: Jamtveit B, Yardley BWD (eds) Fluid flow and transport in rocks: mechanisms and effects. Springer, Berlin, pp 1–14
Johnson WM, Maxwell JA (1981) Rock and mineral analysis, 2nd edn. Wiley, New York
Johnson JW, Oelkers EH, Helgeson HC (1992) SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000 °C. Comput Geosci 18:899–947
Kisch HJ (1991) Illite crystallinity: recommendations on sample preparation, X-ray diffraction settings, and interlaboratory samples. J Metamorph Geol 9:665–670
Kübler B, Jaboyedoff M (2000) Illite crystallinity. Cr Acad Sci II A 331:75–89
Kulik DA, Wagner T, Dmytrieva SV, Kosakowski G, Hingerl FF, Chudnenko KV, Berner U (2013) GEM-Selektor geochemical modeling package: revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Computat Geosci 17:1–24
Manning CE (2007) Solubility of corundum + kyanite in H2O at 700 °C and 10 kbar: evidence for Al-Si complexing at high pressure and temperature. Geofluids 7:258–269
Marsala A, Wagner T, Wälle M (2013) Late-metamorphic veins record deep ingression of meteoric water: a LA-ICPMS fluid inclusion study from the fold-and-thrust belt of the Rhenish Massif, Germany. Chem Geol 351:134–153
Massone H (1995) Metamorphic evolution of the northern phyllite zone. In: Dallmeyer RD, Franke W (eds) Pre-Permian geology of central and eastern Europe. Springer, Berlin, pp 132–137
Masters RL, Ague JJ (2005) Regional-scale fluid flow and element mobility in Barrow’s metamorphic zones, Stonehaven, Scotland. Contrib Mineral Petr 150:1–18
Matte P (1991) Accretionary history and crustal evolution of the Variscan belt in Western Europe. Tectonophysics 196:309–337
McLennan SM (1989) Rare earth elements in sedimentary rocks; influence of provenance and sedimentary processes. Rev Mineral Geochem 21:169–200
Mernagh TP, Bierlein FP (2008) Transport and precipitation of gold in Phanerozoic metamorphic terranes from chemical modeling of fluid-rock interaction. Econ Geol 103:1613–1640
Miron GD, Wagner T, Wälle M, Heinrich CA (2013) Major and trace-element composition and pressure-temperature evolution of rock-buffered fluids in low-grade accretionary-wedge metasediments, Central Alps. Contrib Mineral Petr 165:981–1008
Mountain BW, Williams-Jones AE (1996) Mass transfer and the path of metasomatic reactions in mesothermal gold deposits; an example from Flambeau Lake, Ontario. Econ Geol 91:302–321
Mullis J, Dubessy J, Poty B, OʼNeil J (1994) Fluid regimes during late stages of a continental collision - physical, chemical, and stable-isotope measurements of fluid inclusions in fissure quartz from a geotraverse through the Central Alps, Switzerland. Geochim Cosmochim Ac 58:2239–2267
Nesbitt BE, Muehlenbachs K (1995) Geochemical studies of the origins and effects of synorogenic crustal fluids in the southern Omineca belt of British-Columbia, Canada. Geol Soc Am Bull 107:1033–1050
Newton RC, Manning CE (2008) Solubility of corundum in the system Al2O3-SiO2-H2O-NaCl at 800 °C and 10 kbar. Chem Geol 249:250–261
Oliver NHS (1996) Review and classification of structural controls on fluid flow during regional metamorphism. J Metamorph Geol 14:477–492
Oliver NHS, Bons PD (2001) Mechanisms of fluid flow and fluid-rock interaction in fossil metamorphic hydrothermal systems inferred from vein-wallrock patterns, geometry and microstructure. Geofluids 1:137–162
Oncken O (1984) Relationships in the structural genesis of the Rhenish Massif. Geol Rundsch 73:619–649
Oncken O (1988) Aspects of the reconstruction of the stress history of a fold and thrust belt (Rhenish-Massif, Federal-Republic-of-Germany). Tectonophysics 152:19–40
Oncken O (1991) Aspects of the structural and paleogeothermal evolution of the Rhenish Massif. Ann Soc Géol Belg 113:139–159
