Abstract
Allelopathic compounds released by invasive alien plants can suppress the growth of plants in their vicinity. The aim of this study was to investigate changes in tissue and cell structure in roots of radish seedlings treated with 10% aqueous extracts of rhizomes from the invasive knotweeds Fallopia japonica and F. ×bohemica. After 7 days of growth without and with aqueous extracts from these rhizomes, the anatomical and ultrastructural changes in the radish seedling roots were analyzed with light and transmission electron microscopy, and hydrogen peroxide was localized with diaminobenzidine, to define oxidative stress. The roots of radish seedlings treated with the knotweed extracts were shorter and thicker, due to the shorter and wider shapes of their cortex cells, which were organized in more columns than the control roots. There were signs of cell damage and oxidative stress in the root cap cells, and to a lesser extent in the meristematic zone. As well as the irregularly shaped nuclei and plasma membrane detached from the cell wall, the most prominent ultrastructural effects in the root cap cells of these aqueous rhizome extracts were the ring-shaped form of the mitochondria and large endoplasmic reticulum bodies. Excessive vacuolization was seen for the cells of the root apical meristem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive plants are very successful with their plant–plant interactions, as they have several competitive advantages over the native flora. Consequently, their dominance can lead to reduced species richness (Murrell et al. 2011). Fallopia japonica var. japonica (Houtt.) Ronse Decr. (Japanese knotweed) is a shrub native to East Asia, and is now among the 100 most invasive taxa in Europe and North America (Lowe et al. 2000). Fallopia × bohemica (Chrtek and Chrtková) J. P. Bailey (Bohemian knotweed) is a hybrid of F. japonica and F. sachallinensis (F. Schmidt) Ronse Decr. (giant knotweed), and it has been reported to be even more invasive than Japanese knotweed (Bailey et al. 2009).
Knotweeds have several strategies that allow them to dominate their native plant neighbors: rapid vegetative propagation through underground rhizomes (Bailey et al. 2009); large biomass production (Frantík et al. 2013); and good regeneration capacity (Bímová et al. 2003). However, there is another important additional mechanism behind the success of this invasion of knotweeds. The “novel weapon” hypothesis posits that the advantage of invasive plants is their release of biologically active compounds into the soil, which can have strong negative effects on other nearby plants—the phenomenon known as allelopathy (Callaway and Aschehoug 2000; Murrell et al. 2011). Knotweed rhizomes contain numerous allelochemicals, which include in particular stilbenes and complex polyphenols. Resveratrol, (-)-catechin, (-)-epicatechin, piceid, and emodin represent five of the secondary compounds most frequently isolated from knotweed rhizomes (Fan et al. 2009).
Allelopathic compounds have been reported to have effects on many cellular processes, including photosynthesis, mitochondrial respiration, DNA and RNA synthesis, amino acid metabolism, and mineral uptake (Gniazdowska and Bogatek 2005). These can result in structural and ultrastructural changes to plant roots, such as increased root width, changes in the patterns of cell elongation, reduced numbers of cell organelles, and several anomalies of organelle structure (Burgos et al. 2004). At the organism level, reduced seed germination and reduced growth of exposed plants are the more obvious signs of the allelopathic potential of knotweed (Siemens and Blossey 2007; Fan et al. 2010; Moravcová et al. 2011; Tucker Serniak 2016).
As for many other stresses, allelopathic compounds can also trigger uncontrolled production and accumulation of reactive oxygen species (ROS) (Gechev et al. 2006), such as hydrogen peroxide (H2O2). ROS are known to include signaling molecules that are involved in plant development and in the regulation of plant responses to biotic and abiotic stress. However, excessive production of ROS results in oxidative stress, with the consequent damage to proteins, lipids, and DNA (Das and Roychoudhury 2014). Compared to other toxic by-products of aerobic metabolism, H2O2 is more stable, with a half-life of 1 ms, and it can therefore migrate from its site of synthesis to other cell compartments, or even to nearby cells. At high H2O2 concentrations, programmed cell death can even be induced (Gechev et al. 2006).
