Abstract
Different breeding systems occur in the Gardenieae complex (Rubiaceae), from homoecy to dioecy which is present in two tribes, Gardenieae and Cordiereae. As part of a broad project focused on the reproductive anatomy of the species of these two tribes, we described the structural and functional differences of the gynoecium in the different floral morphs and determined the degree of gynoecium development in the staminate flowers. We conducted a comparative anatomical study focused on the gynoecium of one homoecious species (Tocoyena formosa, with perfect flowers) and three dioecious species (Genipa americana, Randia calycina, and Randia heteromera) of Gardenieae and one dioecious species (Cordiera concolor) of Cordiereae. The dioecious species have flowers that are morphologically perfect and functionally unisexual. Flowers in successive stages of development were collected, photographed, and fixed in formalin-acetic acid-alcohol. The material was examined using light microscopy and scanning electron microscopy. The anatomy of the ovary, style, and stigma was analyzed, and megasporogenesis and megagametogenesis were studied. The results achieved in this study, together with previously obtained data of the androecium, show that dioecy originated from homoecy in these species, since the unisexual flowers conserve some characteristics of the perfect flowers. In addition, a new type of ovule for the Rubiaceae family is described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Rubiaceae family presents a great variety of floral mechanisms for sexual reproduction, characterized by different types of organization of the gynoecium and androecium (Castro et al. 2008). Robbrecht (1988) recognized three main mechanisms: (1) heterodistyly, (2) secondary pollen presentation, and (3) flower unisexuality. Each mechanism is generally associated with a group of the Rubiaceae: Heterostyly commonly occurs in the subfamily Rubioideae, in particular in species of the tribes Spermacoceae (formerly Hedyotideae) and Psychotrieae; secondary presentation of pollen on the style is generally observed in the subfamily Cinchonoideae sensu lato, including Gardenieae and related tribes; and flower unisexuality is present in ca. 10% of the Rubiaceae genera belonging to the two subfamilies, even occurring in groups with stylar presentation of pollen (Robbrecht 1988).
The tribe Gardenieae DC. was originally described by De Candolle (1830) and diagnosed by having large flowers, multiovulate bilocular ovaries, and indehiscent fruits with a fleshy mesocarp. The tribe comprises 33 genera, very diverse morphologically in aspects such as corolla aestivation or the type of placentation. This classical concept of the tribe has been strongly modified in recent years, based on molecular and morphological studies. Many of the genera included by Candolle are now distributed in 13 currently accepted tribes. Schumann (1891) proposed the first infratribal classification based mainly on floral features, recognizing four subtribes: Eugardeniinae, Cordierinae, Bertierinae, and Hamelinae. This classification was later rejected by numerous authors, but some groups have currently been recovered as monophyletic taxa (e.g., Cordierinae as tribe Cordiereae). The most important morphological work in Gardenieae was conducted by Robbrecht and Puff (1986) who recognized two subtribes, Diplosporinae with seven genera restricted to Africa, and the Pantropical subtribe Gardeniinae, next to some allied tribes, such as Pavetteae, Coffeeae, and Hypobathreae. Persson (2000) carried out the first molecular phylogeny of Gardenieae. He revealed that the subtribal classification proposed by Robbrecht and Puff (1986) was artificial, and he united their subtribe Diplosporinae with their Coffeeae. The tetrad group sensu Robbrecht and Puff (1986) was also recovered as monophyletic; however, it should also include some genera with monad pollen grains (e.g., Rosenbergiodendron Fagerl., Sphinctanthus Benth., and Tocoyena Aubl.). Persson (2000) named the latter as the Randia clade, later renamed as the Randia group.
Recently, Mouly et al. (2014) redefined the circumscription of Gardenieae based on a broad molecular sampling and accepted four tribes in a “Gardenieae-Pavetteae clade”: Pavetteae (mainly paleotropical genera), Cordiereae (neotropical; with 12 genera of which Cordiera A. Rich. is studied here), Sherbournieae (the only tropical African genera), and Gardenieae s.s (pantropical; with some 50 genera). In the latter tribe, they identified five clades: Gardenia group (including Genipa L studied here), Rothmannia group, Aidia group, Porterandia group, and Randia group (with Randia and Tocoyena studied here). A further elucidation of the neotropical element in the Randia group was recently provided by Borges et al. (2021) which confirmed the monophyly of Tocoyena studied here, but demonstrated that the genus Randia, as presently circumscribed, is polyphyletic.
In the Gardenieae complex, there are homoecious (hermaphrodite), monoecious, and dioecious species in the tribe Gardenieae, but only dioecious species in the Cordiereae (Mouly et al. 2014). In species of the Rubiaceae with unisexual flowers, the staminate flowers often have a pistillode similar to the gynoecium of the pistillate flowers with an empty ovary, and the pistillate flowers have staminodes with empty and smaller anthers (Robbrecht 1988). Recently, we studied the androecium in different South American species of the Gardenieae complex (Judkevich et al. 2020, in press), including one homoecious species Tocoyena formosa (Cham. & Schltdl.) K.Schum. with perfect flowers (PF) and four dioecious species with functional staminate flowers (FSF) and functional pistillate flowers (FPF): Cordiera concolor (Cham.) Kuntze, Genipa americana L., Randia calycina Cham., and Randia heteromera Judkevich & R.M.Salas. In this study, it was observed that the androecium of PF and FSF presents a similar structure; however, in FPF, the androecium has sterile anthers with different degrees of pollen development depending on the species. While in C. concolor and R. calycina the pollen development stops at the tetrad stage, in G. americana, it occurs at the meiosis stage and in R. heteromera at the microspore mother cell stage.
The gynoecium of Rubiaceae has very constant characteristics: the ovary is small and generally bicarpellate, there is generally one style, and the number of stigmatic branches corresponds to the number of carpels (Robbrecht 1988). In addition, the surface of the stigma may have folds, ridges, or hairs that play a role in the secondary presentation of the pollen (Robbrecht 1988; Puff et al. 1996). As for the type of placentation in Rubiaceae, the most frequent is the axile (Robbrecht 1988). The insertion of the axile placentas is variable; they may be inserted entirely along the septum or only in one part: at the apex, in the middle region, or at the base of the septum (Robbrecht 1988; Groeninckx et al. 2007). Hallé (1967) recognized three types of placentation for the species that are currently found in the Gardenieae complex: (1) typical parietal; (2) typical axile, in which the differentiation of the ovules occurs when the proliferation of the placenta is complete and the ovules are superficial to the placenta; and (3) diffuse placentation, a variant of axile placentation, with the proliferation of the placenta continuous and occurring at the same time as the differentiation of the ovules so that the ovules are immersed in the placenta.
