Abstract
Chrysolaena flexuosa (Sims.) H. Rob. is a South American species in the tribe Vernonieae, with potential ornamental value: it has attractive inflorescences, is suitable for pot cultivation, and its cypselae are useful for dried flower arrangements. Apart from studies on the growth dynamics of this species under cultivation, chromosome number, DNA content, ploidy level, size, pollen viability, and the characterization of phenotypic and genetic variability, it is noteworthy that other aspects regarding the floral architecture, reproductive mode, and gametophyte formation of C. flexuosa have not yet been studied. For this reason, our study encompasses a floral morphoanatomical survey and a comprehensive assessment of gametophyte development in the species. As a result of this study, we report new floral morphotypes, confirming that the morphological variability of the species might be greater than speculated. The morphoanatomy of the androecium and gynoecium and the male and female gametophyte developmental characteristics are uniform in all the populations studied despite the different ploidy levels. Chrysolaena flexuosa has five tetrasporangiate stamens of the dicotyledonous type of development; all the populations studied displayed a unilocular inferior ovary with a single anatropous, unitegumented, and tenuinucellar ovule. Given that all the embryo sacs observed were of the Polygonum-type development regardless of the ploidy level, we infer that the populations analyzed are fertile and undergo sexual reproduction. Our results not only contribute further research in the field of breeding systems and propagation of this species, but also promote the successful introduction of C. flexuosa to the plant ornamental market.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The worldwide market for flowers and ornamental plants is fundamentally shaped by consumer preferences, driving demand in specific directions through their expectations for novel varieties and higher quality standards. Argentina has a great floricultural potential due to the diversity of ecological environments that support plants with varying light, temperature, water, and nutrient requirements. Numerous authors have highlighted the ornamental value of native Argentine species belonging to the Asteraceae family (Barrionuevo et al. 2006; Mazzoni et al. 2006; Negrín and Zalba 2012). They emphasize desirable ornamental qualities, such as life cycle variations, stature, height, foliage pigmentation, and captivating inflorescence characteristics. Chrysolaena flexuosa (Sims.) H. Rob., a South American species in the tribe Vernonieae, thrives in the countryside of northern and south-central Argentina, with the province of Buenos Aires marking the limit of its distribution (Cabrera 1963; Dematteis 2014). Its attractive inflorescences make it suitable for pot cultivation, and its cypselae are used in dried flower arrangements (Fig. 1). Consequently, several authors have suggested its potential as a new ornamental variety for the market (Alonso et al. 2009; Echeverría and Alonso 2012).
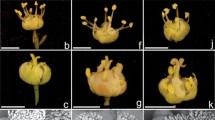
Ambient and floral morphotypes of C. flexuosa analyzed. A Natural habitat in grassland of Tres Cerros, Corrientes province. B Plant in its natural habitat. C Capitulum of diploid specimen with corolla and stamens and styles of purple color associated with brown phyllaries (M1). D Capitulum of diploid specimen with white corolla associated with pink stamens and purple styles (M2). E Capitulum of tetraploid specimen with purplish corolla and purple stamens and styles (M3). F Capitulum of specimen with corolla, stamens, and styles of purple color associated with green phyllaries (M4)
To introduce a novel species into the floricultural market and formulate sustainable utilization strategies, the generation of basic data is indispensable for informing the development of a plant breeding program (Stoskopf et al. 2019; Singh et al. 2021). This basic data encompasses facets of reproductive biology, morphological characteristics, and genetic diversity inherent within the species (Acquaah 2015; Stoskopf et al. 2019). Therefore, a thorough characterization and evaluation of the vegetative and reproductive traits is essential to facilitate the careful selection of genotypes with high ornamental value. The first advances towards fundamental breeding information were previously reported by Echeverría and Alonso (2012), who studied the growth dynamics of this species under cultivation. Subsequently, Echeverria and Camadro (2017) examined the chromosome number, DNA content, ploidy level, size, and pollen viability of different populations spanning the northeastern-southeastern distribution range in Argentina; their findings culminated in the assertion that natural hybridization and sexual polyploidization are strongly related to the origin, establishment, and expansion of those natural populations. Concluding this research, Echeverría and Camadro (2020) studied the characterization of phenotypic and genetic variability; their study not only discovered marked morphological diversity encompassing both qualitative and quantitative traits of ornamental significance but also valuable molecular diversity revealed through AFLP markers, fostering their applicability in ornamental breeding programs. Based on the factorial correspondence analysis of qualitative morphological traits and ploidy level, they also proposed a diploid morphotype with slightly pubescent leaves, green phyllaries with lilac and pink corollas (Corrientes and Misiones province), a tetraploid morphotype which had pubescent leaves, green phyllaries, and white corollas (Entre Ríos province) and a hexaploid morphotype with pubescent leaves, green phyllaries, and purple corollas (Buenos Aires province). Robinson (2007), Via Do Pico et al. (2016), and Marques et al. (2020) have previously analyzed some aspects of floral microcharacters of C. flexuosa with taxonomic purposes. However, it is noteworthy that essential data regarding the floral architecture, reproductive system, and gametophyte formation remains elusive. Understanding the floral architecture of the species becomes important when assessing the need for artificial hybridization in breeding efforts. The mode of reproduction, whether sexual or asexual, affects the genetic structure of plants, enabling breeders to delineate the appropriate breeding method (self-pollination, cross-pollination, or clonal-propagation). Concurrently, the study of gametophyte formation contributes to elucidate the reproductive behavior of the species (Brown and Caligari 2008; Acquaah 2015; Stoskopf et al. 2019; Singh et al. 2021). The species reproductive mode is also relevant to efficient propagation and maintenance of newly cultivated varieties (Acquaah 2015). In this context, understanding the embryology and floral morphoanatomy of the species will lead to developing an efficient and successful breeding protocol (Singh et al. 2021).