While previous allelopathy studies on knotweeds have mainly focused on the inhibition of germination and growth of seedlings, the background of this growth inhibition has not been investigated in depth. Consequently, the effects of knotweed extracts on root morphology are neither well understood nor have been analyzed in any detail. Therefore, the aim of this study was (i) to compare the effects of aqueous extracts of F. japonica and F. ×bohemica rhizomes on radish seedlings; (ii) to define histological and ultrastructural effects on radish root tips following these treatments; and (iii) to define possible sites of H2O2 accumulation in the radish seedling tissues.
Materials and methods
Plant material preparation
The rhizomes of Japanese knotweed and Bohemian knotweed were collected in Ljubljana, Slovenia (46° 2′ 33.98″ N, 14° 27′ 0.91″ E; 46° 3′ 0.3″ N, 14° 28′ 44″ E; respectively) in March and April 2016. The identity of both populations was previously confirmed with genome size analysis (Strgulc Krajšek and Dolenc Koce 2015). The knotweed rhizomes were dried, lyophilized, ground in a cutting mill (SM 200; Retsch, Germany), and stored in the dark at room temperature prior to preparation of the extracts.
The dicot species of radish (Raphanus sativus L. cv. Saxa 2) was selected as the test species because of its rapid growth and frequent use in allelopathic studies (Aliotta et al. 1993; De Feo et al. 2003; Tucker Serniak 2016). For the germination and seedling growth assays, 50 radish seeds (Semenarna Ljubljana, Slovenia) were placed in each Petri dish (diameter, 14 cm) on a layer of filter paper.
Preparation and HPLC analysis of the extract of Fallopia rhizomes
The same aqueous extracts were used for this and previous study when effects on germination and early growth of radish were tested (Šoln et al. 2021). Briefly, the contents of the four main allelochemicals in the rhizomes of F. japonica and F. ×bohemica were determined with high-pressure liquid chromatography (HPLC). Ground rhizomes were suspended in distilled water and 33% methanol to prepare 10% aqueous and methanol extracts, respectively.
For the HPLC analysis, 10 μg/mL of the individual standards of resveratrol, catechin, (-)-epicatechin, and emodin (all Sigma-Aldrich, USA), and the same volume of F. japonica or F. ×bohemica extract were recorded (Alliance 2695 with 2996 PDA and 2475 fluorimetric detectors; Waters, USA) at 320 nm for resveratrol, 435 nm for emodin, and 280/315 nm (kEx/kEm) for catechin and epicatechin. Identification and quantification of the four allelochemicals in the F. japonica and F. ×bohemica extracts were achieved by comparing the HPLC spectra of the standard compounds with the spectra from the extracts, using Empower 4 software (Waters, USA).
Preparation of the aqueous extract of Fallopia rhizomes
The ground rhizome material of F. japonica and F. ×bohemica was suspended in distilled water (5 g rhizome in 50 mL distilled water). The mixtures were shaken at 175 rpm for 24 h at room temperature on an orbital shaker (Laboshake 500; Gerhardt, Germany). After this aqueous extraction, the mixtures were vacuum filtered to provide the 10% (w/v) aqueous extract. These extracts were prepared fresh before the experiment, and stored at 4 °C between the applications, as described previously (Dolenc Koce and Šoln 2018).
Seed germination and early growth of radish
Three independent replicates were performed for each treatment (n = 50). At the beginning of the experiment, the seeds were watered with 10 mL distilled water (control) or the 10% aqueous extracts from F. japonica or F. ×bohemica. The seedlings were left to grow up to 7 days and were watered with 3 mL distilled water on the 3rd and 5th day in all treatments to sustain moisture. After 7 days, the germinated seeds were counted to determine the germination rates, and photographed. The lengths of roots and shoots were measured on the digital photos using the ImageJ 1.x software (Schneider et al. 2012).