The structure of the ovule in angiosperms is determined mainly by their curvature, the thickness of the nucellus, and the number of integuments (Endress 2011). In the Rubiaceae, the first author to study ovule structure was Schleiden (1837), who described the ovule without any integuments; Lloyd (1899) demonstrated the presence of a single integument; and Fagerlind (1937), who made the most extensive study of the family, classified the ovules in six types, taking account of the number of archesporial cells and the form of the nucellar epidermis; in addition, he postulated evolutionary relations between these types. Later studies expanded the number of ovule types to 12 (Galati 1991; Mariath and Cocucci 1997; De Toni and Mariath 2008, 2010; Figueiredo et al. 2013b, 2017). In recent years, more studies on ovule development have been conducted in different tribes of the Rubiaceae family (De Toni and Mariath 2004, 2008, 2010; Li et al. 2010; Figueiredo et al. 2013a,b; Florentin et al. 2016; Figueiredo et al. 2017; Romero et al. 2021). However, De Toni and Mariath (2008) emphasized that the knowledge of ovule differentiation in the Rubiaceae remains fragmentary and there is only data for some isolated genera. For the very large Gardenieae complex, Hallé (1967) described the ovules as anatropous with a single integument; however, apart from our own abovementioned work, recent studies of the embryology of dioecious species in the Gardenieae complex are not known. So far, the only two studies of the Rubiaceae describing the differentiation of the megagametophyte in dioecious species are on Mussaenda pubescens W.T. Aiton (tribe Mussaendeae; Li et al. 2010) and Cephalanthus glabratus (Spreng.) K. Schum. (tribe Naucleeae, Romero et al. 2021).
Continuing our studies of the reproductive system in the Gardenieae complex (Judkevich et al. 2020, in press), the objectives of this paper are as follows: (1) to compare the structure of the gynoecium between one homoecious and four dioecious species of the Gardenieae complex and (2) to identify the degree of development of the gynoecium in staminate flowers. For this purpose, different parts of the gynoecium (ovary, style, and stigma) were analyzed anatomically.
Material and methods
Mature flowers and flower buds were analyzed from one species of the tribe Cordiereae, Cordiera concolor, and four species of the tribe Gardenieae, Genipa americana, Randia calycina, Randia heteromera, and Tocoyena formosa. The material was collected and photographed in the field and preserved in formalin-acetic acid-alcohol (5 mL formalin, 5 mL acetic acid, and 90 mL 70% ethanol). Voucher information is in the Appendix.
Observations were made with a stereoscopic microscope (SM), light microscope (LM), and scanning electron microscope (SEM). Digital images of fresh and fixed material of the stigma were made with a stereoscopic microscope Leica MZ 6.
For LM analysis, the ovaries and style/stigma of 10–15 flowers and flower buds of each species and of each floral morphotype were removed. The dissected material was dehydrated in an ascending series of alcohol and embedded in paraffin (Johansen 1940; modified by Gonzalez and Cristóbal 1997). Serial transverse and longitudinal Sects. (12 μm) were made using a Microm HM350 rotary microtome (Microm International, Walldorf, Germany). The sections were stained with safranin and Astra blue (Luque et al. 1996) and mounted in synthetic Canada balsam. Observations and digital images were made using a Leica DM LB2 (Leica Microsystems) light microscope equipped with polarized filters and a Leica DATA digital camera.
For SEM, the style/stigma of five mature flowers of each species and of each floral morphotype were taken from the fixed flowers. These floral pieces were then dehydrated in an increasing acetone series and then critical point dried using liquid CO2 (Denton Vacuum, DCP-1, Pleasanton, NJ) and sputter-coated with gold–palladium (Denton Vacuum, Desk II, Pleasanton, NJ). The samples were analyzed with a Jeol LV 5800 (JEOL, Tokyo, Japan) at 10 kV in the Service of Electron Microscopy facility at the Universidad Nacional del Nordeste.
Results
Tocoyena formosa is a species with perfect flowers, whereas Cordiera concolor, Genipa americana, Randia calycina, and R. heteromera have unisexual flowers and are dioecious.
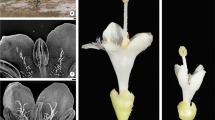
From a morphological point of view, the flowers in all the species analyzed have both sexual whorls well-developed (Figs. 1 and 2). An inferior ovary, one style, and a stigma form the gynoecium. The androecium is composed of anthers attached to the corolla tube by short filaments. Whereas in Tocoyena formosa, a homoecious species (Fig. 1 a-c), both whorls are functional, in the other dioecious species only one whorl is functional so the flowers are functionally unisexual.
Morphology of flowers. a–c Tocoyena formosa. a Flower. b Surface view of the stigma. c Longitudinal section of the ovary. d–k Cordiera concolor. d–g FPF. e Longitudinal section of the flower. f Stigma. g Staminodium. h–k FSF. i Longitudinal section of the flower. j Stigma. k Anther. l-q Genipa americana. l–n FPF. l Flower with exposed stigma. m Longitudinal section of the ovary. n Staminodium. o–q FSF. p Longitudinal section of the rudimentary ovary. q Anther. Scales: a = 1 cm; b, p = 2 mm; c–e, h–i, n, q = 1 mm; f–g, k = 0.5 mm; j = 0.1 mm; l–m, o = 5 mm
Morphology of flowers of Randia. a–j R. calycina. a FPF. b Surface view of the stigma. c Lateral view of the stigma. d Longitudinal section of the ovary. e Staminodium. f FSF. g Surface view of the stigma. h Lateral view of the stigma. i Longitudinal section of the rudimentary ovary. j Anther. k–t R. heteromera. k FPF. l Surface view of the stigma. m Lateral view of the stigma. n Longitudinal section of the ovary. o Staminodium. p FSF. q Superficial view of the stigma. r Lateral view of the stigma. s Longitudinal section of the rudimentary ovary. t Anther. Scales: a, f, k, p = 5 mm; b–d, g–i, l–n, q–s = 1 mm; e, j, o, t = 0.5 mm
In the PF FPF flowers (Figs. 1d,l and 2a,k), the gynoecium was formed of an inferior ovary (Figs. 1e,m and 2d,n) with fully developed ovules and a receptive stigma (Figs. 1f,l and 2b,m). In FSF (Figs. 1h,o and 2f,p), the gynoecium was formed of a small ovary (Figs. 1i,p and 2f,p) without any ovule formation, at most with some parenchymatic bodies, and the stigma is adapted to presenting pollen on its surface (Judkevich et al. 2020, in press; Figs. 1j,o and 2g–h,q–r). The ovary in the FPF is noticeably more voluminous than in the FSF (except for Genipa americana with very similar ovaries in the two floral types; in the FSF of this species, the volume of the parenchymal bodies is similar to that occupied by the ovules in the FPF).
In the PF and FSF, the androecium contains stamens with fully developed anthers that produce pollen (Figs. 1k,q and 2j,t), while in the FPF, the androecium consists of staminodes with smaller empty anthers (Figs. 1g,n and 2) as described by Judkevich et al. (2020, in press).