Oncken O, Massone H, Schwab M (1995) Metamorphic evolution of the Rhenohercynian zone. In: Dallmeyer RD, Franke W (eds) Pre-Permian geology of central and eastern Europe. Springer, Berlin, pp 82–86
Oncken O, von Winterfeld C, Dittmar U (1999) Accretion of a rifted passive margin: the Late Paleozoic Rhenohercynian fold and thrust belt (Middle European Variscides). Tectonics 18:75–91
Penniston-Dorland SC, Ferry JM (2008) Element mobility and scale of mass transport in the formation of quartz veins during regional metamorphism of the Waits River Formation, east-central Vermont. Am Mineral 93:7–21
Plesch A, Oncken O (1999) Orogenic wedge growth during collision - constraints on mechanics of a fossil wedge from its kinematic record (Rhenohercynian FTB, Central Europe). Tectonophysics 309:117–139
Powell R, Holland T (1993) On the formulation of simple mixing models for complex phases. Am Mineral 78:1174–1180
Powell R, Holland T (1999) Relating formulations of the thermodynamics of mineral solid solutions: activity modeling of pyroxenes, amphiboles, and micas. Am Mineral 84:1–14
Ramsay JG (1980) Crack-seal mechanism of rock deformation. Nature 284:135–139
Reed MH (1998) Calculation of simultaneous chemical equilibria in aqueous-mineral-gas systems and its application to modeling hydrothermal processes. Rev Econ Geol 10:109–124
Robie RA, Hemingway BS (1995) Thermodynamic properties of minerals and related substances at 298.15 K and 1 Bar (105 Pascals) pressure and at higher temperatures. United States Geol Survey Bull 2131:1–461
Sharygin AV, Wood RH, Zimmerman GH, Balashov VN (2002) Multiple ion association versus redissociation in aqueous NaCl and KCl at high temperatures. J Phys Chem B 106:7121–7134
Shock EL, Helgeson HC (1988) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: correlation algorithms for ionic species and equation of state predictions to 5 kb and 1000 °C. Geochim Cosmochim Ac 52:2009–2036
Shock EL, Helgeson HC, Sverjensky DA (1989) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: standard partial molal properties of inorganic neutral species. Geochim Cosmochim Ac 53:2157–2183
Shock EL, Sassani DC, Willis M, Sverjensky DA (1997) Inorganic species in geological fluids: correlations among standard molal thermodynamic properties of aqueous ions and hydroxide complexes. Geochim Cosmochim Ac 61:907–950
Sverjensky DA, Shock EL, Helgeson HC (1997) Prediction of the thermodynamic properties of aqueous metal complexes to 1000 °C and 5 kb. Geochim Cosmochim Ac 61:1359–1412
Tagirov B, Schott J (2001) Aluminum speciation in crustal fluids revisited. Geochim Cosmochim Ac 65:3965–3992
Tagirov B, Schott J, Harrichourry JC, Salvi S (2002a) Experimental study of aluminum speciation in fluoride-rich supercritical fluids. Geochim Cosmochim Ac 66:2013–2024
Tagirov B, Schott J, Harrichourry JC (2002b) Experimental study of aluminum-fluoride complexation in near-neutral and alkaline solutions to 300 °C. Chem Geol 184:301–310
Tanger JC, Helgeson HC (1988) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: revised equations of state for the standard partial molal properties of ions and electrolytes. Am J Sci 288:19–98
Van Noten K, Muchez P, Sintubin M (2011) Stress-state evolution of the brittle upper crust during compressional tectonic inversion as defined by successive quartz vein types (High-Ardenne slate belt, Germany). J Geol Soc London 168:407–422
Vidal O, Dubacq B (2009) Thermodynamic modelling of clay dehydration, stability and compositional evolution with temperature, pressure and H2O activity. Geochim Cosmochim Ac 73:6544–6564