Polyploidy is an inherent driving force that triggers and promotes novel phenotypic variation (Udall and Wendel 2006; Sattler et al. 2016). This widespread phenomenon has had a significant impact on the heterogeneity of the chromosome number of the genus Chrysolaena, a fact substantiated by the exhaustive research undertaken (Dematteis 1996, 1997a, 1997b, 2002, 2007, 2009; Angulo and Dematteis 2009a, 2009b; Oliveira et al. 2012; Via Do Pico and Dematteis 2012, 2013, 2014, 2017; Via Do Pico 2015; Echeverria and Camadro 2017; Via Do Pico et al. 2019). The genus has a basic chromosome number x = 10 (Dematteis 2002), but species show variation in their cytotypes with chromosome numbers ranging from 2n = 20 to 2n = 80. Hence, Chrysolaena is recognized for its cytogenetic complexity with significant interspecific and intraspecific chromosomal variation (Via Do Pico et al. 2019). In particular, the chromosome numbers of C. flexuosa range from 2n = 20 to 2n = 60 with diploid, tetraploid, and hexaploid specimens (Echeverria and Camadro 2017; Via Do Pico et al. 2019). According to Echeverria and Camadro (2017), the ploidy level of the species has a geographic pattern of distribution. Via Do Pico et al. (2019) detected diploid C. flexuosa living in sympatry with diploid C. verbascifolia (Less.) H. Rob., diploid C. propinqua (Hieron.) H. Rob., and hexaploid C. cognata (Less.) M. Dematt. in Zaiman Creek (Misiones province, Argentina), an important area of notable cytotype diversity and species richness. Via Do Pico et al. (2019) postulated that this cytotype diversity in confined regions stems from hybridization and polyploidization events.
The enormous cytotype diversity, coupled with the important natural polyploid reservoir, gives C. flexuosa an extensive molecular and morphological spectrum that enhances its commercial value (Echeverría and Camadro 2020). As the mode of reproduction and floral architecture remain unexplored, there is a gap in the knowledge regarding this aspect within the context of ornamental breeding. Therefore, the study of floral morphoanatomy and gametophyte development has the potential to enrich the data needed for formulating a targeted ornamental breeding program for C. flexuosa. For this reason, our study encompasses a floral morphoanatomical survey and comprehensive gametophyte development assessment of the species. Our results will not only contribute further research in the field of breeding systems and propagation of this species, but will also contribute to the reproductive biology of the genus.
Materials and methods
We collected 50–100 mature flowers and flower buds from different localities of Corrientes (Argentina); information about chromosome number, ploidy level, morphotype, location, voucher information and reference of the previous chromosome counts of the studied material is provided in Table 1. Vouchers were deposited in the herbarium of the Instituto de Botánica del Nordeste (CTES). The material was collected, fixed, and stored in formalin-acetic acid-alcohol (5 mL formalin, 5 mL acetic acid, and 90 mL 70% ethanol).
The observations and interpretations of the floral morphoanatomy were made using a light microscope (Leica DM LB2, Leica Microsystems, equipped with Leica ICC50 HD digital camera and polarized filters) and a scanning electron microscope (Jeol LV 5800, Service of Electron Microscopy, Universidad Nacional del Nordeste). The observations of male and female gametophyte development were made using a light microscope.
We analyzed 10–15 flowers and flower buds per population of C. flexuosa. The material was dehydrated with an increasing series of alcohol and embedded in paraffin according to González and Cristóbal (1997). The sample was cut in serial transverse and longitudinal sections of 10–12 μm using a rotary microtome (Microm HM350). These sections were stained with Safranin and Astra blue (Luque et al. 1996) and then mounted with synthetic Canada balsam.
For the scanning electron microscope observations, we used 10–15 mature flowers per population of C. flexuosa. This material was dehydrated in an ascending acetone series and then critical point dried using CO2 (Denton Vacuum, DCP-1, Pleasanton, NJ). Finally, the samples were sputter-coated with gold–palladium (Denton Vacuum, Desk II, Pleasanton, NJ) for observation under the microscope.
Results
Floral morphoanatomy
All the populations analyzed showed a similar floral morphological pattern. The flowers are usually arranged in capitula formed by several imbricate preflowering phyllaries (Fig. 1C–F). The observed variability enabled us to categorize the populations into four floral morphotypes based on the pigmentation of the corolla, stamens, phyllaries, and styles. Morphotype 1 (M1) is a diploid population from the northeast of the province (Santo Tomé dept.) that has a purple corolla, stamens, and styles associated with brown phyllaries (Fig. 1C). Morphotype 2 (M2) is another diploid population, from the northwest of the province (El Sombrero dept.) that has white corollas with pink stamens, purple styles, and green phyllaries (Fig. 1D). Morphotype 3 (M3) is a tetraploid population from Tres Cerros; these flowers have purplish corollas associated with deep purple stamens and styles and green phyllaries (Fig. 1E). Morphotype 4 (M4) is populations in the south-center of the province, with an unknown chromosome number, which have flowers with a purplish corolla, stamens, and styles associated with green phyllaries (Fig. 1F). Each capitulum comprises perfect, tubular, isomorphic, sessile, epigynous flowers, inserted on a receptacle without paleae (Fig. 3A). Table 2 summarizes the variability observed in the analyzed morphotypes and those previously reported for C. flexuosa. Furthermore, Fig. 2 illustrates the observed floral morphotypes and the geographic distribution of Argentine populations of C. flexuosa analyzed to date.
Floral morphotypes and geographic distribution of the Argentinian populations of C. flexuosa analyzed thus far. Ref. asterisk (*) indicates the populations analyzed in this work; VdPDFV 45, VdPDFV 26, VdPDFV 14, AloEch 1, AloEch 2, AloEch 3, and Nu 1: populations examined by Echeverría and Camadro (2020)
Corolla and calyx
The corolla is actinomorphic, pentamerous, gamopetalous, and tubular, with a differentiated tube and pentasect limb (Fig. 3A, B). Anatomically, the corolla is composed of an outer epidermis, a mesophyll, and an inner epidermis. Both epidermises (inner and outer) are unistrata and have large thin-walled cells with dense cytoplasm and conspicuous nuclei (Fig. 3C). The outer epidermis is covered by a cuticle that increases in thickness at the tips, where it is notoriously thick and striated (Fig. 3C). The mesophyll is composed of isodiametric parenchyma cells of homogeneous aspect, with cellular spaces (Fig. 3C). In cross-section, two collateral vascular bundles per petal were observed (Fig. 3C).
Flower, morphology, and anatomy of corolla, calix, style, and stigmatic branches of C. flexuosa. A Flower. B Pentamerous corolla with pentasect limb and staminal tube. C Anatomy of the corolla in cross-section. D Calix represented by heteromorphic biseriate pappus. E Protruding tissue where the pappus is inserted. F Typical “Vernonioid” style. G Detail of sweeping hairs of the external surface of stigmatic branches. H Detail of the small stigmatic papillae of the internal surface of stigmatic branches. I Morphology of the style base. J Base of the style in cross-section. K Anatomy of the style with central transmission tissue and two vascular bundles. L Anatomy of the stigmatic branches in cross-section. Bn, basal node; ca, calix; co, corolla; CoT, corolla tip; Cs, cellular space; CT, corolla tube; cu, cuticle; Eb, external bristle; ep, epidermis; fi, filament; GTr, glandular trichome; Ib, internal bristle; Iep, inner epidermis; ms, mesophyll; Oep, outer epidermis; ov, ovary; SB, stigmatic branches; Sp, stigmatic papillae; ST, staminal tube; st, style; Sh, sweeping hair; Tt, transmission tissue; Vb, vascular bundle. Scales: B 1 cm; D, F 500 µm; C, E, G, I, J, L 50 µm; H, K 20 µm
The calyx of the flowers, represented by the pappus, is located at the upper edge of the ovary and extends to the apex of the corolla (Fig. 3D). It is composed of several filamentous bristles formed by abundant squamose bracts (Fig. 3D). All the species analyzed presented a heteromorphic biseriate pappus: the external series have short and wide bristles, while the second series contains elongated and fine bristles. In the apical portion of the ovary, the pappus is inserted in a protruding tissue differentiated towards the periphery and composed of layers of tangentially flattened, thin-walled cells with an evident nucleus, covered by a uniseriate epidermis (Fig. 3E).