The anatomy of the different parts of the gynoecium is detailed below in a comparison between species and, unless clarified, in both floral types.
Ovary
In all analyzed species and types of flowers, the ovary is inferior and is surrounded by the remaining anthophylls inserted on the floral tube. The descriptions were made in cross section (Fig. 3).
Cross section of the ovary wall. a–d T. formosa. e–h C. concolor. i–l G. americana. m–p R. calycina. q–t R. heteromera. a, d, h–j, m–p, s Portion of the ovary wall (i external portion, j inner portion); in T. formosa and R. calycina, the trichomes have been removed for histological sections. b–c, f, l, n, r Detail of the external epidermis with trichomes. e, k, q Detail of the external epidermis with stomata. g Idioblast with druse. Scales: a, i–j, m, o–p, s = 100 µm; b, d, h = 50 µm; c, e–f, k–l, q, r = 20 µm; g, n = 10 µm
Epidermis
The external epidermis corresponds to the outer layer of the floral tube and has stomata (Fig. 3e,k,q). There are simple, multicellular, uniseriate, and lignified trichomes in T. formosa (Fig. 3b–c) and unicellular and lignified trichomes in C. concolor (Fig. 3f), G. americana (Fig. 3l), R. calycina (Fig. 3n), and R. heteromera (Fig. 3r). The internal epidermis presents smaller cells, delimits the locules, and lacks trichomes.
Mesophyll
The mesophyll strictly corresponds to the wall of the inferior ovary and the extra-gynoecium parts (Figs. 3a and 9d,h–j,m–p,s). The external zone is vascularized, occupying almost the entire thickness of the mesophyll and is formed of polygonal cells; the internal zone is formed of a few layers of elongated cells arranged tangentially and has no vascular bundles. In all species, the inner zone has several cycles of collateral vascular bundles with abundant ramifications. The external bundles correspond to the floral tube and the internal bundles correspond to the carpels.
In C. concolor, the mesophyll presents abundant idioblasts with randomly distributed tannin (Fig. 3d,h). In T. formosa and G. americana, some tanniniferous cells were found. In addition, druses were observed in C. concolor (Fig. 3g), T. formosa, G. americana, and R. heteromera.
Placental tissue
In the ovary of the PF and FPF, two placentas protrude from the central septum and completely occupy the locules of the ovary (Fig. 4a–f,h–j,l–n,p–r). In T. formosa, G. americana, and the two Randia species, each placenta is inserted along the septum (Fig. 4a,h,l,p), whereas in C. concolor, the placentas are inserted in the upper-middle region of the septum (Fig. 4d–e). In C. concolor, each placenta has 5–6 ovules, whereas in the other species, there are numerous ovules (> 10). Two types of placentation were found in the analyzed species: in G. americana (Fig. 4h–j), the placentation is typical axile; in this case, the placenta shows little growth, and the ovules cover the whole surface. In T. formosa (Fig. 4a–c), C. concolor (Fig. 4d–f), and the two Randia species (Fig. 4l–n,p–r), the placenta is diffuse; in this case, the placenta growth is greater than the growth of the ovules, so it ends up protruding towards the locule, and the ovules become immersed in it.
Placentation. a–c T. formosa, PF. d–g C. concolor (d–f FPF; g FSF). h–k G. americana (h–j FPF; k FSF). l–o R. calycina (l–n FPF; o FSF). p–s R. heteromera (p–r FPF; s FSF). a, d, h, l, p Longitudinal section of the ovary at the level of the placenta. b, e, i, m, q Placenta removed with the ovules. c, f–g, j–k, n–o, r–s Cross-section of the ovary at the level of the placenta. c, f, n, r Diffuse axile placentation. j–k Typical axile placentation. g, k, o, s Rudimentary placentas in transection, without ovules. Abbreviations: pl = placental tissue; ov = ovule; ru = rudiment of ovule. Scales: a–b, d, h–i, l–m, p–q = 1 mm; e = 0.5 mm; c, f–g, j–k, n–o, r–s = 100 µm
On the other hand, in the FSF a varied degree of reduction of the ovary and the placental tissue has been observed depending on the species. In G. americana, there is the least degree of reduction, the ovary possessing two locules; the placental tissue is similar to that of the FPF, but it occupies less volume and has several ovule rudiments formed only of parenchyma (Fig. 4k). In C. concolor and both Randia species, the following variations were observed in the ovary: two loculi with a placenta in each one (Fig. 4o), a single locule with one or two placentas (Fig. 4g–s), or a small locule without any placentas or ovule rudiments. In the cases in which ovule rudiments are formed, there is no differentiation of the integuments, the processes of megasporogenesis and megagametogenesis do not occur, and no gametes are formed.
The ovule of the PF and FPF, megasporogenesis, and megagametogenesis
The ovules are hemianatropous, tenuinucellate and unitegmic (Fig. 5). The integument is massive, presenting more than 10 layers of cells. In C. concolor and G. americana (Fig. 5b), the epidermal cells of the integument present tannins. The differentiation of the ovule and the embryo sac is identical in all species. The main stages are described below.
Stage 1: ovule primordium
On the surface of the placenta, there are three layers: dermal, subdermal, and central (Fig. 6a). The differentiation of the ovule begins after the occurrence of divisions in the three layers, thus forming a protrusion, the primordium of the ovule. This primordium is projected towards the locule as it grows (Fig. 6b).
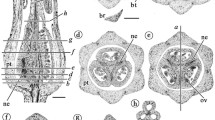
Megasporogenesis and megagametogenesis. a, d–f, i–k G. americana. b–c R. heteromera. g R. calycina. h C. concolor. l T. formosa. a Meristematic layers. b Ovule primordium. c Ovule primordia with archesporial cell. d Megaspore mother cell. e Diade. f Tetrad. g Linear tetrad with collapsed cells near the micropyle. h–i Immature gametophyte. j–k Mature embryo sac with starch grains in central cell. l Mature embryo sac with starch grains observed with polarized light at right side (arrowhead indicates filiform apparatus). Abbreviations: an = antipodals; ar = archesporial cell; cc = central cell; ce = central layer, de = dermal layer, in = integument; mmc = megaspore mother cell; nu = nucellus; su = subdermal layer; sy = synergids. Scales: 50 µm
Stage 2: megaspore mother cell (MMC)
In ovules curved at 90°, the nucellus and the edges of the integument can already be seen differentiated. The single integument is 4 to 7 cells thick; its borders are not yet closed (Fig. 6c). The nucellar epidermis consists of few cells in longitudinal Sect. (6–8) and has a convex shape. Subepidermally, an archesporial cell (Fig. 6c) is differentiated into a megaspore mother cell (Fig. 6 d), which is distinguished from the other cells by being elongated and having a prominent nucleus.
