Abstract
Unusual nectaries were anatomically described as being usual traits for Gentianaceae. They are small, avascularized, and formed by 3 to 5 rosette cells with labyrinthine walls around one central cell. Such as nectaries have been reported for stems, petals, and sepals of different species of the family, however, there is no information on the mechanisms involved with the synthesis and release of secretion. Thus, this work aimed to unravel the mechanism of secretion and exudation of nectar for these curious nectaries using Calolisianthus speciosus as a model. Samples were processed according to standard methods for light and electron microscopy. Leaf and sepal nectaries were described, as were those of the apex of petals where ants were observed patrolling a darkened region. The enzymatic method was used for the detection of sugars, proteins, and amino acids in leaf and sepal exudates. The nectaries of petals of C. speciosus are similar to those of its leaves, sepals, and stem, although their activities are asynchronous. Polysaccharides were detected on the labyrinthine walls of rosette cells and protein in the opposite region of the cytoplasm. Labyrinthine walls increase the contact surface between rosette cells and the central cell, allowing for the transfer of secretion. After accumulation, the secretion is released to the subcuticular space of the central cell through disruption of the cuticle. The secretion and exudation of nectar were elucidated and involve distinct organelles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nectaries are considered useful structures for Gentianaceae taxonomy (Delgado et al. 2011b; Guimarães et al. 2013; Dalvi et al. 2013, 2017) and have been anatomically described for the leaves of 33 species (Delgado et al. 2011a, b; Dalvi et al. 2013, 2014; Guimarães et al. 2013) and stems of 25 species (Dalvi et al. 2014, 2017; Guimarães et al. 2013). The presence of glands visible to the naked eye in the calyx has been cited as a common trait of Gentianaceae and considered taxonomically relevant (Struwe and Albert 2002; Dalvi et al. 2020). However, such structures have been anatomically described for only Irlbachia Mart. (Vogel 1998), Calolisianthus pedunculatus (Cham. & Schltdl) Gilg. (Dalvi et al. 2020), and Chelonanthus viridiflorus (Mart.) Gilg (El Ajouz et al. 2022). The presence of glands on the external surface of the calyx was mentioned for Hockinia montana Gardner (monospecific genus) (Gilg 1895) yet only nectaries dispersed throughout the leaf and on the stem surface have been anatomically described (Dalvi et al. 2013, 2014). Glands present on the external surface of the calyx of C. pedunculatus and C. viridiflorus have been classified as extrafloral nectaries (Dalvi et al. 2020; El Ajouz et al. 2022), although these nectaries are on the floral part. In addition, the position of nectaries on the leaf blade can vary and has been considered a taxonomically useful feature for Gentianaceae (Delgado et al. 2011a, b; Dalvi et al. 2013).
Despite morphoanatomical variation, nectaries are usually macroscopic structures that are mostly vascularized by phloem, and the nectar they produce is exuded via nectarostomata (stomata that lost the ability to close the ostiole) or by partial or total rupture of the cuticle from epidermal secretory cells (Pacini and Nepi 2007). The mechanism of nectar exudation in epidermis devoid of stomata has been an issue of extensive debate (Gaffal 2012), with a new recently proposed model to explain this mechanism (Paiva 2017).
The nectaries described for Gentianaceae were considered unusual (Delgado et al. 2011a, b; Dalvi et al. 2013, 2014, 2017, 2020; El Ajouz et al. 2022). They are very tiny and avascularized structures, being formed by only 3 to 5 cells with labyrinthine walls and are arranged in a rosette around one central region. This central region was first described as a central channel with a pore (Delgado et al. 2011a, b), but later was observed to contain one cell — the central cell (Dalvi et al. 2013, 2014, 2017, 2020; El Ajouz et al. 2022). Nonetheless, the mechanism of secretion of this unique structure, the role of labyrinthine walls of the rosette cells, and the involvement of the central cell in the exudation of nectar have not been clarified.
Floral and extrafloral nectaries secrete nectar that is involved in the interaction between plants and animals (Rudgers 2004; Oliveira and Freitas 2004; Nicolson 2007; Do Nascimento and Del-Claro 2010), acting as a resource for pollinators and/or defensive insects, mainly ants (Torezan-Silingard 2012; Del-Claro et al. 2016). Nectar composition can vary according to the location and function of the nectary (Cruden et al. 1983), containing mainly glucose, fructose, and sucrose in different proportions (Fahn 1979b; Nicolson and Thornburg 2007; Nepi 2017). There is also a direct relationship between nectar composition and visitation events (Baker and Baker 1977; Cruden et al. 1983; Nicolson 2007). Thus, chemical characterization of secretion provides important data for ecological studies that aim to evaluate the interaction between plants and visiting animals (pollinators or defenders). Chemical characterization is also important for distinguishing secretory structures that occur in the same region, such as the presence of colleters and nectaries on the leaf blade, as shown for Euphorbiaceae by Feio et al. (2016) and Meira et al. (2020). Since nectar is a costly energy resource for plant metabolism, asynchrony among nectaries can promote the presence of nectar for longer periods at a lower cost (Cardoso-Gustavson et al. 2013).
Nectaries in Calolisianthus speciosus (Cham. & Schltdl.) Gilg were described as clusters of structures on leaves and sepals (Delgado et al. 2011a, b), as well as on the surface of the stem (Dalvi et al. 2017). Delgado (2008) described the glands on the surface of the leaf and calyx of C. speciosus as being composed of secretory units, denominated nectarioles, consisting of 3–8 cells disposed in a rosette with a mid-channel. Field observations recorded ants patrolling the apices of pre-anthesis and anthesis flower petals of C. speciosus, suggesting the existence of secretory structure(s) in this region (El Ajouz et al. 2022).