Light and electron microscopy of radish roots
The experimental system for analysis of the seedling roots is shown in Fig. 1. The root samples used for light microscopy and transmission electron microscopy (TEM) were ~3-mm-long pieces of the root tips, which consisted of the root apical meristem, the root cap, and the nearby tissues. The samples were fixed in 3% glutaraldehyde in 75 mM phosphate buffer (pH 7.2) for 2 h to 3 h on a rotor at 20 °C, and then overnight at 4 °C. After washing in 75 mM phosphate buffer (pH 7.2), the samples were postfixed in 1% osmium tetroxide for 1.5 h, washed again, and dehydrated through a graded series of ethanol (30%, 50%, 70%, 90%, 100%) and then acetone (100%). The samples were embedded in Agar 100 resin (Agar Scientific, UK). Semi-thin (1 μm) longitudinal sections were cut with a glass knife, stained with Azure II–methylene blue, and imaged under light microscopy (Axioskop 2 MOT; Carl Zeiss, Germany) equipped with a color digital camera (AxioCam MRc; Carl Zeiss, Germany). Light microscopy examination was performed to compare the structures of the root tips of the control and treated seedlings, and to measure the sizes of the cortex cells. Root width and number of cell columns were determined at 650 μm from the distal end of the root tip (n = 5). The lengths and widths of 25 randomly selected cortex cells were measured at 650 μm to 900 μm from the root tip. The measurements were performed on micrographs using the ImageJ 1.x software (Schneider et al. 2012). The ratios between cell length and width were calculated. Ultrathin longitudinal sections were also cut (Reichert Ultracut S ultramicrotome; Leica, Austria), collected on Formvar-coated slotted grids, contrast treated with 1% uranyl acetate for 10 min and 10% lead citrate for 5 min, and examined by TEM (CM100; Philips, The Netherlands), using a digital camera (BioScan 792; Gatan, USA). The first three cell layers of the root cap and the meristematic zone were analyzed in detail. Images were processed using the Photoshop CS5 and Illustrator CS5 software (Adobe System, USA). The experiments were repeated twice, independently.
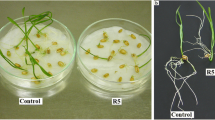
Radish seedling after 7 days of germination (a), fresh microscopic preparation of the radish root (b), longitudinal section of the radish root (c), and longitudinal section of the radish root tip (d). Abbreviations: apical meristem (AM), cortex (C), epidermis (E), root (R), root cap (RC), root hair (RH), root tip (RT), shoot (S), vascular cylinder (VC)
Localization of hydrogen peroxide
After 5 days and 7 days of control and Fallopia aqueous extract treatments, the radish seedlings were stained with 3,3′-diaminobenzidine (DAB) to localize H2O2 (Jambunathan 2010). Briefly, 15 seedlings were placed in a Petri dish with DAB solution (1 mg DAB/mL, pH 3.0), vacuum infiltrated for 10 min, and incubated for 4 h on an orbital shaker (40 rpm, room temperature). After the incubation, the brown precipitate associated with H2O2 was observed. The stained seedlings were fixed in a solution of 96% ethanol:acetic acid:glycerol (3:1:1; v/v/v), and photographed under a stereo microscope (Stemi SV; Carl Zeiss, Germany) equipped with a color digital camera (AxioCam MRc; Carl Zeiss, Germany) using the AxioVison 4.8 software (Carl Zeiss, Germany). The images were processed with the Photoshop CS5 and Illustrator CS5 software (Adobe System, USA). Five independent replicates were performed for each treatment (n = 5).
Statistical analysis
For the statistical analyses, means and standard errors were calculated for each measured parameter. The means were compared between treatment groups using one-way ANOVA, and Holm–Sidak post hoc tests when the differences were statistically significant. Two-way ANOVA was used to compare the effects of the knotweed taxa, extract concentrations, and their interactions (Excel with XL Toolbox 7.2.13; Microsoft). Differences in the content of the three main active compounds in the rhizomes between the two knotweed taxa were evaluated by one-way ANOVA and Duncan’s post hoc adjustments using the statistical software R (version R x64 3.6.3) and Library Agricolae 1.3-3. The level for statistical significance was defined as P <0.05.