Stage 3: meiosis
When the growth of the integument delimits the micropyle, the megaspore mother cell undergoes meiotic division, producing a dyad and then a linear tetrad of megaspores (Fig. 6e–f). Of the four cells resulting from meiosis, the three near the micropyle degenerate (Fig. 6g), and the remaining one forms a functional megaspore.
Stage 4: embryo sac
The integument increases notably in thickness, becoming massive due to divisions of its cells in different planes. The nucellus begins to be consumed. The functional megaspore undergoes three mitotic divisions (Fig. 6h–i), resulting in a Polygonum-type embryo sac with 7 cells and 8 nuclei (Fig. 6j–l) that has one egg cell, two synergids, one central cell, and three antipodals. Both the synergids and the antipodal cells present dense cytoplasm. In the synergids, the vacuole can be distinguished towards the chalazal end and the nucleus near the micropylar end. The filiform apparatus is evident in these cells. The cytoplasm of the central cell is filled with starch grains (Fig. 6l).
Style
It is solid in all species and flower types (Fig. 7a,d–e,h–i,k–l). The epidermis is glabrous (Fig. 7c,f,j,m) except in the FSF of C. concolor which has simple unicellular trichomes (Fig. 7g). Tannins are present in T. formosa (Fig. 7b), C. concolor (Fig. 7d–g), and G. americana (Fig. 7h–i). In the center of the style of the PF and FPF, it is possible to distinguish the solid transmitting tissue formed of smaller secretory cells with dense cytoplasm (Figs. 7a,d–e,h–i,k–l; 8 g; 9f–g; and 10 g). This tissue runs through the whole style and continues in the stigmatic branches where it is continued as the internal epidermis (Figs. 8 and 9). In the FSF, this tissue is reduced to its minimum expression (Fig. 10). In all flowers, there are two collateral vascular bundles that run through the entire style ending at the base of the stigmatic branches (Figs. 7–10).
Styles in cross section. a–c T. formosa, PF. d–g C. concolor. h–j G. americana. k–m R. heteromera. d, f, h, j, k, m. FPF. e, g, i, l FSF. a, d–e, h–i, k–l Transection of the middle zone of the style. b Detail of the vascular bundle. c, f–g, j, m Detail of the epidermis, note in g the unicellular trichome. Abbreviations: vb = vascular bundle; tn = tannin; tr = transmitting tissue. Scales: a–e, h–m = 50 µm, f–g = 10 µm
Course of the transmitting tissue in the stigma and style on the PF of Tocoyena formosa. a Surface view of style and stigma. b–d, f–g Cross section to the levels indicated in (a). e Detail of the transmitting tissue. Abbreviations: vb = vascular bundle; tr = transmitting tissue. Scales: a = 1 cm; b–g = 50 µm
Course of the transmitting tissue in the style and stigma of the FPF of Randia heteromera. a Surface view of upper portion of ovary covered by nectary, style, and bilobed stigma. b–d, f–g Transections to the levels indicated in (a). e Detail of the transmitting tissue. Abbreviations: vb = vascular bundle; tr = transmitting tissue. Scales: a = 1 mm; b–g = 50 µm
Course of the transmitting tissue in the style and stigma of the FSF of Randia heteromera. a Surface view of style and stigma. b–d, f–g Cross section of the levels indicated in (a). e Detail of the transmitting tissue. Abbreviations: vb = vascular bundle; tr = transmitting tissue. Scales: a = 1 mm; b–g = 50 µm
Stigma
It consists of two stigmatic branches that are in close contact at the base and middle region, and free towards the apex (Fig. 11a,e,g,i,k,m,o,q,s). Only in T. formosa these branches spread in their entirety after the anthesis (Fig. 11a). The stigma is dry in C. concolor, G. americana, and T. formosa and is wet in the Randia species.
Stigma morphology. a–d T. formosa. e–h C. concolor (e–f FPF, g–h FSF). i–l G. americana (i–j FPF, k–l FSF). m–p R. calycina (m–n FPF, o–p FSF). q–t R. heteromera (q–r FPF, s–t FSF). a, e, g, i, k, m, o, q, s General view of the stigma. b External surface of a stigmatic lobe. c Inner surface of a stigmatic lobe. d, f, j, l, n, r Detail of the smooth inner surface and the papillose outer surface of the stigma. h Outer surface of stigma with attached pollen grain. p, t Detail of the external surface of the stigma with stomata in both species of Randia. Abbreviations: es = external surface; is = inner surface; po = pollen grain; s = stomata. Scales: a, i, k = 2 mm; b, c, e = 500 µm; d, f–g, j, n, p, r, t = 50 µm; h = 20 µm; l = 5 µm; m, o, q, s = 200 µm
Two epidermises are distinguished, the external and the internal epidermis (Figs. 11b–d,f–g,j,l,n,o,r and 12a–q). The external epidermis of T. formosa (Fig. 12b), G. americana (Fig. 12i,l), and of the FSF of R. calycina (Fig. 11p) and R. heteromera shows stomata (Fig. 11t). This epidermis is more developed than the internal epidermis in the FSF of C. concolor (Fig. 12g) and in the Randia spp. (Fig. 12q–r). In T. formosa (Fig. 12a–c), both epidermises are well developed, being much more voluminous than the parenchyma cells of the stigma. On the other hand, the internal epidermis of the FPF of C. concolor (Fig. 12d,f), G. americana (Fig. 12h,j), and Randia spp. (Fig. 12n,p) has cells that are much more developed than those of the outer epidermis.
Cross section of stigma. a–c T. formosa. d–f C. concolor. g–l G. americana. m–p Randia heteromera. a, d, h, k, n, q Cross section of a stigmatic branch (n and q are details of Figs. 9 c and 10 b). g Cross section of the base of stigma. b, e, i, l, o, r Detail of the epidermis of the external surface of the stigma. c, f, j, m, p Detail of the epidermis of the inner surface of the stigma. Abbreviations: dr = druse; es = external surface; is = inner surface; s = stomata, tn = tannin; vb = vascular bundle. Scales: a, h, k = 100 µm; b–g, i–j, l = 50 µm; r = 20 µm
In all flowers, the internal epidermis continues the transmitting tissue in the style indicating that it is the receptive surface of the stigma. In each stigmatic branch, there is a collateral vascular bundle (Fig. 12a,d,h,k,n,q).
On the surface of the style of the FSF of C. concolor and on the stigma (mainly) of T. formosa and the FSF of G. americana and Randia spp., the pollen is exposed to pollinators in a secondary pollen presentation mechanism.