The present work aimed to describe the ontogeny of the nectaries of C. speciosus, and determine the type of secretory structure(s) present in the apices of the petals and whether there is asynchrony in the secretory phase of the nectaries. In addition, this work aimed to chemically describe the exudate nectar and evaluate the relationship between nectar composition and visitors. Ultrastructural analyses are used to unravel the mechanism of secretion and exudation of nectar, as well as to identify the cell organelles involved, correlating the function of the labyrinthine walls of the rosette cells and the involvement of the central cell in the transport and exudation of nectar.
Material and methods
Sampling and collection
Leaf samples were collected from the third node towards the apex and sepal and petal samples from floral buds and expanded flowers of Calolisianthus speciosus in Serra do Ouro Branco, municipality of Ouro Branco, state of Minas Gerais, Brazil. The area in Serra de Ouro Branco is part of a 7520-hectare state park environmental protection area, where rocky field vegetation predominates (IEF 2022).
Field expeditions were carried out in both the dry period (from May to October) and the rainy period (from November to April). Exudate from sepals and leaves of C. speciosus was collected in the field for chemical analysis. Ants seen feeding on exudate were photographed with a Nikon model D7000 camera with a resolution of 16.2 megapixels. No exudation was observed in petals during field expeditions, despite ants being seen patrolling the apex. Voucher material was deposited in the herbarium of Universidade Federal de Viçosa under the number VIC 49,368.
Light microscopy
For anatomical and histochemical analyses, samples of fully expanded leaves, sepals, and petals were fixed in neutral buffered formalin (Lillie 1965) and conserved in 70% ethanol (Jensen 1962). Samples of these materials were transversely sectioned using a table microtome (model LPC; Rolemberg & Bhering Trade and Bhering LTDA, Belo Horizonte, Brazil) and the sections subjected to tests for the detection of phenolic compounds with ferric trichloride (Johansen 1940); for lipids compounds with both Sudan Black B and Sudan IV (Pearse 1951, 1980). The sections were mounted on slides with glycerinated gelatin. Fresh samples were also sectioned, and the sections submitted to vanillin–hydrochloric acid for tannins (Mace and Howell 1974). Respective controls were processed simultaneously for the histochemical tests using standard procedures. Samples not subjected to reagents (white) were also analyzed.
For structural characterization, part of the material was dehydrated in an ethanol series and embedded in methacrylate (Historesina Leica Microsystems Nussloch GmbH, Heidelberg, Germany). Cross and longitudinal sections of 5 µm thickness were obtained using an automatic rotary microtome (model RM2155, Leica Microsystems Inc., Deerfield, USA). The sections were stained with toluidine blue at pH 4.7 (O’Brien et al. 1964). Some of these sections were subjected to the following histochemical tests: xylidine Ponceau (O’Brien and McCully 1981) and coomassie blue (Fisher 2004) for total proteins; periodic acid — Schiff (PAS) (O’Brien and McCully 1981) for total polysaccharides; ruthenium red for pectins and mucilage (Johansen 1940); Lugol reagent (IKI) (Pearse 1972) for starch. Slides were mounted with synthetic resin (Permount, Fisher Scientific, Fair Lawn, NJ).
Observations and photographic documentation were performed using a light microscope (AX70TRF, Olympus Optical, Japan) equipped with an image capture system (Ax Cam, Zeiss, Germany). Diagrams were drawn based on pictures of anatomical sections.
Electron microscopy
For scanning electron microscopy (SEM), samples from secretory regions of fully expanded sepals and petals of C. speciosus were fixed in FAA (formalin, glacial acetic acid, 50% ethanol at 1:1:18), dehydrated in an ethanol series, and subjected to critical point drying using CO2 (model CPD 030 Bal-Tec, Balzers, Liechtenstein). The materials were then mounted on stubs with double-sided adhesive tape and sputter-coated with gold (model SCA 010, Bal-Tec, Balzers, Liechtenstein). Observations and photographic recordings were performed using a scanning electron microscope (model LEO 1430 VP-Zeiss, Cambridge, England).
For transmission electron microscopy (TEM), samples of secretory regions of the sepals of C. speciosus were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.2 (10–12 h), post-fixed in 1% osmium tetroxide for 2 h, dehydrated in an ethanol series, and embedded in LR White acrylic resin (Sigma Aldrich). Sections of 50–60-nm thickness were obtained using an ultramicrotome (Leica EM UC6, Vienna, Austria). These sections were collected on copper grids and contrasted with 5% uranyl acetate aqueous solution and lead citrate (Reynolds 1963). Observations and images were obtained using a Zeiss EM 109 transmission electron microscope (Carl Zeiss Microscopy, Jena, Germany) at 80 kV at NMM/UFV.
Chemical analysis of nectar
Exudate from sepals and leaves of C. speciosus was collected in microtubes, frozen in liquid nitrogen, and kept in an ultra-freezer at − 80 °C until analysis. The sepal exudate was collected in April, the month of high flowering, and the leaf exudate in November, when the exudate in this region is more intense. Sugars (glucose, fructose, and sucrose), proteins, and amino acids were chemically evaluated. Five microliters of secretion were transferred to a microtube containing 5 µL of 70% ethanol. A 5-μL aliquot of this volume was taken for reaction in medium containing NAD + , ATP, imidazole buffer, and glucose-6-phosphate dehydrogenase. The concentrations of hexoses (glucose, fructose, and sucrose), total amino acids, and proteins (Bradford method) were quantified in three steps by adding the enzymes hexokinase, phosphoglucoisomerase, and finally invertase, with the reduction of NAD + to NADH being quantified (Praxedes et al. 2006).
Results
Nectary visitors and exudate appearance
In C. speciosus, clusters of microscopic nectaries form structures visible to the naked eye on the external surface of the calyx (Fig. 1A−B), petal apices (Fig. 1A, C), leaf blade base, and leaf blade apex (Fig. 1D). These regions were visited by ants that feed on the accumulated droplets which had aggressive and ongoing behavior during the dry and the rainy periods. The activity of floral and extrafloral nectaries is asynchronous. During the floral phenophase, there was greater visitation to the sepals and petals, where nectar drops were more voluminous, than to the leaves. During the vegetative phenophase, exudation from leaf nectaries was abundant, especially for young leaves.