Results
HPLC analysis of aqueous extracts from Fallopia rhizomes
The HPLC spectra of aqueous and methanol extracts from rhizomes of F. japonica and F. ×bohemica showed that both species contained all four allelochemicals (Fig. 2). The F. ×bohemica extract contained 26.1-fold more catechin, 18.2-fold more epicatechin, and 1.8-fold more emodin than F. japonica extract. However, more resveratrol was present in F. japonica extract with 2.8-fold higher concentration than in F. ×bohemica.
Seed germination and early growth of the radish seedlings
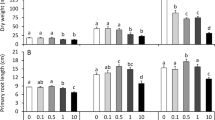
Germination of the radish seeds overall was >95% and was not affected by the knotweed extracts (P = 0.697). However, both extracts significantly and strongly inhibited the growth of the radish roots (Table 1). Treatment with the F. japonica aqueous extract showed significantly shorter root lengths by 65.9% (P <0.001), and for F. ×bohemica by 63.5% (P <0.001). The roots of the treated seedlings were also significantly thicker compared to the control samples (Fig. 3, Table 1); when treated with the F. japonica aqueous extract, by 84.7% (P = 0.003), and with F. ×bohemica, by 72.1% (P = 0.002). These effects were similar for both of the aqueous extracts, and were thus not related to the knotweed taxa.
Primary root of 7-day-old radish seedlings (left) and longitudinal section of the root tip (right): control seedlings (a, b), and treatment with the aqueous extracts of Fallopia japonica (c, d) and F. ×bohemica (e, f) rhizomes. Abbreviations: apical meristem (AM), cortex (C), root cap (RC). Bars: 1 mm (a, c, e), 100 μm (b, d, f)
The shoots were less affected by these knotweed aqueous extracts than the roots (Table 1), as they were only significantly shorter with the F. japonica aqueous extract, by 21.5% (P <0.001); the F. ×bohemica aqueous had no significant effect on shoot growth (P = 0.229).
Histological characteristics of the root tip morphology
Histological analysis showed strong effects of the F. japonica and F. ×bohemica aqueous extracts on the morphology of these radish roots when compared to the control treatment, especially for the cortex, root cap, and apical meristem.
Effects on the root cortex were evaluated in the region from 650 to 900 μm above the most distal part of the root tip. These knotweed aqueous extracts influenced the shape of the cortex cells: the cells were 56.0% and 41.0% shorter, and 44.9% and 32.3% wider (P <0.001, for both) for the seedlings treated with the aqueous extracts of F. japonica and F. ×bohemica, respectively (Table 1). These aqueous extracts also significantly increased the number of cell columns by 85.6% and 88.9%, respectively (P <0.001, for both), which thus resulted in densely packed multiple columns.
The tissues in the root tips following these treatments were disorganized. The structures of the root cap and root apical meristem were greatly altered, such that it was difficult to delineate the cell types (Fig. 3), especially for the boundary between the root cap and the root apical meristem. In the control seedling roots, there were 3 or 4 layers of root cap cells, which were larger in the outer layers (Fig. 3b). Most of the roots of the seedlings treated with the F. japonica aqueous extract had 5 or 6 layers of flattened and disintegrated root cap cells and 3 or 4 layers of normal shaped root cap cells (Fig. 3d). Treatment with the F. ×bohemica aqueous extract resulted in 5 or 6 layers of root cap cells that were even larger than the cells of the control seedlings (Fig. 3f). Both of these extracts also influenced the root apical meristem. While the meristematic zone in the control seedlings was clearly distinguished by the darkly stained cells, the meristematic zone could not be clearly identified in the seedlings treated with the aqueous extracts of F. japonica (Fig. 3d) and F. ×bohemica (Fig. 3f).
Ultrastructure of root tip cells
The TEM analysis of the longitudinal sections of the root tips was focused on two regions: the cells of the root cap and the root apical meristem.