Discussion
In the Rubiaceae family, homoecy is the most common breeding system (Razafimandimbison et al. 2009). However, there are also cases of monoecy and dioecy (Robbrecht 1988). In the Gardenieae complex several genera have unisexual flowers (Robbrecht and Puff 1986; Robbrecht 1988; Mouly et al. 2014). Such flowers (pistillate and staminate flowers) may be similar or very different (Pacini 1996). In the dioecious species analyzed here, the flowers present great morphological similarity since they have both sexual whorls; the main characteristics that differentiate them externally are the smaller size of the ovary in the FSF and of the anthers in the FPF. Mayer and Charlesworth (1991) defined the term cryptic or functional dioecy as a breeding system of species with unisexual flowers, in which one or both morphs appear to be perfect, retaining non-functional organs, such as gynoecium in the FSF and androecium in the FPF. The case of the species with unisexual flowers analyzed in this paper matches this definition. It should be noted that in unisexual species of the Rubiaceae, including those studied here, it is often possible to identify the sexuality of the plants despite the morphological similarity between the FPF and the FSF, since the FSFs are arranged in multiflorous inflorescences, whereas the FPF are placed in 1- or pauciflorous inflorescences (Robbrecht and Puff 1986; Robbrecht 1988; Judkevich et al. 2020). In addition, fruits are observed in plants with FPF (Robbrecht and Puff 1986; Robbrecht 1988; Judkevich 2019).
In different species of Rubiaceae (mainly in the subfamily Rubioideae in which heterostyly is very common; Robbrecht and Manen 2006), cryptic dioecy combined with heterostyly has been described; several authors have suggested that in these cases, dioecy was originated from heterostyly (Pailler et al. 1998; Naiki and Kato 1999; Naiki 2008; Sugawara et al. 2011; Terra-Araujo et al. 2012; Watanabe et al. 2014). Those studies focused on the pollination of these species or their morphology. However, the only embryological studies known with cryptic dioecy in the Rubiaceae species are those by Li et al. (2010) in Mussaenda pubescens (tribe Mussaendeae, the only tribe of the subfamily Cinchonoideae sensu lato with heterostyly) and Romero et al. (2021) in Cephalanthus glabratus (tribe Naucleeae, subfamily Cinchonoideae). In M. pubescens (Li et al. 2010), the long-styled (L) morph (that has sterile pollen and functions as a pistillate flower) and the short-styled (S) morph (that is pistillate sterile and functions as a staminate flower) are recognized. The L morph has normal differentiation of the ovule and embryo sac (Polygonum type). However, the S morph shows abnormal megasporogenesis and megagametogenesis, with an arresting of the processes at different stages and the consequent absence of functional ovules. The authors propose that the condition prior to cryptic dioecy in M. pubescens is stigma-height dimorphism. In C. glabratus (Romero et al. 2021), the FPF and FSF are described; they differ morphologically in the corolla and stigma/style length and ovary size; also, in the FPF, the ovules develop normally, and the anthers are sterile, whereas in the FSF, the ovules are atrophied, and the anthers are fertile. Both flowers share the first stages of ovule development, but in the FSF, it stops before the embryo sac is formed. The authors do not go so far as to hypothesize a possible origin of dioecy in this species. In the analyzed species of the Gardenieae complex, ovules only develop in the PF and in the FPF, whereas in the FSF, there are no ovules (there may only be rudiments of ovules formed by undifferentiated parenchyma, without differentiation of megaspores). The studies carried out by Judkevich et al. (2020), on the androecium of the same species as those analyzed in the present study, indicated that in the FPF, the pollen development is arrested at different stages leading to sterile anthers, unlike the FSF in which there is normal pollen development. Given that heterostyly and stigma-height dimorphism are absent in the Gardenieae complex (and most other Cinchonoideae sensu lato, Robbrecht and Manen 2006), it is believed that cryptic dioecy derives from homoecy, at least in these species, since, in addition, unisexual flowers conserve characteristics of the PF (Table 1). This origin was also supposed by Razafimandimbison et al. (2009) in the Rubiaceae tribe Vanguerieae (another tribe of the Cinchonoideae sensu lato without heterostyly).
In the Rubiaceae, the gynoecium can present differences in the morphology and structure of the stigma/style and the ovary, position of the placenta, the number of ovules, and their orientation (Robbrecht 1988; Svoma 1991; De Toni and Mariath 2010).
The anatomical structure of the ovary wall is similar in all the species studied. The differences found between the species are mainly the amount and type of indumentum and the occurrence and distribution of tannins and druses in the ovary. The presence of collateral vascular bundles and the arrangement of cells in the inner region of the ovary wall coincide with that described in other Rubiaceae, such as Oldenlandia salzmannii (DC.) Benth. & Hook. f. ex B.D. Jacks. (tribe Spermacoceae, Florentin et al., 2016), Nichallea soyauxii (Hiern) Bridson, and species of Rutidea DC. (tribe Pavetteae, De Block 1995). Although the appendicular origin of the extracarpellar tissues of the ovary is mentioned historically in this family (Douglas 1957), the histological study carried out here did not make it possible to differentiate the areas, in cross section, that strictly correspond to the ovary from the extracarpellar tissues, for which a detailed analysis of the floral vascularization would be necessary.
Among the analyzed species of the Gardenieae complex with PFs and FPFs, two types of placentation were found by taking account of the proliferation of placentas: typical axile placentation in G. americana and diffuse axile placentation in T. formosa, C. concolor, and Randia species. This is in agreement with the types of placentation described by Hallé (1967) for species of the Gardenieae complex. Among the species with this diffuse axile placentation, there are differences in the attachment of each placenta to the septum, whereas in T. formosa and Randia spp., it is arranged along the entire septum; in C. concolor it is in the upper half.
In the species analyzed here, the ovule is only formed in the PFs of T. formosa and in the FPFs of dioecious species. This ovule is hemianatropous, unitegmic, has a single archesporial cell, and the nucellar epidermis is convex. Anatropous ovules are the most frequent in the Rubiaceae (Robbrecht 1988; De Toni and Mariath 2004, 2008, 2010; Li et al. 2010; Figueiredo et al. 2013a,b, 2017; Romero et al. 2021) and particularly in species of the Gardenieae complex (Hallé 1967; Robbrecht and Puff 1986); however, campylotropous (Wunderlich 1971; Robbrecht et al. 1991; De Block 1995) and hemianatropous ovules (Maheswari Devi and Krishnam Raju 1980; Von Teichman et al. 1982; De Toni and Mariath 2008; Florentin et al. 2016) may also exist in the family. In angiosperms, the integuments vary in number and thickness. The basic number is two, but they can be reduced to one and exceptionally to zero (Endress 2011). In the analyzed species, the ovules have only one integument, which would support the conclusions of other authors who mentioned that the unintegumented condition of the ovule is a constant character in the Rubiaceae (Fagerlind 1937; Robbrecht 1988; De Toni and Mariath 2008, 2010), there being few cases in the family where the presence of vestiges of the external integument is recorded during the formation of the ovule (Fagerlind 1937; Andronova 1977; De Toni and Mariath 2004). The thickness of the integument is a relatively stable character and represents a character of importance at the macrosystematic level (Endress 2011). In the species studied here, the integument is massive from its origin and the product of divisions of its cells in different planes. Ovules with massive integuments have also been described in other Rubiaceae, such as in Chomelia obtusa Cham. & Schltdl., Guettarda pohliana Müll. Arg. (Figueiredo et al. 2013b), Ixora coccinea L. (De Toni and Mariath 2008), Relbunium (Endl.) Hook. f. species (De Toni and Mariath 2010), and Rutidea DC. species (De Block 1995).