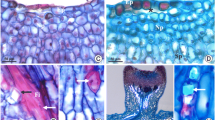
Leaf and flower nectaries of Calolisianthus speciosus. A. Field specimen and details of the clustering of nectaries of sepals (B), petals (C), and leaves (D). Anatomy of the sepal nectaries in frontal view (staining with toluidine blue). Note the more intense staining in the region of the labyrinthine walls (asterisk) and the central cell (arrowhead). F. Sepal nectary in longitudinal section. n, nucleus; np, nectariferous parenchyma. Bars = A, C–D: 1 cm; B: 0.5 cm; E–F: 30 μm
Sepal, petal, and leaf nectaries and exudate composition
Nectaries observed on sepals, petals, and leaves of C. speciosus have a diameter approximately of 50 μm, and they are made up of 3 to 5 cells with prominent nuclei and invaginated walls — labyrinthine walls — around a central cell, forming a rosette configuration (Fig. 1E) and the glandular tissue are non-vascularized (Fig. 1F).
Nectary origin is common for all organs since they are formed by protodermal activity (Fig. 2). The initial stage is marked by a set of cells that become radiated, which have prominent nuclei, dense cytoplasm, and thin, pectocellulosic walls (Fig. 2A). Three to four cells begin to arrange themselves in a radiated way with their nuclei facing centrally. The central portion contains one tiny cell with dense cytoplasm and no labyrinthine walls (Fig. 2B). In the next stage, the externally located cells become larger than the central cell and, after the displacement of their nuclei to the opposite pole, each forms a labyrinthine wall in the portion facing the central cell (Fig. 2C). In the last stage, the external, or rosette, cells become even larger, with most of their cytoplasm volume being occupied by their labyrinthine wall while their nucleus settles at a position opposite to the central cell (Fig. 2D). The cell walls of the central cell are thicker than those of the cell walls of the rosette cells (Fig. 2D).
Illustration of the ontogeny of floral and extrafloral nectaries of Calolisianthus speciosus. A. Common epidermal cells. B. Arrangement of radiating secretory cells delimiting the central cell (arrowhead). Note the nuclei of the rosette cells facing the central cell. C. Beginning of the development of labyrinth wall and the shift of the nucleus to the opposite pole, note the relatively diminutive size of the central cell compared to the rosette cells. D. Final stage of nectary differentiation with rosette cells larger than the central cell, labyrinthine walls occupying much of the rosette cells and numerous vacuoles. ec, epidermal cell; lw, labyrinthine wall; n, nucleus; rc, rosette cell; v, vacuole. Bars = 30 μm
Floral (sepals and petals) and extrafloral (leaves) nectaries reacted similarly to the histochemical tests (Table 1). The only exception was a positive test for starch in the rosette cells and the central cell only of leaf nectaries (Fig. 3A). The test for polysaccharides (PAS) showed a strong reaction in the cytoplasm, especially where the labyrinthine wall is arranged (Fig. 3B). Pectins/mucilage were detected in a manner similar to that for polysaccharides (Fig. 3C). The most intense reaction for proteins was in the cytoplasmic region opposite to the labyrinthine wall (Fig. 3D).
Positive results (asterisk) of histochemical tests performed on sepal nectaries of Calolisianthus speciosus. A. Presence of starch in both rosette cells and central cell evidenced by Lugol reagent. B. Strong PAS reaction in the region of labyrinthine wall (asterisk). C. Pectins stained pink in the region of labyrinthine wall (asterisk), especially in the region facing the cytoplasm, evidenced by ruthenium red reagent. D. Cytoplasmic content rich in proteins (asterisk) evidenced by xylidine Ponceau reagent. cc, central cell; ec, epidermal cell; lw, labyrinthine wall; n, nuclei; rc, rosette cell; s, starch. Bars = 30 μm
Ultrastructural complexity of the nectary
The largest rosette cells are in direct contact with the tiny central cell (Fig. 4A). The walls of the rosette cells that have contact with the central cell are labyrinthine; the walls between rosette cells are thin, with evident middle lamella and some plasmodesmata (Fig. 4B, C). Mitochondria and granular endoplasmic reticulum (GER) accompany the ingrowths of the labyrinthine wall (Fig. 4D). The nucleus of each rosette cell is in the region opposite the labyrinth wall and shows decondensed chromatin (Fig. 4E). Many small vacuoles are present in the cytoplasm and are concentrated close to the adjacent walls of rosette cells (Fig. 4B, E).
Anatomy and ultrastructure of calyx nectaries of Calolisianthus speciosus. A. Radiated arrangement of rosette cells with labyrinthine walls around the central cell, with the accumulation of nectar (asterisk). B. Rosette cells with numerous vacuoles and thin primary walls communicating by plasmodesmata (C). D. Detail of a rosette cell showing numerous mitochondria and GER accompanying the labyrinthine wall. E. Overview of rosette cell showing numerous vacuoles in parietal position and the nucleus surrounded by labyrinthine wall. F. Longitudinal section of nectary with nectar exudation evidenced by the arrowhead (toluidine blue staining). G. Secretion (asterisk) directed by the labyrinthine wall to cross the cell wall towards the central cell. cc, central cell; cw, cell wall; ger, granular endoplasmic reticulum; gm, granular material; lw, labyrinthine wall; m, mitochondria; n, nuclei; v, vacuole. Bars = A: 30 μm; B, D–E: 5 μm; C: 500 nm
The central cell possesses a large vacuole containing granular material (Fig. 4A, F), causing the other cytoplasmic organelles and the nucleus to occupy a parietal position (Fig. 4E, F). The wall of the central cell is thick and exhibits a striated appearance by the loosening of structural features not observed in the rosette cells (Fig. 4G). Secretion is directed to the labyrinthine walls of rosette cells to cross the wall of the central cell (Fig. 4G).