Gynoecium
The gynoecium is gamocarpellar, with two carpels fused to form a unilocular inferior ovary with a single anatropous, unitegumented, and tenuinucellar ovule (Figs. 3A and 4A). The style is cylindrical; it is divided into two linear branches in its upper part (Fig. 3F). The branches are acute, flat towards the inner sides, and curved outwards. They are covered with sweeping hairs from the apex of the linear branches and slightly below the point of division (Fig. 3F, G). The inner part of the style branches is completely covered with small stigmatic papillae (Fig. 3H). At the base of the style, there is a group of conspicuous, isomorphic, rectangular-shaped epidermal cells with a conspicuous nucleus and a lignified cell wall forming a basal stylar nodule (Fig. 3I, J). Internally, the branches are composed of an epidermis, parenchymatous tissue, and transmission tissue in the central zone (Fig. 3K). Two vascular bundles are observed near the transmission tissue in the central zone, and then, each branch of the stigma is innervated by a vascular bundle coming from the style (Fig. 3L).
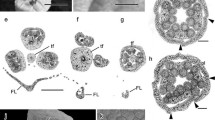
Female gametophyte development of C. flexuosa. A Anatropous ovule with a megaspore mother cell. B Enlarged megaspore mother cell. C One functional megaspore and three crushed megaspores of micropile orientation. D Young embryo sac with two nucleus. E Seven-nucleate embryo sac. F–I: Mature female gametophyte. F Egg cell of a mature embryo sac. G Two synergids with a developed filiform apparatus. H Mature embryo sac with two polar nuclei fused. I Detail of the antipodals. an, antipodals; ec, egg cell; fa, filiform apparatus; FM, functional megaspore; IT, integumentary tapetum; mmc, megaspore mother cell; n, nucleus; Ov, ovule; po, fusioned polar nuclei; sy, synergids. Scales: A, D, E, F, G, H, I 50 µm; B, C 20 µm
Development of the female gametophyte, sporogenesis, and gametogenesis
Initially, a subdermally nucellar cell differentiates into an archesporial cell, distinguished by its size, thin cell wall, dense cytoplasm, and conspicuous nucleus (Fig. 4A). Subsequently, this cell elongates, acquires a rectangular shape, and directly differentiates into the megaspore mother cell (mmc; Fig. 4B).
The mmc divides by meiosis: in meiosis I, a dyad of reduced megaspores is formed; at meiosis II, a linear tetrad of haploid megaspores aligned on the chalazal-micropylar axis is formed. The megaspore of the chalazal side grows, elongates, and crushes the remaining three micropylar-oriented megaspores (Fig. 4C). The crushed megaspores degenerate, and the chalazal megaspore becomes the functional megaspore and gives rise to the embryo sac. The functional megaspore undergoes three consecutive cycles of mitotic divisions: in the first division, two nuclei are formed that are separated by a centrally placed vacuole which gives polarity to the immature embryo sac (Fig. 4D); in the second mitotic division, an immature embryo sac forms with four nuclei; in the third division, the four nuclei formed previously divide once again forming a disorganized seven-nucleate coenocitic megagametophyte (Fig. 4E). The inner epidermis of the integument becomes the integumentary tapetum: an endothelium of radially elongated cells, with dense cytoplasm and prominent nucleus, which remains uniseriate throughout the development of the megagametophyte (Fig. 4D, E, F). Simultaneously, the nucella cells collapse and disintegrate. Finally, the eight nuclei organize and differentiate to form the mature embryo sac (Fig. 4F–I), surrounded by an integumentary tapetum. All mature embryo sacs observed consisted of an oospheric apparatus located at the micropylar pole, a central cell, and two antipodals oriented toward the chalazal pole (Fig. 4F–I). The oospheric apparatus consists of the conspicuous ovocellula and two elongated synergids (Fig. 4F, H). The central cell, which occupies most of the embryo sac, contains the two polar nuclei located towards the ovocellula. Before fertilization of the ovocellula, the two polar nuclei fuse (Fig. 4H). The two antipodals observed are small in size (Fig. 4I).
Androecium
The five anthers are connate forming a staminal tube around the style (synanthereous androecium); they are united by a thin cuticle that covers the dorsal face of the stamens (Fig. 5A). The anthers are oblong in shape and present longitudinal dehiscence (Fig. 5B). Each stamen is introrse, deep purple, with two thecae, and its dorsal apex is covered with glandular trichomes (Fig. 5C). The sterile prolongations of the anthers (apex and base of the anther) do not participate in the formation of the staminal tube (Fig. 5D).
Androecium morphology and filament anatomy of C. flexuosa. A Flower morphology showing the synanthereous androecium with introrse anthers forming a staminal tube around the style. B Two anthers showing its longitudinally dehiscence; observe in the apical portion the sterile apical prolongation. C Dorsal view of anther apex with glandular trhichomes. D Apical portion of the staminal tube where we visualize that the sterile prolongations are free from the staminal tube. E Anther dorsal view showing the insertion of the filament and the sterile prolongation of the base. F Filament observed in cross-section. AnA, anther apex; AnB, anther base; CoT, corolla tip; co, corolla; ep, epidermis; fi, filament; GTr, glandular trichome; lo, locule; pa, parenchyma; ST, staminal tube; st, style; Vb, vascular bundle. Scales: A 500 µm; B, E 300 µm; C, D 100 µm; F 50 µm
The filament is the free and sterile part of the anther; it is attached to the corolla in the medial portion of the corolla tube and is inserted in the dorsal part of the anther (dorsifixed anthers; Fig. 5E). It is formed by a uniseriate epidermis of papillose nature covered by a thin cuticle; in subepidermal position, we observed very compact isodiametric parenchymatous cells. It is innervated by a closed collateral bundle derived from the corolla bundles (Fig. 5F).