Finally, the embryo sac of both the PF and FPF is of the Polygonum type, which is the most common type in Angiosperms (Johri 1984) and in the Rubiaceae (Maheswari Devi and Krishnam Raju 1980; Von Teichman et al. 1982; De Toni and Mariath 2004; De Toni and Mariath 2008; De Toni and Mariath 2010; Li et al. 2010; Figueiredo et al. 2013a,b; Florentin et al. 2016; Romero et al. 2021). The embryo sac described in this study presents starch grains in the central cell, which agrees with Hallé (1967) who mentioned that this is a common feature in species of the Gardenieae complex, although it has also been mentioned in the embryo sacs of other Rubiaceae species of the tribes Pavetteae (Von Teichman et al. 1982; De Block 1995) and Rubieae (De Toni and Mariath 2010).
Regarding the type of ovules in the Rubiaceae, several types have been described over the years. Based on the number of integuments, the number of archesporial cells, and the shape of the nucellar epidermis, Fagerlind (1937) determined six different types of ovules and proposed evolutionary links between them. Based on that classification, subsequent studies by Galati (1991), De Toni and Mariath (2008, 2010), and Figueiredo et al. (2017) extended the number of ovule types found in the family (Table 2).
In the species of the Rubiaceae analyzed here, the ovules present a single archesporial cell, one integument without vestiges of an external integument and a convex nucellar epidermis. These ovules differ from the types previously described, so we assign the Randia type (Fig. 13) to represent the species of the present study. We named it after Randia because our first observation of the type was made in that genus.
De Toni and Mariath (2008), based on previous work, mentioned that the evolutionary trends in Rubiaceae ovule types are as follows: the disappearance of the external integument, the transition of the shape of the nucellar epidermis from convex to flat, the reduction in the number of archesporial cells, and the elongation of the cells of the nucellar epidermis. We here add the Randia type to the diagram of ovule types (Figuereido et al. 2013b), as a state derived from the Ixora type, due to the reduction in the number of archesporial cells to only one. In addition, the Dialypetalanthus type (described by Figueiredo et al. 2017) had not been previously incorporated into the evolutionary diagram. This ovule is characterized by an integument without vestiges of an external one, a dome-shaped nucellar epidermis is, three to four archesporial cells, and a conical chalaza projection. We add this type of ovule as a second derivation from the Ixora type due to the decrease in the number of archesporial cells to three or four and the presence of the conical chalaza projection. The Chomelia type (Figueiredo et al. 2013b) is removed here, since it is only distinguished from the Ixora type by its pendulous position. Indeed, in previous literature, the orientation of the ovule in the ovary had not been taken into account (Fagerlind 1937; Galati 1991; De Toni and Mariath 2008, 2010).
All species and floral types analyzed have a structurally massive style, a feature that had already been described in other species of Rubiaceae (De Block and Igersheim 2001, Florentin et al. 2016, Romero et al. 2021). The stigma of angiosperms can be classified as dry (when the secretion forms a thin film on the surface) or wet (when it presents a fluid secretion on the surface), with some families in which both types can be found, such as the Rubiaceae (Heslop-Harrison and Shivanna 1977). Of the species analyzed, only Randia spp. have wet stigmata, while the rest have dry stigmata.
In the stigmata of the PF of T. formosa and in the FPF of dioecious species, a smooth external surface and a papillose internal surface with well-developed cells are clearly distinguished, whereas in the FSF, the external surface is smooth or slightly papillose and has more developed cells than the internal surface. The internal epidermis of the stigma, in the species analyzed in this study, has continuity with the transmitting tissue, which indicates that this is the receptive surface of the stigma and is in agreement with De Block and Igersheim (2001) for the homoecious Rutidea DC. and Nichallea Bridson (tribe Pavetteae, Rubiaceae).
In the FSF of the Gardenieae complex, the transmitting tissue is poorly developed and continuous with the internal surface of stigma, and it has no receptive function. However, the external surface of the stigma in the species of Gardenieae (external surface of the style in C. concolor) participates in the secondary presentation of pollen, already previously described in these species by the authors of this paper (Judkevich et al. 2020, in press). Robbrecht and Puff (1986) mentioned that in species of the Gardenieae complex, secondary pollen presentation on the stigma occurs in the same way in both the PF and FSF, so this would be further evidenced that cryptic dioecy in species of this tribe has originated from homoecy. The secondary pollen presentation on the stigma would be a function of the stigma that is still preserved in the FSF but has been lost in FPF.
In T. formosa, as well as in both types of G. americana flowers and in the FSF of the Randia species, stomata were observed on the external surface of the stigma. Their presence in the PF and FSF could possibly be related to the secretion of some substance that helps pollen adherence for presentation on the stigma, while in the FPF of G. americana, it is perhaps only a relict. Further studies would be needed to explain the role of these stomata on the stigma.
Conclusion
Cryptic dioecy is confirmed as a breeding system in Cordiera concolor, Genipa americana, Randia calycina, and R. heteromera. The results obtained in the present study, added to those of previous studies, allow us to conclude that cryptic dioecy originated from homoecy in these species. Of the species analyzed, four correspond to the tribe Gardenieae, and only one corresponds to the recently separated tribe Cordiereae. The results of this study show that these species share many similar embryological characters, for example, the same type of ovule, referred to in this study as the Randia type. Therefore, it provides the basis for future studies that include a larger number of species and ones that have a higher inference at the tribal level. The recent phylogenetic framework for the Randia group (Borges et al. 2021) can therefore serve as a framework for efficient sampling.