The mechanism of nectar secretion and exudation
Different compartments are involved in the secretion process of nectaries of C. speciosus (Fig. 5). Mitochondria (Fig. 5A, B, C, D), free ribosomes (Fig. 5C, D), GER (Fig. 5C, D), and dictyosomes (Fig. 5D, F) are present in the cytoplasm of rosette cells and are involved with the synthesis of polysaccharides and proteins (as histochemically detected), packaging (Fig. 5F), and the granulocrine pathway (Fig. 5E) of exocytosis of nectar from rosette cells to the central cell (Fig. 5C, E).
Ultrastructure of calyx nectaries of Calolisianthus speciosus evidencing the cytoplasm of rosette cells. A. Rosette cells in contact with parenchymal vacuolated cells. B. Region of the nectary where the labyrinth wall cannot yet be seen but the central cell is evident. C–D, F. Rosette cells in high metabolic activity evidenced by associations between mitochondria, a large amount of GER, ribosomes, and dictyosomes with numerous vesicles plus the nucleus with decondensed chromatin. C, E. Cytoplasm of rosette cell rich of GER, free ribosomes, vacuoles, mitochondria, and some plastids. cc, central cell; cw, cell wall; d, dictyosome; ger, granular endoplasmic reticulum; m, mitochondria; ml, middle lamella; n, nuclei; r, ribosomes; p, plastid; v, vacuole; vs, vesicles. Bars: A–C, E = 2 μm, D = 1 μm, F = 500 nm
As shown in Fig. 6, pre-nectar is derived from photoassimilates, water, and mineral salts that are transported by xylem and phloem. As the nectary is non-vascularized, these components are transported by both symplastic and apoplastic pathways, cell by cell, to rosette cells. After entering the cytoplasm of rosette cells, the pre-nectar is modified into nectar, after which it is transferred to the central cell via labyrinthine walls. The ingrowing of the labyrinthine wall increases the surface area in contact with cytoplasm, allowing the transportation of a large amount of nectar to the central cell. Once in the central cell, the secretion accumulated in vacuoles presses the cytoplasm against the cell wall.
Model proposed for understanding the routes of photoassimilates from parenchyma cells to rosette cells and nectar exudation in the nectaries of leaves, sepals, and petals of Calolisianthus speciosus. Pre-nectar from the parenchyma is transferred to rosette cells via apoplast (blue arrow) and symplast (red arrow), where it will be converted into nectar. The nectar is then conducted through the labyrinthine wall to the central cell (green arrow). The accumulation of secretion in the subcuticular space of the central cell causes the cuticle to rupture and subsequently release nectar into the external environment (orange arrow)
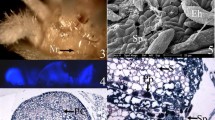
Nectaries occur in small depressions on the surface (Fig. 7A). The cuticle on the external wall of the central cell becomes separated from the wall (Fig. 7B−F) and, in some regions of the cuticle, a pore (Fig. 7B, E) and/or slit can be seen (Fig. 7C, D). In some regions, overlapping cuticular layers are evident (Fig. 7F). Fungal hyphae were common on the nectaries of both older leaves and older sepals (Fig. 7A, B).
Nectaries of sepals (A, C, E) and petals (B, D, F) of Calolisianthus speciosus visualized by scanning electron microscopy (SEM) showing different stages of nectar exudation. A. Clusters of nectaries in depressions on the external surface of the calyx. After the secretory phase, the nectaries are blocked by hyphae. B. Accumulation of secretion is evidenced by the distended cuticle. C–D. Cuticle ruptured after the accumulation of secretion in the subcuticular space, allowing secretion to be released to outside of the organ. E. Secretion exudation (white arrow). F. Cuticle ruptured during the release of accumulated nectar. bc, broken cuticle; dc, distended cuticle; ec, epidermal cell; h, hyphae; n, nectary; ns, nectar secretion; pc, peripheral cell. Bars = A–B: 20 μm, C–F: 10 μm
Chemical composition of the nectar
Droplets exuded by both sepal and leaf nectaries of C. speciosus are colorless and translucent. However, discrepant variations in sugar concentration were detected by the chemical analyses (Table 2). The overall concentration of sugars in the secretion of sepals is 25 times higher than that of the secretion of leaves (Table 2). This difference may be correlated with the higher viscosity of the secretion produced by sepals compared to the secretion produced by leaves (more liquid), as it was noticed in the field. Glucose was not detected in leaf exudate, but was in sepal exudate, with 100.53 g/L of glucose, the highest concentration among all the sugars analyzed. Fructose concentration was 1.39 times higher in sepal compared to leaf exudate, and sucrose was much higher in sepal than in leaf exudate. Although proteins were not detected in any exudate, equivalent concentrations of amino acids were present in both sepal and leaf exudates, being just 1.10 times higher in the former (Table 2).
Discussion
The nectaries present in the leaves, sepals, and petals of Calolisianthus speciosus are anatomically similar and possess a general structure previously described for other species of Gentianaceae (Vogel 1998; Delgado et al. 2011a, b; Dalvi et al. 2013, 2014, 2017, 2020). Dejean et al. (2011) observed such structures on the petal apex and calyx exterior of Chelonanthus alatus (Aubl.) Pulle (Gentianaceae), and classified them as stomatal pores through which nectariferous secretion is exuded. However, the observations of the present study indicate that this classification seems to be improper. From the characteristics observed to be in common between Chelonanthus alatus and Calolisianthus speciosus, it is possible to infer, as is widely observed in Gentianaceae, that these structures of C. alatus are stomata-free nectaries.