The root cap cells of the control seedlings contained a large central vacuole (Fig. 4a), electron-dense mitochondria, and numerous dictyosomes and cisternae of the endoplasmic reticulum (ER), which were often oriented parallel to the plasma membrane. Individual ring-shaped mitochondria and mitochondria with dilated cristae were only rarely observed. The nuclei were spherical. The cells were interconnected with thin plasmodesmata oriented perpendicular to the cell wall (Fig. 4b). In contrast, F. japonica and F. ×bohemica aqueous extracts strongly influenced the ultrastructure of the root cap cells. Here, the cells had thickened and wrinkled cell walls, with the plasma membrane detached from the cell walls (Fig. 4c, d). In the F. japonica–treated seedlings, the inner cells of the root cap were of normal shape with thickened cell walls, while most of the outer cells were flattened, with disintegrated and unrecognizable organelles (Fig. 4e). In the F. ×bohemica–treated seedlings, all of the root cap cells were large and had retained their shape, and they contained large vacuoles (Fig. 4f).
Ultrastructure of radish root cap cells from control seedlings (a, b) and seedlings treated with the aqueous extracts of Fallopia japonica (c–e) and F. ×bohemica (f) rhizomes after 7 days of growth. Abbreviations: cell wall (CW), endoplasmic reticulum (ER), mitochondrion (M), plasmodesma (P), vacuole (V). Asterisk (*) indicates ER body; arrow indicates plasma membrane detached from the cell wall
Ultrastructural effects to the nuclei, ER, mitochondria, and plasmodesmata were also seen for the root cap cells of the treated seedlings (Fig. 5). The nuclei were irregularly shaped (Fig. 5a), and the ER mainly consisted of dilated cisternae filled with electron-dense material, i.e., ER bodies (Fig. 5b). In the control radish seedling roots, only a few ER bodies were seen in each cell (i.e., 1–3 per cell), which were spherical, with a diameter that did not exceed 1 μm. In the treated roots, the ER bodies were more abundant (8–12 per cell) and were longitudinal in shape. Their lengths were generally ~2 μm, although for the F. ×bohemica–treated seedlings, some ER bodies were up to 10 μm long. Mitochondria with dilated cristae (Fig. 5c) and ring-shaped mitochondria (Fig. 5d) were an outstanding feature of these root cap cells in the treated seedlings. Also, the plasmodesmata were curved and filled with electron-dense material in both the F. japonica–treated (Fig. 5e) and F. ×bohemica–treated (Fig. 5f) seedlings. The dictyosomes were similar to those in the control seedling roots. Amyloplasts and starch grains were rarely observed in either the control or the treated samples.
Ultrastructural changes in radish root cap cells after 7 days of growth after the treatment with the aqueous extracts of Fallopia japonica and F. ×bohemica rhizomes. TEM micrographs show irregularly shaped nuclei (a), dilated ER cisternae with dense content, i.e., ER bodies (b), mitochondria with dilated cristae (c), ring-shaped mitochondria (d), curved plasmodesmata filled with electron-dense material (e), and detached plasma membrane (f). Abbreviations: cell wall (CW), dictyosome (D), endoplasmic reticulum (ER), mitochondrion (M), nucleus (N), nucleolus (Nu), plasmodesma (P), vacuole (V). Asterisk (*) indicates ER body; arrow indicates plasma membrane detached from the cell wall. The panels b, c, and e were photographed in F. japonica–treated seedlings and panels a, d, and f in F. ×bohemica–treated seedlings
The apical meristem was clearly identified in the control seedling roots, where the meristem cells contained dense cytoplasm with well-developed organelles, as mitochondria, dictyosomes, ER cisternae, and several small vacuoles (Fig. 6a, b). In the roots of the seedlings treated with the knotweed extracts, either the apical meristem zone was not distinguishable or the meristem cells were larger (Fig. 6c) with many large vacuoles (Fig. 6d) and light cytoplasm. In some of the roots of the seedlings treated with the F. japonica aqueous extract, the meristem cells were triangular in shape with broken down cell content (Fig. 6e), and the plasma membrane was detached from the cell wall, and multivesicular bodies were seen (Fig. 6f).