References
Andronova NN (1977) On the structure of the ovule of Rubiaceae. Bot Zeitung 62:1461–1469
Borges R, Razafimandimbison S, Roque N, Rydin C (2021) Phylogeny of the Neotropical element of the Randia clade (Gardenieae, Rubiaceae, Gentianales). Plant Ecology and Evolution 154(3):458–469. https://doi.org/10.5091/plecevo.2021.1889
de Candolle AP (1830) Prodromus systematis naturalis regni vegetabilis, vol 4. Treuttel & Würz, Paris. https://doi.org/10.5962/bhl.title.286
Castro CC, Oliveira PEAM, Pimentel RMM (2008) Reproductive biology of the herkogamous vine Chiococca alba (L.) Hitchc. (Rubiaceae) in the Atlantic Rain Forest, SE Brazil. Rev Brasil Bot 31(2):317–321
De Block P (1995) Ovary, seed and fruit of Rutidea (Rubiaceae, Pavetteae). Pl Syst Evol 196:1–17
De Block P, Igersheim A (2001) Stigma of the African Genera Rutidea and Nichallea (Rubiaceae-Ixoroideae-Pavetteae): highly modified receptive surfaces. Int J Pl Sc 162:567–578
De Toni KLG, Mariath JEA (2004) Desenvolvimento do rudimento seminal em Borreria verticillata (L.) G. Mey. (Rubiaceae – Rubioideae – Spermacoceae). Rev Brasil Bot 27:185–219
De Toni KLG, Mariath JEA (2008) Ovule ontogeny in Rubiaceae (Juss.): Chomelia obtusa (Cinchonoideae–Guettardeae) and Ixora coccinea (Ixoroideae–Ixoreae). Pl Syst Evol 272:39–48
De Toni KLG, Mariath JEA (2010) Ovule ontogeny of Relbunium species in the evolutionary context of Rubiaceae. Austral J Bot 58:70–79
Douglas GE (1957) The inferior ovary. Bot Rev 23:1–46
Endress PK (2011) Angiosperm ovules: diversity, development, evolution. Ann Bot 107:1465–1489
Fagerlind F (1937) Embryologische, zytologische und bestäubungsexperimentelle studien in der familie Rubiaceae nebst Bemerkugen über einige polyploiditätsprobleme. Acta Horti Berg 2:196–470
Figueiredo RC, Masullo FA, Vieira RC, De Toni KLG (2013a) Development of carpels and ovules in Psychotria carthagenensis (Psychotrieae) and Rudgea macrophylla (Palicoureeae) (Rubioideae, Rubiaceae). S African J Bot 84:110–114
Figueiredo RC, Vieira RC, De Toni KLG (2013b) Development of the gynoecium of Guettarda pohliana in the context of Rubiaceae evolution. Botany 91:562–567
Figueiredo RC, Vieira RC, Mariath JEA, Moço MCC, De Toni KLG (2017) Development of carpels and ovules in Dialypetalanthus fuscescens Kuhlm. (Rubiaceae): an enigmatic taxon. Acta Bot Bras 31:128–133
Florentin MN, Cabaña Fader A, Gonzalez AM (2016) Morpho-anatomical and morphometric studies of the floral structures of the distylous Oldenlandia salzmannii (Rubiaceae). Acta Bot Bras 30:585–601
Galati BG (1991) Estudios embriológicos en la tribu Spermacoceae (Rubiaceae). Parte 1: Anatomia floral. Megasporogénesis Megagametogénesis Bol Soc Argent Bot 27:7–20
Gonzalez AM, Cristóbal CL (1997) Anatomía y ontogenia de semillas de Helicteres lhotzkyana (Sterculiaceae). Bonplandia 9:287–294
Groeninckx I, Vrijdaghs A, Huysmans S, Smets E, Dessein S (2007) Floral Ontogeny of the Afro-Madagascan Genus Mitrasacmopsis with Comments on the Development of Superior Ovaries in Rubiaceae. Ann Bot 100:41–49
Hallé F (1967) Étude biologique et morphologique de la tribu des Gardéniées (Rubiacées). Mém ORSTOM 22:1–146
Heslop-Harrison Y, Shivanna KR (1977) The receptive surface of the angiosperm stigma. Ann Bot 41:1233–1258
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Johri BM (1984) Embryology of angiosperms. Springer-Verlag
Judkevich MD (2019) Anatomía reproductiva en especies de Cordiera, Genipa, Randia y Tocoyena (Gardenieae - Rubiaceae), doctoral thesis
Judkevich MD, Salas RM, Gonzalez AM (2020, in press) Anther structure and pollen development in species of Rubiaceae and anatomical evidence of pathway to morphological dioecy. An Acad Bras Ciênc
Li A, Wu X, Zhang D, Barrett S (2010) Cryptic dioecy in Mussaenda pubescens (Rubiaceae): a species with stigma-height dimorphism. Ann Bot 106:521–531
Lloyd FE (1899) The comparative embryology of the Rubiaceae. Mem Torrey Bot Club 8:1–112
Luque R, Sousa HC, Kraus JE (1996) Métodos de coloração de Roeser (1972) - modificado - de Kropp (1972) visando a substituição do azul de astra por azul de alcião 8 GS ou 8 GX. Acta Bot Bras 10:199–212
Maheswari Devi H, Krishnam Raju PVSNG (1980) Embryology of two species of Dentella (Dentella repens and Dentella serpyllifolia). Proc Indian Acad Sci (plant Sci) 89:213–218
Mariath JEA, Cocucci AE (1997) The ovules of Relbunium hypocarpium in the context of the Rubiaceae. Kurtziana 25:141–150
Mayer SS, Charlesworth D (1991) Cryptic dioecy in flowering plants. Trends Ecol Evol 6:320–325
Mouly A, Kainulainen K, Persson C, Davis AP, Wong KM, Razafimandimbison SG, Bremer B (2014) Phylogenetic structure and clade circumscriptions in the Gardenieae complex (Rubiaceae). Taxon 63:801–818
Naiki A (2008) Breeding system in Mussaenda shikokiana (Rubiaceae). Bull Am Mus Nat Hist 62:21–26
Naiki A, Kato M (1999) Pollination system and evolution of dioecy from distyly in Mussaenda parviflora (Rubiaceae). Plant Species Biol 14:217–227
Pacini E (1996) Reproductive strategies in dioecious plants. Giorn Bot Ital 130:68–72
Pailler T, Humeau L, Figier J (1998) Reproductive trait variation in the functionally dioecious and morphologically heterostylous island endemic Chassalia corallioides (Rubiaceae). Biol J Linn Soc 64:297–313
Persson C (2000) Phylogeny of Gardenieae (Rubiaceae) based on chloroplast DNA sequences from the rps16 intron and trnL (UAA)-F(GAA) intergenic spacer. Nord J Bot 20:257–270
Puff C, Robbrecht E, Buchner R, De Block P (1996) A survey of secondary pollen presentation in the Rubiaceae. Opera Bot Belg 7:369–402