The anatomical studies of the nectaries of C. speciosus presented here reveal that the walls of the central cell are twice as thick as those of the rosette cells. Labyrinthine walls function to increase the absorption area and more efficiently promote a series of intense short-distance transports (Gunning and Pate 1969). This thinner wall of rosette cells may favor the transport of material to be exuded. In general, the plasma membrane follows the arrangement of the labyrinthine walls.
Chemical analysis of exudate collected on sepals and leaves of studied species demonstrated the presence of sugars and amino acids in varying concentrations. Glucose was not detected in the nectar secreted on leaves, while fructose and sucrose showed concentrations of 4.81 and 0.02 μM, respectively. Delgado et al. (2011a) also chemically analyzed nectar exuded from the leaves of C. speciosus and found the concentrations of glucose, fructose, and sucrose to be 3.34, 3.38, and 1.04 μM, respectively. The different composition of sugars found in the nectar produced by foliar nectaries of this species may be due to the difference in collection procedure, as well as to environmental factors, since both studies collected nectar in November. In the present work, drops of nectar were collected from directly on the leaf and immediately transferred to microtubes, which were stored in a thermal container with liquid nitrogen. This procedure prevents the breakdown of sucrose into glucose and fructose (Vollhardt and Schore, 2013). The drops collected by Delgado et al. (2011a) were subjected to dilution to proceed with the analysis and samples were not frozen immediately after collection, which may have caused a breakdown of sucrose. The high dilution in water may have also influenced the results. Thus, these procedural differences may explain the presence of glucose in the analyses of Delgado et al. (2011a) and its non-detection in the present study for nectar secreted by leaves at the same time of the year. In the sepal nectaries, glucose, fructose, and sucrose were detected at concentrations of 100.53, 6.73, and 16.38 μM, respectively, a higher concentration compared to the nectar collected in the leaf nectaries. The composition of nectar is highly variable among species, in addition to being influenced by several environmental factors (Nicolson 2007; Nicolson and Thomburg 2007). Petal nectaries of C. speciosus of the present study accumulated insufficient nectar for collection.
The secretory activity of the nectaries occurs at an early stage, which is easily observed in the field by the presence of ants visiting floral buds to open flowers. Analysis of anatomical development revealed that still undifferentiated sepals have fully differentiated and functional nectaries. The activity of nectaries in attracting ants fits the hypothesis proposed by Del-Claro et al. (2016), whereby the plant responds to ecological pressure exerted by herbivores in a chemically defensive manner, secreting, through extrafloral nectaries, food that will attract predators, such as ants, spiders, or wasps. For C. speciosus, this hypothesis extends to floral nectaries located in sepals and petals, which perform the same function as leaf nectaries, attracting ants that forage uninterruptedly during flowering. Likewise, during the vegetative period, leaf nectaries are visited continuously.
The secretory phase is asynchronous, with the extrafloral nectaries being more active during the vegetative phenophase, when there is no demand for photoassimilates to be destined for fruit production at a high source-to-sink ratio. On the other hand, from the beginning of flowering to the complete senescence of the flowers, nectar exudation is visibly greater in sepals and petals in relation to leaves. The increase in nectar production in floral organs attracts more ants, widening the protection of flowers until seed production. Despite the low production of nectar in leaves during the flowering period, protection against herbivores is still provided by ants.
Nectar composition also influences visitation events. Studies suggest further hypotheses about the function of nectar in its different compositions (Heil 2011). However, it is known that secretion composition involves two main factors, namely, the mutualistic attraction or the repellence of nectar robbers (Torezan-Silingard 2012). The diet of nectar consumers varies, and it is the composition of the secretion that determines the type of consumer (Nicolson 2007; Nepi et al. 2012), with carbohydrates and amino acids contained in nectar being most important in the attraction function. Although some ant species lack invertase, the sucrose-cleaving enzyme, thus opting for nectar without sucrose (Heil et al. 2005; Martínez del Rio 1990), nectariferous ants generally prefer nectar with considerable amounts of sugars and the presence of amino acids (Nicolson et al. 2007). However, according to Pacini et al. (2003), more watery nectar is an important resource for visitors, such as ants, as in the leaf nectaries of C. speciosus, which secrete nectar with lower concentrations of sugars. The nectar exuded by the floral nectaries of the species in the floral phenophase has a 25-times higher concentration of sugars than the nectar they exude in the vegetative phenophase.
It is uncommon to observe floral nectaries not involved in the pollination function. The present study highlights these characteristics attributed to the floral nectaries present in sepals, which present the same defense function offered by ants that visit the extrafloral nectaries in leaves. It was not possible to chemically analyze the nectar secreted in petals. Observations of ants having the same behavior in leaf, sepal, and petal nectaries indicate that the nectaries present in the three organs have the same function in attracting ants that protect the plant from possible attacks by herbivores. Prevention of microorganism attack is related to the presence of secondary compounds and antimicrobial proteins (Heil 2011), although fungal spores still form hyphae.
Histochemical tests detected the presence of polysaccharides, including mucilage, both in the region of the labyrinthine walls and in the cytoplasm of the rosette cells of the nectaries of the studied species. The more intense reaction for polysaccharides in the region of the labyrinthine walls of the rosette cells was expected since polysaccharides are the main constituent of primary walls (Evert 2013). Mucilage is a polysaccharide compound that has previously been indicated in the composition of nectar and is related to higher viscosity (Santos et al. 2017). Considering the environment of the campos rupestres where the studied plants of C. speciosus were collected, the higher viscosity of the secretion contributes to a decrease in the evaporation of water present in nectar and, thus, makes it accessible to visitors for a longer period of time. The present study detected starch grains only in leaf nectaries in samples collected during the floral phenophase, when leaf exudation was imperceptible and sepal and petal exudation intense. Thus, it is possible that the starch reserve in leaves guarantees the initial re-establishment of secretion in leaves after the senescence of flowers. Reserve starch can be broken down at any time of day, ensuring rapid nectar production (Peng et al. 2004; Nicolson et al. 2007). Proteins, as well as mucilage, can also be nectar constituents (Paiva and Machado 2006; Rocha and Machado 2009).