Ultrastructure of radish root meristem cells from control seedlings (a, b) and seedlings treated with the aqueous extracts of Fallopia ×bohemica (c, d) and F. japonica (e, f) rhizomes after 7 days of growth. Abbreviations: cell wall (CW), endoplasmic reticulum (ER), mitochondrion (M), multivesicular body (MVB), nucleus (N), nucleolus (Nu), vacuole (V)
Hydrogen peroxide distribution
The localization of H2O2 in the 5-day-old and 7-day-old radish seedlings that was revealed using DAB showed intense staining in the roots (Fig. 7). After 5 days of growth, the older and more differentiated root tissues were light brown in color, while the root tips were dark brown, which indicated a higher H2O2 content. The staining was even more pronounced after 7 days, when the seedlings had dark brown roots and almost black root tips. However, there was no difference in H2O2 accumulation between the treatments with the F. japonica (Fig. 7e) and F. ×bohemica aqueous extracts (Fig. 7f). On the other hand, the control seedlings showed less intense staining, which suggested lower H2O2 content.
The H2O2 was also localized in the cotyledons of the 7-day-old seedling (Fig. 8d–f), although there were no differences between the control and treated seedlings. The hypocotyls did not show any H2O2 staining (Fig. 8a–c).
Discussion
The allelopathic effects of Fallopia rhizome aqueous extracts on radish seedlings were mainly expressed in their roots. They were shorter and thicker due to the smaller and wider cells that were packed in more columns than in the control roots. The cells in the root tips showed prominent ultrastructural effects that can be assigned to necrotic processes that can lead to cell death.
Many biologically active compounds have been identified in knotweed, as mainly phenols, such as resveratrol, catechin, epicatechin, emodin, and piceid (Fan et al. 2009). Their contents vary in the different parts of the plants, with the rhizomes being the richest for phenols. Among these phenols, resveratrol, emodin, and epicatechin have been reported to reduce radish root length (Tucker Serniak 2016). These compounds and additionally catechin were also identified in our previous study in the rhizome aqueous and methanol extracts using HPLC (Šoln et al. 2021). The F. ×bohemica aqueous extract generally contained more compounds than F. japonica extract. The highest concentration was measured for epicatechin (36.28 mg/mL), then followed catechin (10.95 mg/mL), emodin (1.34 mg/mL), and resveratrol with the lowest concentration (0.54 mg/mL). In F. japonica extract, the concentrations of all allelochemicals were lower than 2.00 mg/mL but the resveratrol content was 2.8-fold higher than in F. ×bohemica extract. Higher content of resveratrol, and some other phenols, has been reported for F. japonica rhizomes previously (Chen et al. 2013; Frantík et al. 2013). In extracts, which are a mixture of many compounds, it is not only their absolute concentration that is relevant to the effects and biological activity, but also the ratios of the components to each other (De Feo et al. 2003). The differences in the allelochemical contents across these aqueous extracts in the present study might be the cause for the slight morphological and ultrastructural effects in radish seedlings after the treatment with these extracts of F. japonica and F. ×bohemica rhizomes. The radish roots would be more affected than the shoots because they are the first to interact with the allelopathic compounds (Aliotta et al. 1993).
In the present study, the roots of the radish seedlings treated with the knotweed aqueous extracts were not only shorter but also thicker than for the control treatment, due to the shorter length and increased width of the cells, and also more cell columns in the cortex. Inhibition of cell elongation was documented in radish roots after exposure to the allelopathically active coumarin (Aliotta et al. 1993). An altered pattern of cell elongation was also observed in the roots of cucumber (Cucumis sativus) seedlings as a response to treatment with the rye (Secale cereale) allelochemicals 2(3H)-benzoxazolinone (BOA) and 2,4-dihydroxy-1,4(2H)-benzoxazin-3-one (DIBOA) (Burgos et al. 2004).