Microsporogenesis and development of the male gametophyte
The individuals analyzed presented the same development pattern. At first, the young tetralobed anther has an epidermis and a row of archesporial cells differentiated in each corner (Fig. 6A). The archesporial cells divide and form two cell lines: the internal primary sporogenous cell which directly becomes sporogenous tissue and the external primary parietal cell (Fig. 6B). This layer divides and gives rise to the internal secondary parietal layer and the external secondary parietal layer (Fig. 6C). The first differentiates into tapetum; meanwhile, the second layer is divided into the external layer that gives rise to the endothecium and the internal layer that gives rise to the middle layer (Fig. 6D). In this way, the mature anther wall consists of four layers: epidermis, endothecium, middle layer, and tapetum (Fig. 6E). The epidermis consists of rectangular cells that are compressed and persistent; at later stages, it is barely distinguishable as a thin layer (Fig. 6E). The endothecium is a layer of rectangular cells with prominent nuclei. By the microspore stage, lignified thickenings begin to deposit on the tangential and radial walls of the cells. Finally, the differential thickening of the endothecium cell walls contributes to anthesis (Fig. 6G–J). The middle layer is ephemeral; it is observed only during the MMC (microspore mother cell) stage, formed by elongated cells with dense cytoplasm (Fig. 6D, E). The tapetum is a layer of isodiametric-shaped cells with dense cytoplasm and a conspicuous nucleus, which persist in situ at the beginning during the MMC stage (Fig. 6E, F); later, they deform and invade the locule completely, enveloping the young microspores (Fig. 6G, H). In the following stages, the tapetum is completely consumed.
Anther development of C. flexuosa. A Tetralobed anther with archesporial cell. B Microsporangium with primary sporogenous cell and primary parietal cell. C Microsporangium with sporogenous tissue, external secondary parietal cell, and internal secondary parietal cell. D Microsporangium with microspore mother cell; the asterisk points to the division of the external parietal cell. E Anther wall formed by epidermis, endothecium, middle layer, and tapetum. F Anther locule with tetrad of microspores with callose. G Microsporangium with tapetum starting to invade the anther locule. H Tapetum invaded the anther locule and surrounded the microspores. I Stamen in anthesis with prominent endothecium and obliterated epidermis. J Endothecium showing lignified thickenings; cross-section observed with polarized light. K Three-celled pollen grain. ac, archesporial cell; en, endothecium; ep, epidermis; esp, external secondary parietal cell; isp, internal secondary parietal cell; lt, lignified thickenings; mi, microspore; ml, middle layer; MMC, microspore mother cell; nu, nucleus; po, pollen grain; ppc, primary parietal cell; psc, primary sporogenous cell; sp, sporogenous tissue; ta, tapetum; va, vacuole; vb, vascular bundle; vc, vegetative cell. Scales: A, I, K 50 µm; B, C, D, E, F, G, H, J 20 µm
The sporogenous tissue of the young anther consists of a single row of isodiametric cells vertically placed within the anther. These cells differentiate into large, spherical, thin-walled microspore mother cells (MMC) with prominent nuclei (Fig. 6D, E). They divide by meiosis: after meiosis I, two haploid reduced nuclei are formed; in meiosis II, one cell with four haploid nuclei is formed and then separated by cytokinesis into four small cells forming a callose-covered tetrad (Fig. 6F). In all the species analyzed, the cytokinesis observed was of the simultaneous type. The callose dissolves releasing the microspores in the locule of the immature anther, and each microspore recently released has dense cytoplasm and a prominent centrally located nucleus (Fig. 6G). These cells grow in size, incorporate large amounts of water, and increase in volume, forming a prominent vacuole within them, which displaces the nucleus toward the periphery of its walls (Fig. 6H). As the microspore continues to mature, the vacuole disappears. The first mitotic division within the young pollen grain gives rise to a vegetative cell and a generative cell. The vegetative cell grows and incorporates the generative cell into its cytoplasm. Shortly after, the vegetative cell divides mitotically, and two sperm cells are formed. At the dehiscence stage, the mature pollen grains released were three-celled in all the specimens analyzed (Fig. 6I, K).
Discussion
This is the first analysis of the floral morphoanatomy of different populations of C. flexuosa. While some specific traits of floral morphology have been previously analyzed such as floral microcharacters (Robinson 2007; Via Do Pico et al. 2016; Marques et al. 2020), their anatomical perspective remained unstudied until now.
Floral morphology and morphotypes
The floral morphology is uniform among the specimens analyzed; however, the color of the phyllaries, stamens, style, and corolla vary, resulting in four floral morphotypes. The results of this study reflect the chromatic variability of the populations analyzed. In the province of Corrientes, there are populations of C. flexuosa displaying purple, purplish, or white corollas and stamens ranging from pink to purple and purple styles. The four floral morphotypes previously described in the results are usually observed together with green phyllaries, and to a lesser extent, brown phyllaries.
Echeverría and Camadro (2020) performed a survey related to qualitative floral traits such as leaf pubescence, color of the corolla, and phyllaries. They described three other floral morphotypes based on the factorial correspondence analysis of qualitative morphological traits and ploidy level: the diploid population of Corrientes and Misiones province had slightly pubescent leaves and green phyllaries with lilac and pink corollas; the tetraploid population belonging to Entre Ríos province had pubescent leaves, green phyllaries, and white corollas; and hexaploid populations from Buenos Aires province were associated with pubescent leaves, green phyllaries, and purple corollas. Considering the findings of Echeverría and Camadro (2020), it is evident that the pigmentation of the corolla represents the most variable character, ranging from white, pink, lilac, purplish, to purple color.
Furthermore, our analysis reveals that the flowers with white corollas are not limited to populations in Entre Ríos, nor are they exclusively linked to tetraploid cytotypes. This study also involved the observation and description of color variations concerning the styles and stamens, which represents a substantial contribution to the floral characterization of wild populations of C. flexuosa. The populations examined in this study exhibited various floral morphotypes associated with the color of the floral whorls, distinct from the floral morphotypes documented by Echeverría and Camadro (2020). In this study, we not only document new floral morphological variations but also confirm that the species exhibits substantial diversity in flower colors. Since the color of the floral whorls varied independently of their chromosome number, we can conclude that the observed phenotypic variation is not associated with ploidy levels of the specimens studied.
Gynoecium, ovule, and embryo sac
This study characterizes and reports the female gametophyte development of C. flexuosa for the first time. Female embryological characteristics, such as the architecture of the ovary, the shape of the ovule, and the number of teguments, are conserved among the populations studied.