The strong reaction to proteins in the nectaries of C. speciosus indicates the intense metabolic activity of these cells and corroborates the presence of amino acids detected in the nectar. Experimental work by other researchers has shown that ants prefer nectar with a high concentration of amino acids (Lanza et al. 1995; Wagner and Kay 2002). Ants are common visitors of leaf, sepal, and petal nectaries of C. speciosus (Delgado et al. 2011a) and the existence of amino acids in nectar can act as an attractant for these animals.
Ultrastructural features similar to those observed by us have been reported for nectaries of species belonging to different families (Fahn 1979a, b), such as Leguminosae (Davis et al. 1988) and Sapindaceae (Avalos et al. 2017). Nectar production is influenced by environmental and physiological factors such as insect visitation events and photosynthetic rates (Del-Claro et al. 2016; Pacini et al. 2003). According to Peng et al. (2004), the center of the dynamic transformation of nectar involves a combined complex of amyloplasts and vacuoles, with the disappearance of amyloplasts being observed to the detriment of vacuolar growth in floral nectaries of Cucumis sativus L., as evidenced in the present study, where vacuoles are numerous and full of granular content, and amyloplasts are not evident.
The transfer of nectar components from rosette cells to the central cell through wall invaginations is an unusual mechanism that has been reported for nectar exudation by secretory trichomes (Fahn 1979a). Transfer cells have been mentioned as occurring in secretory structures where active transport occurs, in high amounts, over a short distance, since the invaginations increase the contact surface with the cytoplasm (Nguyen and McCurdy 2017). Thus, nectar that is produced by rosette cells is transferred to the central cell and then exuded from it. Although the ultrastructure of nectaries of C. speciosus has been investigated previously, the role of labyrinthine walls and the existence of a central cell have not been addressed (Delgado et al. 2011a).
In our study, we show that in C. speciosus, the nectar that accumulates in the subcuticular space of the central cell was observed to be exuded by cuticle breakage. This form of secretion and exudation is not common, as in most cases the secretion release route involves modified stomata, palisaded cells, or secretory trichomes (Fahn 1979a; Cruden et al. 1983; Nepi 2007). Although uncommon, this nectar exudation mechanism of the nectaries of C. speciosus is compatible with the model proposed by Paiva (2017) for nectaries devoid of nectarostomata, where the process is cyclic and depends on accumulation in the central cell to enable the elimination process by pressure.
Conclusion
All the studied secretory structures of Calolisianthus speciosus were recognized as nectaries. There is asynchrony of the secretion phases of the species floral and extrafloral nectaries. Evident differences were found in the concentrations of sugars in the nectar of sepals and leaves. Amino acid production in the exudate of leaf and sepal nectaries of C. speciosus was similar, although proteins were not detected in the analyses.
The mechanism of secretion and exudation of nectar by the nectaries of C. speciosus were elucidated, as was the functional role of the central cell of this structure. Floral and extrafloral nectaries of C. speciosus present a curious nectar production and elimination mechanism that involves the granulocrine pathway. Labyrinthine walls increase the contact surface of rosette cells, which are responsible for the transformation of pre-nectar into nectar. The central cell accumulates nectar in the subcuticular space before elimination by cuticle rupture.
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Avalos AA, Lattar EC, Galati BG, Ferrucci MS (2017) Nectary structure and ultrastructure in two floral morphs of Koelreuteria elegans subsp. formosana (Sapindaceae). Flora 226:29–37. https://doi.org/10.1016/j.flora.2016.11.003
Baker HG, Baker I (1977) Intraspecific constancy of floral nectar amino acid complements. Bot Gaz 138:183–191. https://doi.org/10.1086/336914
Cardoso-Gustavson P, Andreazza NL, Sawaya AC, de Moraes CM (2013) Only attract ants? The versatility of petiolar extrafloral nectaries in Passiflora. Am J Plant Sci 4:460–469. https://doi.org/10.4236/ajps.2013.42A059
Cruden RW, Herman SM, Peterson S (1983) Patterns of nectar production and plant pollination coevolution. In: Bentley B, Elias T (eds) The biology of nectaries. Columbia University Press, New York, pp 80–125
Dalvi VC, de Faria GS, Azevedo AA (2020) Calycinal secretory structures in Calolisianthus pedunculatus (Cham. & Schltdl) Gilg (Gentianaceae): anatomy, histochemistry, and functional aspects. Protoplasma 257:275–284. https://doi.org/10.1007/s00709-019-01436-5
Dalvi VC, Meira RMSA, Azevedo AA (2013) Extrafloral nectaries in neotropical Gentianaceae: occurrence, distribution patterns, and anatomical characterization. Am J Bot 100:1779–1789. https://doi.org/10.3732/ajb.1300130
Dalvi VC, Meira RMSA, Azevedo AA (2017) Are stem nectaries common in Gentianaceae Juss.? Acta Botanica Brasilica 31:403–410. https://doi.org/10.1590/0102-33062016abb0404
Dalvi VC, Meira RMSA, Francino DMT, Silva LC, Azevedo AA (2014) Anatomical characteristics as taxonomic tools for the species of Curtia and Hockinia (Saccifolieae–Gentianaceae Juss.). Plant Syst Evol 300:99–112. https://doi.org/10.1007/s00606-013-0863-1