The underlying mechanisms for altered cell growth can be diverse, but only some of them have been directly linked to allelopathic compounds. Some allelochemicals can affect auxin transport, as shown for weisiensin B, which reduces gene expression for auxin PIN transporters, to lead to increased auxin concentrations in the root apex and suppressed growth of the primary root (Li et al. 2019). Coumarin modulates the auxin distribution in roots of Arabidopsis seedlings by affecting auxin transporters (Lupini et al. 2014). Cytokinins are also involved in cell wall remodeling, thus driving differentiation through the mechanical control of the cell wall (Pacifici et al. 2018).
The lower length-to-width ratios and higher numbers of cell columns here indicate that these knotweed aqueous extracts might have effects on the cytoskeleton. The allelochemical cyanamide was shown to affect the arrangements of microtubules of preprophase bands and phragmoplasts, which resulted in alterations to cell division and to the cell cycle (Soltys et al. 2011). Cortical microtubules might also be shifted, resulting in different arrangements of cellulose microfibrils in the cell wall, which will affect the direction of cell elongation. In addition, cell growth might be affected by loosening and stiffening of the cell wall, due to the activation of cell wall peroxidases by allelopathic compounds (Gonzáles and Rojas 1999).
Next, we show that both of these knotweed aqueous extracts had a strong influence on the root cap of the radish seedlings. The growth of the root cap cells determines the growth of the root, and therefore any changes in root cap cells will have a strong influence on elongation of the root (Kumpf and Nowack 2015). The aqueous extract from F. japonica was more destructive than that from F. ×bohemica, which might be attributable to their differences in resveratrol content and the ratio between these different compounds in complex mixtures like these aqueous extracts. We noted that for most of the radish roots treated with the F. japonica aqueous extract, there were several layers of disintegrated cells with abnormal shapes and with degenerated organelles. Such a pronounced destructive effect on the root cap was not seen for BOA, which strongly reduced the number of cell layers in the root caps of cucumber seedlings (Burgos et al. 2004), nor for an extract from the cucurbit Sicyos deppei, which resulted in smaller, but undamaged, root cap cells of treated bean seedlings (Cruz-Ortega et al. 1998). The reason for more destructive effects in the present study could be the other allelochemicals and higher, phytotoxic concentrations.
On the other hand, the root caps of the F. ×bohemica–treated radish had 2 or 3 additional cell layers, the cells were larger, and there were no newly formed cells. This indicates that the F. ×bohemica aqueous extract stimulated differentiation of the root cap cells or slowed down their disposal. Changes in the patterns of cell differentiation are a common sign of stress-induced morphogenic responses, as a general structural response of plants to various types of stress (Potters et al. 2007), including allelopathy (Burgos et al. 2004).
At the ultrastructural level, the rhizome aqueous extracts of F. japonica and F. ×bohemica strongly influenced the structure of the mitochondria in the root cap cells of the radish seedlings. While the mitochondria in the control radish roots usually had round or oval cross-sectional profiles, the mitochondrial profiles in the treated seedlings were far from this classical shape, and were often ring-shaped. Such mitochondria have rarely been recorded for plant cells. Some studies reported their occurrence during some developmental processes in egg cells of the fern Pteridium aquilinum (Bell and Mühlethaler 1964) and after various stresses, which included cold stress in the root tip cells of cucumber (Cucumis sativus) (Lee et al. 2002), and salt stress in embryogenic calli of lemon (Citrus limon) (Piqueras et al. 1994). Effects on the mitochondrial structure might also be reflected in their function, as shown by a previous study of six inhibitors of mitochondrial respiration, which changed the normal shape of the mitochondria in the root cells into ring shapes (Ponomareva and Polygalova 2012). In animal cells, however, ring-shaped mitochondrial profiles have been recorded for a variety of normal tissues, as well as under pathological and stress conditions (Miyazono et al. 2018). The significance of this shape transformation is not clear, but it has often been interpreted as a morphological feature of functional impairment of mitochondria, and especially as disruption of the mitochondrial membrane potential (Miyazono et al. 2018). At this point, it is worth noting that ring-shaped profiles of mitochondria might well represent cup-shaped mitochondria, but in a different sectional plane (Ponomareva and Polygalova 2012; Miyazono et al. 2018).