The specimens studied showed homogeneity in the morphology of the style and stigmatic branches. Additionally, no significant relationship was observed between the morphology and the ploidy levels of the three populations analyzed, although this assertion cannot be generalized to all populations due to the unknown ploidy levels of certain groups at present. They all present the Vernonioid morphotype previously described for the tribe (Bremer 1987). This type of style plays an important role in the secondary presentation of pollen characteristic of the species (Howell et al. 1993). In the Asteraceae family, the pollen can be exposed from the anther tube in three different ways: pulled by adherence to small papillose hairs, pushed by collecting hairs located at the apex of the stigmatic branches, or brushed by collecting hairs (Anderberg et al. 2007; Funk et al. 2009). Chrysolaena flexuosa exhibits the pollen brushing system characteristic of the Vernonieae (Anderberg et al. 2007), in which the style and the collecting hairs on the outer face of the stigmatic branches function as a brush (Anderberg et al. 2007).
The basal stylar node observed in all the specimens studied has been extensively analyzed in different tribes, such as the Lactuceae, Senecioneae, Heliantheae, Anthemideae, Mutisieae, Inuleae, Millerieae, Vernonieae, Eupatorieae, Astereae, Tageteae, Arctoteae, Calenduleae, and Cynareae (Angulo and Dematteis 2014; Jana and Mukherjee 2015; Via Do Pico et al. 2016; Angulo et al. 2018; Marques et al. 2020); however, its anatomy has not been recorded so far. The size of this structure varies between species; sometimes, it may be poorly represented and form a cluster of sclerified cells forming a ring or several rows of sclerified cells forming a nodule. In the specimens analyzed in this study, the base of the style presents a group of cells forming a ring, which agrees with the observations of Via Do Pico et al. (2016). This structure gains significance when we contemplate its potential role in imparting solidity to the style. As the style elongates and brushes the pollen grains, it encounters resistance as it passes through the staminal tube. Furthermore, it is plausible to hypothesize that the intraspecific variation observed for this character may be associated with the degree of resistance presented by the staminal tube as the style passes through it. Additionally, the results of this study allow us to determine that the variation in this character is not related to the ploidy level of the specimen studied.
All specimens analyzed in this study of C. flexuosa presented a unilocular, bicarpellar, and syncarpous ovary. These characters agree with those described for other members of the Old World tribe Vernonieae, such as Vernonia elaeagnifolia DC., V. divergens (DC.) Edgew., Elephantopus scaber L., Adenostemma rugosum Wt., and A. lavenia (L.) Kuntze (Pullaiah 1979a). In general, this type of gynoecium is uniform in all taxa within the Asteraceae family as no deviations from this pattern have been documented so far (Davis 1966; Johri et al. 1992; Anderberg et al. 2007). Based on these previous observations and the outcomes of our study, we consider that these features remain conserved within the family.
The ovules observed in this study presented anatropous ovules with a single integument, as do all previously studied representatives of the family (Johri et al. 1992). Unitegmic ovules are considered a derived character that dominates and are almost exclusive in euasterids (Endress 2011). This condition is observed in several lineages, indicating that it has arisen several times during the evolution of angiosperms (Stebbins 1974; Bouman 1984). Furthermore, tenuinucellate ovules, such as those observed in Chrysolaena species, are also typical of the Asteraceae family and are representative of the euasterid clade (Endress 2011). Bonifacio et al. (2018) proposed that anatropous, unitegmic, and tenuinucellate ovules are plesiomorphic characters for the family, because they are characters common to the Asteraceae and Asterales (Johri et al. 1992; Tobe and Morin 1996). Considering the comprehensive literature available, we agree with Bonifacio et al. (2018) in asserting that anatropous, unitegmic, and tenuinucellate ovules are conserved and representative traits within the family.
The monosporic embryo sac is one of the most complex structures originating from a single megaspore (Willemse and Van Went 1984). Given that all the embryo sacs observed in this study originated from a single megaspore of chalazal orientation, the C. flexuosa embryo sacs are of the Polygonum-type of development. Furthermore, the integumentary tapetum developed in all analyzed populations of this work is a characteristic tissue that surrounds the embryo sacs of several members within the Asteraceae family (Bhojwani and Bhatnagar 1974; Lersten 2008). Although all the embryo sacs observed in this study were of monosporic origin, there is empirical evidence that other types of development can be observed in the Asteraceae family. There are embryo sacs of bisporic (Taraxacum type), tetrasporic (Antennaria type), or aposporic (Hieracium type) development (Anderberg et al. 2007). However, in light of the high frequency of the monosporic Polygonum type of development observed among the entities within the studied entities of tribe Vernonieae, we regard it as the typical pattern of embryo sac development (Tiagi and Taimni 1960, 1963; Pullaiah 1979a; Pérez et al. 2021).
In this study, we confirmed that all the populations analyzed formed reduced haploid embryo sacs regardless of the ploidy level. Therefore, we infer that these populations are fertile and undergo sexual reproduction. This behavior is compatible with that observed in L. plantaginoides (Pérez et al. 2021), a natural tetraploid with sexual reproduction. In the case of C. flexuosa, both the diploids and the polyploids studied produced reduced haploid gametes; therefore, we infer that they have sexual reproduction. Breeding methods are classified in three categories according to the mode of reproduction (sexual or asexual): self-pollinated, cross-pollinated, or clonally propagated (Acquaah 2015; Allard 1960). Breeders usually choose one of these three methods with the main objective of retaining the natural genetic structure of the species in the new cultivar (Acquaah 2012, 2015). Considering the sexual mode of reproduction and the high morphological variability of C. flexuosa, we are tempted to recommend artificial hybridization or crossing as breeding methods to create new cultivars with desirable commercial traits. The newly created cultivars could be maintained through self-pollinated methods in case the cultivars are self-compatible.
Androecium and male gametophyte development
This is the first description of the morphoanatomy of the androecium and the male gametophyte development of a species of the genus. The morphoanatomy of the androecium and male gametophyte developmental characteristics are uniform, with no variability despite the ploidy levels of C. flexuosa. Our results allow us to infer a trend in which high ploidy levels do not significantly affect normal anther development; nonetheless, to confirm this assumption, the ploidy level of all morphotypes would have to be determined.
The synanthereous androecium, a distinctive trait within the family (Bremer 1994; Anderberg et al. 2007), is closely linked to secondary pollen presentation (Leins and Erbar 1990; Erbar and Leins 1995). However, this unique feature observed in Asteraceae specimens has potential advantages, particularly in the context of artificial hybridization efforts. To prevent self-pollination, breeders commonly eliminate the anthers prior to stigma receptivity. This process, referred to as emasculation, serves not only to prevent self-pollination but also to facilitate controlled pollination by depositing the desired parental pollen on the stigma (Priyadarshan 2019). Emasculation would be easy to apply in C. flexuosa by breeders.