Davis AR, Peterson RL, Shuel RW (1988) Vasculature and ultrastructure of the floral and stipular nectaries of Vicia faba (Leguminosae). Can J Bot 66:1435–1448. https://doi.org/10.1139/b88-198
Dejean A, Corbara B, Leroy C, Delabie JH, Rossi V, Céréghino R (2011) Inherited biotic protection in a Neotropical pioneer plant. PLoS ONE 6:18071. https://doi.org/10.1371/journal.pone.0018071
Del-Claro K, Rico-Gray V, Torezan-Silingardi HM, Alves-Silva E, Fagundes R, Lange D, Rodriguez-Morales D (2016) Loss and gains in ant-plant interactions mediated by extrafloral nectar: fidelity, cheats, and lies. Insectes Soc 63:207–221. https://doi.org/10.1007/s00040-016-0466-2
Delgado MN (2008) Caracterização morfoanatômica de espécies de Gentianaceae ocorrentes em áreas de cerrado e de campo rupestre em Minas Gerais. Dissertation, Universidade Federal de Viçosa
Delgado MN, Azevedo AA, Valente GE, Kasuya MCM (2011a) Comparative anatomy of Calolisianthus species (Gentianaceae-Helieae) from Brazil: taxonomic aspects. Edinb J Bot 68:139–155. https://doi.org/10.1017/S0960428610000284
Delgado MN, Silva LC, Báo SN, Morais HC, Azevedo AA (2011b) Distribution, structural and ecological aspects of the unusual leaf nectaries of Calolisianthus species (Gentianaceae). Flora 206:676–683. https://doi.org/10.1016/j.flora.2010.11.016
Do Nascimento EA, Del-Claro K (2010) Ant visitation to extrafloral nectaries decreases herbivory and increases fruit set in Chamaecrista debilis (Fabaceae) in a Neotropical savanna. Flora 205:754–756. https://doi.org/10.1016/j.flora.2009.12.040
El Ajouz B, Valentin-Silva A, Francino DMT, Dalvi VC (2022) A flower with several secretions: anatomy, secretion composition, and functional aspects of the floral secretory structures of Chelonanthus viridiflorus (Helieae—Gentianaceae). Protoplasma 259:427–437. https://doi.org/10.1007/s00709-021-01652-y
Evert RF (2013) Anatomia das plantas de Esau: meristemas, células e tecidos do corpo da planta: sua estrutura, função e desenvolvimento. Blucher, São Paulo
Fahn A (1979a) Secretory tissues in plants. Academic Press, New York
Fahn A (1979b) Ultrastructure of nectaries in relation to nectar secretion. Am J Bot 66:977–985. https://doi.org/10.2307/2442240
Feio AC, Riina R, Meira RMSA (2016) Secretory structures in leaves and flowers of two dragon’s blood Croton (Euphorbiaceae): new evidence and interpretations. Int J Plant Sci 177:511–522. https://doi.org/10.1086/685705
Fisher DB (2004) Protein staining of ribboned epon sections for light microscopy. Histochemie 16:92–96. https://doi.org/10.1007/BF00306214
Gaffal KP (2012) How common is the ability of extrafloral nectaries to produce nectar droplets, to secrete nectar during the night and to store starch? Plant Biol 14:691–695. https://doi.org/10.1111/j.1438-8677.2012.00616.x
Gilg E (1895) Ueber die Blüthenverhältnisse der Gentianaceengattungen Hockinia Gardn. und Halenia Borckh. Ber Deutschen Botanischen Gesellschaft 13:114–126
Guimarães EF, Dalvi VC, Azevedo AA (2013) Morphoanatomy of Schultesia pachyphylla (Gentianaceae): a discordant pattern in the genus. Botany 91:830–839. https://doi.org/10.1139/cjb-2013-0077
Gunning BES, Pate JS (1969) “Transfer cells” plant cells with wall ingrowths, specialized in relation to short distance transport of solutes - their occurrence, structure, and development. Protoplasma 68:107–113
Heil M, Rattke J, Boland W (2005) Post secretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science 308:560–563. https://doi.org/10.1126/science.1107536
Heil M (2011) Nectar: generation, regulation and ecological functions. Trends Plant Sci 16:191–200. https://doi.org/10.1016/j.tplants.2011.01.003
IEF (2022) Instituto Nacional de Florestas. http://www.ief.mg.gov.br/parque-estadual/1411. Accessed 24 May 2022
Jensen WA (1962) Botanical histochemistry: principles and practice. WH Freeman, San Francisco
Johansen DA (1940) Plant microtechnique. McGraw- Hill, New York
Lanza J, Smith GC, Sack S, Cash A (1995) Variation in nectar volume and composition of Impatiens capensis at the individual, plant, and population levels. Oecologia 102:113–119
Lillie RD (1965) Histopathologic technic and practical histochemistry. McGrawHill, New York
Mace ME, Howell CR (1974) Histochemistry and identification of condensed tannin precursor in roots of cotton seedlings. Can J Bot 52:2423–2426. https://doi.org/10.1139/b74-314
Martínez del Rio C (1990) Phylogenetic and ecological correlates of intestinal sucrase and maltase activity in birds. Physiol Zool 63:987–1011. https://doi.org/10.1086/physzool.63.5.30152625
Meira RMSA, Miranda JD, Coutinho IAC (2020) Anatomical reevaluation and novelties on the leaf marginal tooth glands in Sapium glandulosum (L.) Morong. (Euphorbiaceae): the importance of distinguishing colleters from nectaries. In: Demarco D (ed) Plant ontogeny: studies, analyses and evolutionary implications. Nova Science Publishers, New York, pp 63–83
Nepi M, Soligo C, Nocentini D, Abate M, Guarnieri M, Cai G, Pacini E (2012) Amino acids and protein profile in floral nectar: much more than a simple reward. Flora: Morphol Distrib Funct Ecol Plants 207:475–481. https://doi.org/10.1016/j.flora.2012.06.002
Nepi M (2007) Nectary structure and ultrastructure. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht, pp 129–166