Wagner T, Cook NJ (2000) Late-orogenic alpine-type (apatite)-quartz fissure vein mineralization in the Rheinisches Schiefergebirge, NW Germany: mineralogy, formation conditions and lateral-secretionary origin. Mineral Mag 64:539–560
Wagner T, Jochum J (2002) Fluid-rock interaction processes related to hydrothermal vein-type mineralization in the Siegerland district, Germany: implications from inorganic and organic alteration patterns. Appl Geochem 17:225–243
Wagner T, Boyce AJ, Erzinger J (2010) Fluid-rock interaction during formation of metamorphic quartz veins: a REE and stable isotope study from the Rhenish Massif, Germany. Am J Sci 310:645–682
Wagner T, Kulik DA, Hingerl FF, Dmytrieva SV (2012) GEM-Selektor geochemical modeling package: TSolMod library and data interface for multicomponent phase models. Can Mineral 50:1173–1195
Walther JV (1997) Determination of activity coefficients of neutral species in supercritical H2O solutions. Geochim Cosmochim Ac 61:3311–3318
Walther JV (2001) Experimental determination and analysis of the solubility of corundum in 0.1 and 0.5 m NaCl solutions between 400 and 600 °C from 0.5 to 2.0 kbar. Geochim Cosmochim Ac 65:2843–2851
Warren CJ, Waters DJ (2006) Oxidized eclogites and garnet-blueschists from Oman: P-T path modelling in the NCFMASHO system. J Metamorph Geol 24:783–802
Wei C, Powell R (2003) Phase relations in high-pressure metapelites in the system KFMASH (K2O-FeO-MgO-Al2O3-SiO2-H2O) with application to natural rocks. Contrib Mineral Petr 145:301–315
Wilson AD (1960) The micro-determination of ferrous iron in silicate minerals by a volumetric and a colorimetric method. Analyst 85:823–827
Wolf M (1978) Inkohlungsuntersuchungen im Hunsruück (Rheinisches Schiefergebirge). Z Dtsch Ges Gowiss 129:217–227
Yardley BWD (1983) Quartz veins and devolatilization during metamorphism. J Geol Soc London 140:657–663
Yardley BWD (1997) The evolution of fluids through the metamorphic cycle. In: Jamtveit B, Yardley BWD (eds) Fluid flow and transport in rocks. Springer, Berlin, pp 99–121
Yardley BWD, Bottrell SH (1992) Silica mobility and fluid movement during metamorphism of the Connemara schists, Ireland. J Metamorph Geol 10:453–464
Acknowledgments
This study was carried out within the framework of the DGMK (German Society for Petroleum and Coal Science and Technology) research project 718 (Mineral Vein Dynamics Modeling), which is funded by the companies ExxonMobil Production Deutschland GmbH, GDF SUEZ E&P Deutschland GmbH, RWE Dea AG and Wintershall Holding GmbH, within the basic research program of the WEG Wirtschaftsverband Erdöl- und Erdgasgewinnung e.V. We thank the companies for their financial support and their permission to publish our results. We thank Lydia Zehnder for help with the XRD and XRF measurements, and Markus Wälle for support during LA-ICPMS analysis. Dan Miron and Stephan Nagel are thanked for the assistance during the preparation of the illite and chlorite crystallinity samples, and the data processing. The authors sincerely thank Chris Heinrich for his outstanding support during this research. We thank an anonymous Mineralogy and Petrology reviewer for constructive comments that have helped to improve this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: L. Danyushevsky
Rights and permissions
About this article
Cite this article
Marsala, A., Wagner, T. Mass transfer and fluid evolution in late-metamorphic veins, Rhenish Massif (Germany): insight from alteration geochemistry and fluid-mineral equilibria modeling. Miner Petrol 110, 515–545 (2016). https://doi.org/10.1007/s00710-016-0424-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-016-0424-8