The anther wall anatomy of the Asteraceae was previously studied by Davis (1966), Pullaiah (1979b), and Johri et al. (1992). The anther wall of the species studied here shows certain general characters of the family, such as the persistent epidermis, the presence of fibrous thickening in the endothecium, and the ephemeral middle layer. The same characters mentioned for the closely related genus Lessingianthus (Pérez et al. 2021, 2023) are common to other species of the family (Davis 1966; Johri et al. 1992; Yurukova-Grancharova 2004; Anderberg et al. 2007; Ao 2007; Gotelli et al. 2008; Deng et al. 2010; Franca et al. 2015) and to angiosperms in general (Davis 1966; Johri et al. 1992).
The principal function of the tapetum is to nourish and promote the development of microspores (Papini et al. 1999). The tapetum of the populations analyzed remains in situ during the early stages, and at the vacuolated pollen grain stage, it invades the locule and surrounds the microspores. The behavior of this tissue is variable within the family: in Cosmos bipinnatus Cav. and Cichorium intybus L., the tapetum is non-syncytial amoeboid; in Adenostemma rugosum Wt., A. lavenia (L.) Kuntze, Elephantopus scaber L., Vernonia elaeagnifolia DC., and V. divergens Benth., the tapetal cell walls become disorganized and the cytoplasm flows into the locule forming a true periplasmodium (Pullaiah 1979a); in Helianthus annuus (Pacini 1997), the cells fuse their cytoplasms to form a syncytium that completely obliterates the locule. In all the species of the tribe Vernonieae studied so far, the tapetum shows an invasive behavior that varies between species, forming a periplasmodium or syncytium. In C. flexuosa, we observed that the cells retain their individuality and do not fuse to form a true periplasmodium or syncytium; for this reason, we considered it as a non-syncytial invasive tapetum. This type of tapetum was initially described in other members of the tribe Vernonieae by Pullaiah (1979a) and subsequently in diploid and polyploid species of Lessingianthus, a closely related genus (Pérez et al. 2023). This data will be a significant contribution to future studies in the field of taxonomy and evolution.
Taking into account the behavior of the parietal layer defined by Davis (1966), the gametophyte development in the specimens analyzed in this study was of the dicotyledonous type because the middle layer and the endothecium share the same origin: the division of the outer secondary parietal layer. This type of development is common in the Asteraceae family and has been previously reported in several tribes, such as Inulae, Heliantheae, Cichorieae, Lactuceae, Senecioneae, the genus Opisthopappus, and the genus Lessingianthus (Pullaiah 1978, 1979b, 1981; 1983; Yurukova-Grancharova 2004; Yurukova-Grancharova and Dimitrova 2006; Gotelli et al. 2008; Jiana et al. 2009; Chehregani et al. 2011; Chehregani and Hajisadeghian 2014; Pérez et al. 2021, 2023). Two other uncommon developmental types have been reported: the basic type in basal Asteraceae species (Bonifacio et al. 2018) and the monocotyledonous type in Ageratum conyzoides L. (Franca et al. 2015). We agree with Bonifacio et al. (2018) in considering that dicotyledonous development is a plesiomorphic character because it is conserved in the Asteraceae family.
Conclusions: mode of reproduction and ornamental breeding
This study represents the first contribution to the embryology of the species, and it is the first comprehensive survey of floral morphoanatomy and gametophyte development in relation to the ploidy level.
The current investigation documents new variations in floral morphology, confirming the high diversity within the floral whorls of the species. Through this study, we identified and characterized four distinct morphotypes in Corrientes Province, distinguished by the pigmentation of the floral whorls. This characterization supplements the previously recorded data. Our observations indicate that phenotypic variation is not associated with the reported ploidy levels.
This survey confirms that the morphoanatomical traits of the androecium and male gametophyte development do not display any variations associated with ploidy levels in three of the examined populations. Similarly, female embryological characteristics, such as ovary structure and ovule shape, appear to be consistent across the populations studied, as found in other species of the family. Hence, we propose that these attributes are evolutionarily conserved within the family.
It is noteworthy that C. flexuosa only presented sexual embryo sacs, and no mature or immature asexual embryo sacs were recorded. In this context, both diploids and polyploids studied had reduced haploid gametes, implying that the studied individuals of C. flexuosa undergo sexual reproduction.
On the other hand, the sexual mode of reproduction of C. flexuosa increases the possibility of obtaining new cultivars that combine desirable attributes. In this regard, ornamental breeding by hybridization is not only feasible for C. flexuosa but also has the ability to diversify the range of floral morphotypes that exhibit the desired commercial features. The next steps in C. flexuosa ornamental breeding involve the study of pollen-stigma compatibility between entities of different ploidy levels.
This study provides crucial information on the reproductive biology and flower structure of C. flexuosa, which is essential for conventional breeding methods, especially when flower manipulation or artificial crosses are required to achieve the desirable traits.
Data availability
All data generated or analysed during this study are included in this published article.