Razafimandimbison SG, Lantz H, Mouly A, Bremer B (2009) Evolutionary trends, major lineages, and new generic limits in the dioecious group of the tribe Vanguerieae (Rubiaceae): insights into the evolution of functional dioecy. Ann Missouri Bot Gard 96:161–181
Robbrecht E (1988) Tropical Woody Rubiaceae Opera Bot Belg 1:1–271
Robbrecht E, Puff C (1986) A survey of the Gardenieae and related tribes (Rubiaceae). Bot Jahrb Syst 108:63–137
Robbrecht E, Puff C, Igersheim A (1991) Evidence for the close alliance between Mitchella and Damnacanthus, with comments on the campylotropy in the Rubiaceae and the circumscription of the Morindeae. Blumea 35:307–345
Robbrecht E, Manen JF (2006) The major evolutionary lineages of the coffee family (Rubiaceae, angiosperms). Combined analysis (nDNA and cpDNA) to infer the position of Coptosapelta and Luculia, and supertree construction based on rbcL, rps16, trnL-trnF and atpB-rbcL data. A new classification in two subfamilies. Cinchonoideae and Rubioideae Syst Geogr Pl 76:85–145
Romero MF, Salas RM, Gonzalez AM (2021) Floral anatomy, embryology, seed, and fruit development in Cephalanthus (Naucleeae-Rubiaceae), with emphasis on C. glabratus. Protoplasma https://doi.org/10.1007/s00709-021-01664-8
Schleiden MJ (1837) Einige Blicke auf die Entwicklungsgeschichte des vegetabilischen Organismus bei den Phanerogamen. Arch Naturgesch 1:289–414
Schumann K (1891) Rubiaceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol 4. Engelmann, Leipzig, pp 1–156
Sugawara T, Tanaka N, Murata J (2011) Dioecy Derived from Distyly in Morinda villosa Hook. f. (Rubiaceae) Occurring in Hukaung Valley, Kachin State. Myanmar J Jap Bot 86:9–14
Svoma E (1991) The development of the bicarpellate gynoecium of Paederia L. species (Rubiaceae – Paederieae). Opera Bot Belg 3:77–86
Terra-Araujo MH, Webber AC, Vicentini A (2012) Pollination of Pagamea duckei Standl. (Rubiaceae): a functionally dioecious species. Biota Neotrop 12:98–104
Von Teichman I, Robbertse PJ, Van der Merwe CF (1982) Contributions to the floral morphology and embryology of Pavetta gardeniifolia A. Rich. Part 2. The ovule and megasporogenesis. S Afr J Bot 1:22–27
Watanabe K, Shimizu A, Sugawara T (2014) Dioecy derived from distyly and pollination in Psychotria rubra (Rubiaceae) occurring in the Ryukyu Islands, Japan. Plant Species Biol 29:81–191
Wunderlich B (1971) Die systematische Stellung von Theligonum. Oesterreichische Botanische Zeitschrift 119:329–394
Acknowledgements
We thank Javier Florentín, Mariela Núñez Florentín, Victor Dávalos, and Walter Medina for providing fixed materials used in this work. Also we thank Rosemary Scoffield for critically reading the English manuscript.
Funding
The first author thanks CONICET by its doctoral grant. This work was funded by PICTO-UNNE 0199/2011, PICT 2016–3517, and CONICET PIP-112–2011-0100906 grants.
Author information
Authors and Affiliations
Contributions
RMS provided the plant material and supplied taxonomic data of the species and the tribe. MDJ processed the plant material to make the histological preparations, took the microscopy photos, and prepared the figures. MDJ and AMG did the anatomical interpretations. MDJ and AMG contributed in the discussions and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Handling Editor: Dorota Kwiatkowska.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
List of the analyzed species of Gardenieae and their voucher information
Cordiera concolor (Cham.) Kuntze. Argentina. Misiones, San Ignacio, Teyú Cuaré, 01 Mar 2013, Judkevich MD & Salas RM 11 (FPF). Idem, 23 Apr 2016, Judkevich MD & Salas 73 (FSF). Idem, 23 Apr 2016, Judkevich MD & Salas 74 (FPF).
Genipa americana L. Argentina. Formosa, Guaycolec, Estancia “Bella Mar”, 11 Sep 2014, Judkevich MD & Salas RM 53 (FPF). Idem, Estancia Miriquiná, 28 Jan 2015, Judkevich MD & Salas RM 58 (FPF). Idem, Judkevich MD & Salas RM 59 (FPF). Idem, 22 Dic 2015, Judkevich MD et al. 73 (FPF). Idem, Reserva Biológica Guaycolec, 16 Nov 2016, Judkevich MD et al., 84 (FPF). Paraguay. Asunción, Campus UMA, 24 Nov 2016, Judkevich MD et al. 85 (FSF).
Randia calycina Cham. Argentina. Formosa, Guaycolec, Estancia “Bella Mar”, 10 Sep 2014, Judkevich MD & Salas RM 49 (FPF). Idem, Judkevich MD & Salas RM 52 (FSF). Idem, “Monte Lindo Chico”, 11 Sep 2014, Judkevich MD & Salas RM 54 (FPF). Chaco, Primero de Mayo, Colonia Benitez, 28 Nov 2014, Judkevich MD & Salas RM 57 (FPF). Idem, 01 Oct 2015, Judkevich MD & Salas RM 70 (FPF). Idem, 01 Oct 2015, Judkevich MD & Salas RM 71 (FPF). Idem, 01 Oct 2015, Judkevich MD & Salas RM 72 (FPF).
Randia heteromera. Argentina. Corrientes, Riachuelo, 17 Sep 2014, Judkevich MD & Salas RM 55 (FSF). Idem, Judkevich MD & Salas RM 56 (FPF). Idem, Puente Pexoa, 14 Sep 2016, Judkevich MD et al. 75 (FSF). Idem, 14 Sep 2016, Judkevich MD et al. 77 (FPF). San Cosme, Las Lomas, Ensenada Grande, 29 Aug 2015, Judkevich MD et al. 61 (FSF). Idem, Judkevich MD et al. 62 (FSF). Dpto: Dan Miguel, Estancia “Tranquita”, 11 Sep 2015, Judkevich MD et al. 63 (FPF). Idem, 11 Sep 2015, Judkevich MD et al. 64 (FSF). Idem, 11 Sep 2015, Judkevich MD et al. 65 (FPF). Idem, Estancia “Santa Julia”, 11 Sep 2015, Judkevich MD et al. 66 (FSF). Idem, 11 Sep 2015, Judkevich MD et al. 67 (FPF). Idem, 11 Sep 2015, Judkevich MD et al. 68 (FSF).
Tocoyena formosa (Cham. & Schltdl.) K. Schum. Paraguay. Asunción, Cerro Tobatí, 25 Nov 2016, MM et al. 187 (PF). Idem, 25 Nov 2016, MM et al. 188 (PF).
Rights and permissions
About this article
Cite this article
Judkevich, M.D., Salas, R.M. & Gonzalez, A.M. Embryology of some flowers of the Gardenieae complex (Rubiaceae). Protoplasma 259, 1233–1254 (2022). https://doi.org/10.1007/s00709-022-01734-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-022-01734-5