Nepi M (2017) New perspectives in nectar evolution and ecology: simple alimentary reward or a complex multiorganism interaction? Acta Agrobotanica 70:1704. https://doi.org/10.5586/aa.1704
Nguyen ST, McCurdy DW (2017) Wall ingrowth deposition in phloem parenchyma transfer cells in Arabidopsis: heteroblastic variations and a potential role in pathogen defense. Plant Signal Behav 12:1676–1691. https://doi.org/10.1080/15592324.2017.1338226
Nicolson SW (2007) Nectar consumers. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht, pp 289–342
Nicolson SW, Thornburg RW (2007) Nectar chemistry. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht, pp 215–264
Nicolson SW, Nepi M, Pacini E (2007) Nectaries and nectar. Springer, Dordrecht
O‘Brien TP, McCully ME (1981) The study of plant structure principles and select methods. Termarcarphi Pty, Ltd, Melbourne
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue. Protoplasma 59:367–373
Oliveira PS, Freitas AVL (2004) Ant–plant–herbivore interactions in the neotropical cerrado savanna. Naturwissenschaften 91:557–570. https://doi.org/10.1007/s00114-004-0585-x
Pacini E, Nepi M (2007) Nectar production and presentation. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht, pp 167–214
Pacini E, Nepi M, Vesprini JL (2003) Nectar biodiversity: a short review. Plant Syst Evol 238:7–21. https://doi.org/10.1007/s00606-002-0277-y
Paiva ÉAS, Machado SR (2006) Ontogenesis, anatomy, and ultrastructure of Hymenaea stigonocarpa Mart. ex Hayne (Fabaceae-Caesalpinioideae) extrafloral nectaries. Acta Botanica Brasilica 20:471–482. https://doi.org/10.1590/S0102-33062006000200022
Paiva EAS (2017) How does the nectar of stomata-free nectaries cross the cuticle? Acta Botanica Brasilica 31:525–530. https://doi.org/10.1590/0102-33062016abb0444
Pearse AGE (1951) A review of modern methods in histochemistry. J Clin Pathol 4:6–36
Pearse AGE (1972) Histochemistry: theoretical and applied. The Williams and Wilkins Company, Baltimore
Pearse AGE (1980) Histochemistry. Williams and Wilkins, Baltimore
Peng YB, Li YQ, Hao YJ, Xu ZH, Bai SN (2004) Nectar production and transportation in the nectaries of the female Cucumis sativus L. flower during anthesis. Protoplasma 224:71–78. https://doi.org/10.1007/s00709-004-0051-9
Praxedes SC, DaMatta FM, Loureiro ME, Maria MA, Cordeiro AT (2006) Effects of long-term soil drought on photosynthesis and carbohydrate metabolism in mature robusta coffee (Coffea canephora Pierre var. kouillou) leaves. Environ Exp Bot 56:263–273. https://doi.org/10.1016/j.envexpbot.2005.02.008
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque staining in electron microscopy. J Cell Biol 17:208–212. https://doi.org/10.1083/jcb.17.1.208
Rocha JF, Machado SR (2009) Anatomy, ultrastructure and secretion of Hibiscus pernambucensis Arruda (Malvaceae) extrafloral nectary. Brazilian J Bot 32:489–498. https://doi.org/10.1590/S0100-84042009000300008
Rudgers JA (2004) Enemies of herbivores can shape plant traits: selection in a facultative ant-plant mutualism. Ecology 85:192–205. https://doi.org/10.1890/02-0625
Santos VH, Minatel IO, Reco PC, Garcia A, Lima GP, Silva RM (2017) Peptide composition, oxidative and insecticidal activities of nectar from flowers of Spathodea campanulata P. Beauv Ind Crops Prod 97:211–217. https://doi.org/10.1016/j.indcrop.2016.12.025
Struwe L, Albert VA (2002) Gentianaceae: systematics and natural history. Cambridge University Press, Cambridge
Torezan-Silingard HM (2012) Flores e animais: uma introdução a história natural da polinização. In: Del-Claro K, Torezan-Silingard HM (eds) Ecologia das Interações Plantas-Animais: uma abordagem ecológico-evolutiva. Technical Books, Rio de Janeiro
Vogel S (1998) Remarkable nectaries: structure, ecology, organophyletic perspectives: II. Nectarioles Flora 193:1–29. https://doi.org/10.1016/S0367-2530(17)30798-3
Vollhardt P, Schore N (2013) Química orgânica: estrutura e função. Bookman, Porto Alegre
Wagner D, Kay A (2002) Do extrafloral nectaries distract ants from visiting flowers? An experimental test of an overlooked hypothesis. Evol Ecol Res 4:293–305
Acknowledgements
We thank Centro de Microscopia Eletrônica da Universidade Estadual de Santa Cruz (UESC) and Centro de Microscopia e Microanálise da Universidade Federal de Viçosa (UFV) for the assistance, and Dr. Rodrigo T. Ávila from Laboratório de Nutrição e Metabolismo de Plantas (UFV) for the chemical analysis. We also thank the two anonymous reviewers for the comments and suggestions that have improved our manuscript.
Funding
RMSAM received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). AZA received a PhD scholarship from CAPES. RMSAM received productivity grants from CNPq — Conselho Nacional de Desenvolvimento Científco e Tecnológico (306740/2019–2).
Author information
Authors and Affiliations
Contributions
RMSAM designed the research project and advised the first author; AZA and KAB collected the samples and performed light microscopy and the histochemical analyses; VFF and AZA performed the scanning and transmission electron microscopy analyses; RMSAM, AZA, VFF, AAA and VCD wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Handling Editor: Dorota Kwiatkowska.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zanotti-Ávila, A., Fernandes, V.F., Barros, K.A. et al. Unraveling the secretion mechanism of the curious nectaries in Gentianaceae. Protoplasma 260, 637–649 (2023). https://doi.org/10.1007/s00709-022-01804-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-022-01804-8