References
Acquaah G (2012) Principles of plant genetics and breeding, 2nd edn. Wiley-Blackwell, Oxford
Acquaah G (2015) Conventional plant breeding principles and techniques. In: Al-Khayri J, Jain S, Johnson D (eds) Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools. Springer, Cham. https://doi.org/10.1007/978-3-319-22521-0_5
Allard RW (1960) Principles of plant breeding. John Wiley and Sons, New York
Alonso SI, Guma IR, Nuciari MC, Van Olphen A (2009) Flora de un área de la Sierra La Barrosa (Balcarce) y fenología de especies nativas con potencial valor ornamental. Rev Fac Cienc Agrar UNCuyo 41(2):23–34
Anderberg AA, Baldwin BG, Bayer RG et al (2007) Compositae. In: Kadereit JW, Jeffrey C (eds) Flowering Plants · Eudicots. The Families and Genera of Vascular Plants, vol 8. Springer, Berlin, Heidelberg
Angulo MB, Dematteis M (2009a) Karyological analysis of South American species of Vernonia (Vernonieae, Asteraceae). Plant Biosyst 143:20–24
Angulo MB, Dematteis M (2009b) Karyotype analysis in eight species of Vernonia (Vernonieae, Asteraceae) from South America. Caryologia 62:81–88
Angulo MB, Dematteis M (2014) Floral microcharacters in the genus Lessingianthus (Vernonieae, Asteraceae) and its taxonomic implications. Plant Syst Evol 300:1925–1940
Angulo MB, Chalup L, Dematteis M (2018) Systematics value of micromorphological and palynological characters in Stenocephalum Sch. Bip.(Vernonieae, Asteraceae). Turk J Bot 42(4):478–490
Ao C (2007) Comparative anatomy of bisexual and female florets, embryology in Calendula officinalis (Asteraceae), a naturalized horticultural plant. Sci Hortic 114:214–219
Barrionuevo V, Fuentes E, Planchuelo AM (2006) Asteráceas silvestres de las sierras de Córdoba promisorias como ornamentales. Acta del III Congreso Argentino de Floricultura y VIII Jornadas Nacionales de Floricultura. INTA, Buenos Aires, pp 325–328
Bhojwani SS, Bhatnagar SP (1974) The embryology of Angiosperms. Vikas Publishing, New Delhi
Bonifacio SK, Moura L, Marzinek J, De-Paula OC (2018) Comparative embryology of Stifftia and Wunderlichia and implication for their evolution in Asteraceae. Bot J Linn Soc 189(2):169–185
Bouman F (1984) The ovule. In: Johri MB (ed) Embryology of the Angiosperms. Springer-Verlag, New York
Bremer K (1987) Tribal interrelationships of the Asteraceae. Cladistics 3(3):210–253
Bremer K (1994) Asteraceae: cladistics and classification. Timber Press, Portland
Brown J, Caligari P (2008) An introduction to plant breeding. Blackwell Publishing Ltd., Ames/Oxford
Cabrera AL (1963) Flora de la Provincia de Buenos Aires. Compuestas, Colección Científica del INTA, Buenos Aires, Argentina
Chehregani A, Hajisadeghian S (2014) Microsporogenesis, megasporogenesis and gametophyte development in Senecio glaucus L. Thaiszia J Bot 24(2):89–100
Chehregani A, Mohsenzadeh F, Ghanad M (2011) Male and female gametophyte development in Cichorium intybus. Int J Agric Biol 13:603–606
Davis GL (1966) Systematic embryology of the angiosperms. John Wiley & Sons, New York
Dematteis M (1996) Estudios cromosómicos en especies argentinas de Vernonia (Asteraceae). Bonplandia 9:103–110
Dematteis M (1997a) Cromosomas en Vernonia platensis y especies afines (Asteraceae). Bonplandia 9(3–4):259–264
Dematteis M (1997b) Números cromosómicos y cariotipos de algunas especies de Vernonia (Asteraceae). Bol Soc Argent Bot 33(1–2):85–90
Dematteis M (2002) Cytotaxonomic analysis of South American species of Vernonia (Vernonieae: Asteraceae). Bot J Linn Soc 139(4):401–408
Dematteis M (2007) Taxonomic notes on the genus Chrysolaena (Vernonieae, Asteraceae), including a new species endemic to Paraguay. Ann Bot Fenn 44(1):56–64
Dematteis M (2009) Revisión taxonómica del género sudamericano Chrysolaena (Vernonieae, Asteraceae). Bol Soc Argent Bot 44:3–7
Dematteis M (2014) Tribu Vernonieae Cass. In: Zuloaga FO, Belgrano MJ, Anton AM (eds) Flora Argentina. Flora vascular de la República Argentina, IBODA-CONICET, Buenos Aires, pp 229–287
Deng Y, Chen S, Teng N, Chen F, Li F, Song A, Guan Z (2010) Flower morphologic anatomy and embryological characteristics in Chrysanthemum multicaule (Asteraceae). Sci Hortic 124:500–505
Echeverría ML, Alonso SI (2012) Crecimiento Inicial bajo cultivo de Chrysolaena flexuosa (Sims) H. Rob., Asteraceae nativa de valor ornamental potencial. Rev Fac Cienc Agrar UNCuyo 44(2):89–98
Echeverría ML, Camadro EL (2020) Morphological and molecular variability of wild diploid and polyploid populations of Chrysolaena flexuosa (Sims) H. Rob.: relevance for ornamental breeding. Sci Hortic 260:108875
Echeverria ML, Camadro EL (2017) Número de cromosomas, anomalías meióticas y formación de polen 2n en accesiones de la especie silvestre Chrysolaena flexuosa (Vernonieae, Compositae) de su rango de distribución en Argentina. Bol Soc Argent Bot 52(4):737–752
Endress PK (2011) Angiosperm ovules: diversity, development, evolution. Ann Bot 107(9):1465–1489
Erbar C, Leins P (1995) Portioned pollen release and the syndromes of secondary pollen presentation in the Campanulales-Asterales-complex. Flora 190(4):323–338
Franca RO, De-Paula OC, Carmo-Oliveira R, Marzinek J (2015) Embryology of Ageratum conyzoides L. and A. fastigiatum R. M. King & H. Rob. (Asteraceae). Acta Bot Bras 29:8–15
Funk VA, Susanna A, Stuessy T, Robinson H (2009) Classification of Compositae. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds) Systematics, evolution, and biogeography of Compositae. International Association for Plant Taxonomy, Vienna, pp 171–189
González AM, Cristóbal CL (1997) Anatomía y ontogenia de semillas de Helicteres lhotzkyana (Sterculiaceae). Bonplandia 9(3–4):287–294
Gotelli M, Galati B, Medan D (2008) Embryology of Helianthus annus (Asteraceae). Ann Bot Fenn 45:81–96
Howell GJ, Slater AT, Knox RB (1993) Secondary pollen presentation and its biological significance. Aust J Bot 41:417–438
Jana BK, Mukherjee SK (2015) Stylopodial diversity of some species of Asteraceae with the help of SEM. J Econ Taxon Bot 39(1):138–146
Jiana L, Nianjun T, Fadi C, Sumei C, Chunqing S, Weimin F (2009) Reproductive characteristics of Opisthopappus taihangensis (Ling) Shih, an endangered Asteraceae species endemic to China. Scient Hortic 121:474–479
Johri BM, Ambegaokar B, Srivastava PS (1992) Comparative embryology of angiosperms, vol 2. Springer-Verlag, Berlin
Leins P, Erbar C (1990) On the mechanisms of secondary pollen presentation in the Campanulales-Asterales-complex. Bot Acta 103(1):87–92
Lersten NR (2008) Flowering plant embryology: with emphasis on economic species. Blackwell Publishing, USA
Luque R, Sousa HC, Kraus JE (1996) Métodos de coloracao de Roeser (1972) –modificado- E. Krop, 1972 visando a susticao do Azul do Astra por Azul de Alciao 8GS ou 8GX. Acta Bot Bras 10(2):199–212
Marques D, Oliveira Franca R, Angulo M, Via Do Pico G, Dematteis M, Marzinek J (2020) Comparative anatomy of the cypselae in the complex group Chrysolaena, Echinocoryne, Lepidaploa and Lessingianthus: contributions to the systematics of Vernonieae (Compositae). Syst Bot 45(3):668–680
Mazzoni A, Mora J, Segui M, Oliva G, Kofalt R (2006) Prueba piloto comercial de Senecio candidans, especie ornamental nativa de la Patagonia Sur Argentina. Actas del III Congreso Argentino de Floricultura y VIII Jornadas Nacionales de Floricultura. INTA, Buenos Aires, pp 85–88
Negrín AL, Zalba SM (2012) Descripción de la cipsela y de la plántula de Grindelia ventanensis (Asteraceae), especie endémica con potencial ornamental. Rev Fac Cienc Agrar UNCuyo 44(1):13–25
Oliveira VM, Semir J, Forni-Martins ER (2012) Chromosome numbers and karyotypes of species of Vernonia sect. Lepidaploa (Asteraceae: Vernonieae). Folia Geobot 47:93–103
Pacini E (1997) Tapetum character states: analytical keys for tapetum types and activities. Canad J Bot 75(9):1448–1459
Papini A, Mosti S, Brighigna L (1999) Programmed-cell-death events during tapetum development of angiosperms. Protoplasma 207(3):213–222
Pérez YJ, Angulo MB, Honfi A, Dematteis M (2021) Embryology and fertility of the natural tetraploid Lessingianthus plantaginoides (Asteraceae, Vernonieae): taxonomic implications. Rodriguésia 72:e01322019
Pérez YJ, González AM, Angulo MB (2023) Male gametophyte development and cytogenetics of natural diploid and polyploid species of the South American genus Lessingianthus (Asteraceae, Vernonieae). Darwiniana, 11(1):71–91
Priyadarshan PM (2019) Plant breeding: classical to modern. Springer Nature Singapore Ptd Ltd, p 570
Pullaiah T (1978) Studies in the embryology of Compositae III. The Tribe Astereae Bot Mag (tokyo) 91:197–205
Pullaiah T (1979a) Embryology of Adenostemma, Elephantopus and Vernonia (Compositae). Bot Not 132:51–53
Pullaiah T (1979b) Studies in the embryology of Compositae IV The Tribe Inuleae. Am J Bot 66:1119–1127
Pullaiah T (1981) Studies in the embryology of Heliantheae (Compositae). Plant Syst Evol 137:203–214
Pullaiah T (1983) Studies in the embryology of Senecioneae (Compositae). Plant Syst Evol 142:61–70
Robinson H (2007) Tribe Vernonieae. In: Kadereit J, Jeffrey C (eds) The families and genera of vascular plants, vol 8. Asterales, Springer, Berlin, pp 165–192
Sattler MC, Carvalho CR, Clarindo WR (2016) The polyploidy and its key role in plant breeding. Planta 243:281–296
Singh DP, Singh AK, Singh A (2021) Plant breeding and cultivar development. Academic Press
Stebbins GL (1974) Flowering plants: evolution above the species level. Harvard University Press
Stoskopf NC, Tomes DT, Christie BR (2019) Plant breeding: theory and practice. CRC Press
Tiagi B, Taimni S (1960) Embryo sac development in Vernonia cinerascens and seed development in V. cinerea. Curr Sci 29:406
Tiagi B, Taimni S (1963) Floral morphology and embryology of Vernonia cineresens Schult. and V. cinerea Less. Agra University Journal of Research 12:123–138
Tobe H, Morin NR (1996) Embryology and circumscription of Campanulaceae and Campanulales: a review of literature. J Plant Res 109:425–435
Udall JA, Wendel JF (2006) Polyploidy and crop improvement. Crop Sci 46:S-3
Via Do Pico GM, Dematteis M (2012) Chromosome number, meiotic behavior and pollen fertility of six species of Chrysolaena (Vernonieae, Asteraceae). Caryologia 65:176–181
Via Do Pico GM, Dematteis M (2013) Taxonomic implications from the pollen morphology in the genus Chrysolaena (Vernonieae, Asteraceae). Palynology 37:177–188
Via Do Pico GM, Dematteis M (2017) Meiotic behaviour and B chromosomes in Argentine populations of Chrysolaena verbascifolia (Vernonieae: Asteraceae). Webbia 72(1):139–147
Via Do Pico GM, Vega A, Dematteis M (2016) Systematic consideration of floral microcharacters of the South American genus Chrysolaena (Vernonieae, Asteraceae). System Biodivers 14(2):224–243
Via Do Pico GM, Pérez YJ, Angulo MB, Dematteis M (2019) Cytotaxonomy and geographic distribution of cytotypes of species of the South American genus Chrysolaena (Vernonieae, Asteraceae). J Syst Evol 57(5):451–467
Via Do Pico GM, Dematteis M (2014) Cytotaxonomy of two species of genus Chrysolaena H. Robinson (Vernonieae, Asteraceae) from Northeast Paraguay. Comp Cytogenet 8(2):125–137
Via Do Pico GM (2015) Estudios biosistemáticos en especies del género Chrysolaena (Vernonieae, Asteraceae). Tesis Doctoral, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Argentina
Willemse MT, Van Went JL (1984) The female gametophyte. In: Johri MB (ed) Embryology of Angiosperms. Springer, Berlin, Heidelberg, pp 159–196
Yurukova-Grancharova PD (2004) On the embryology of Leontodon autumnalis (Asteraceae). Phytol Balc 10:85–91
Yurukova-Grancharova P, Dimitrova D (2006) Cytoembryological study of Crepis bithynica (Asteraceae) from Bulgaria. Flora Mediterr 16:33–43
Acknowledgements
We would like to thank Rosemary Scoffield for critically reading the English manuscript. The author is also very grateful for the doctoral fellowship awarded by CONICET.
Funding
This research has been supported by the Secretaría General de Ciencia y Técnica de la Universidad Nacional del Nordeste (PI No. 19-P005) and the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP No. 112–2020-0102838).
Author information
Authors and Affiliations
Contributions
YJP collected the plant material; performed the histological preparations; analyzed, interpreted, and photographed the results to prepare the figures; and finally wrote the initial version of the manuscript. GVP supplied the taxonomic data of the analyzed species and reviewed and collaborated in the drafting of the manuscript. AMG helped to interpret the results and reviewed and contributed to the discussions. MBA reviewed the manuscript, collaborated to interpret the results, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Dorota Kwiatkowska
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pérez, Y.d., Via Do Pico, G., González, A.M. et al. Exploring floral morphoanatomy and embryology in wild populations of Chrysolaena flexuosa (Vernonia, Asteraceae): a contribution to understanding its ornamental potential. Protoplasma 261, 831–845 (2024). https://doi.org/10.1007/s00709-024-01937-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-024-01937-y