The second mitochondrial alterations observed in the root cap cells of the treated radish seedlings in the present study were mitochondria with dilated cristae, which indicates intracristal swelling. These swollen mitochondria were also reported for root cap cells of bean (Phaseolus vulgaris) after treatment with aqueous leachates of S. deppei (Cruz-Ortega et al. 1998). The general swelling of the organelles is a sign of necrotic cell death (van Doorn et al. 2011).
As structural changes are closely related to functional changes, we consider that these knotweed aqueous extracts not only affected the mitochondrial structure in the root cap cells, but also had an impact on mitochondrial functions, and contributed to the inhibition of root growth. For several allelochemicals, their effects on mitochondria and cellular respiration are known: quercetin inhibits substrate oxidation and phosphate uptake in isolated mitochondria of soybean (Glycine max) hypocotyl (Takahashi et al. 1998); juglone disrupts mitochondrial membrane potential in the root tip of lettuce (Lactuca sativa) seedling (Babula et al. 2014); α-pinene inhibits both basal and coupled respiration in isolated mitochondria of maize (Zea mays) (Abrahim et al. 2000); and the rye allelochemicals BOA and DIBOA reduce the number of mitochondria in the roots of cucumber (Cucumis sativus) seedling (Burgos et al. 2004).
Furthermore, numerous dilated ER cisternae with dense contents were seen for the root cap cells of the treated radish seedlings. This peculiar ER structural type was described earlier in radish seedlings in the zone of root hair formation (Bonnett and Newcomb 1965), and these are now known as ER bodies. ER bodies are considered to be specific ER domains that accumulate ß-glucosidases, and numerous studies of their structure and function have been conducted in plants of the order Brassicales (Hara-Nishimura and Matsushima 2003; Nakano et al. 2014). Their role is not well understood, however, although they are believed to be involved in plant responses to biotic stress (Hara-Nishimura and Matsushima 2003), and in particular in protection against wounding (Matsushima et al. 2002). Recently, the role of ER bodies as a chemical defense against herbivores based on the activity of ß-glucosidase has been reported in Brassicaceae (Yamada et al. 2020).
The abundance of ER bodies is upregulated by stress hormones, such as methyl jasmonate, in particular, as has been shown in radish roots (Gotté et al. 2015) and Arabidopsis thaliana leaves (Matsushima et al. 2002). Our results show that the formation of ER bodies in radish roots is enhanced in number and size in response to this allelopathic stress. In the treated radish roots, the ER bodies were more than twice the length compared to the control, and their shapes were more elongated (oval), which indicates a higher content of accumulated substances. According to these ultrastructural effects, we assume that these knotweed aqueous extracts triggered the cell death cascade in the roots of these radish seedlings.
Some other cell structures in the radish root cap were also altered by these knotweed aqueous extracts. The cell wall was mainly thickened, and the plasma membrane mostly detached from the cell wall. These effects also indicate programmed cell death (Huysmans et al. 2017). In addition, the treated radish seedlings contained curved plasmodesmata that were filled with electron-dense material. The openness of plasmodesmata depends on developmental and environmental signals, which include stress, such as wounding and pathogen infections, which can lead to their obstruction (Benitez-Alfonso et al. 2010). The aqueous extracts of both of these knotweeds also influenced the shape of the nuclei, as many nuclei in the treated radish seedlings were of irregular shape. This effect is comparable to the effects of a S. deppei extract (Cruz-Ortega et al. 1998) and rye allelochemicals (Burgos et al. 2004), which result in irregularly shaped nuclei and, in the case of S. deppei, smaller and denser nucleoli.
As well as regulation of root growth, the root cap also protects the apical root meristem. If the root cap is damaged, it can no longer provide effective protection, and the meristem zone can also be affected (Kumpf and Nowack 2015). In the present study, the extracts of F. japonica and F. ×bohemica blurred the boundary between the root cap and the meristem, on the one hand, and the meristem and the elongation zone with the cortex cells, on the other. The effects were more distinct for the F. japonica aqueous extract.
Many cells with prominent vacuolization and elongation were observed in the root meristem of the treated radish. Excessive vacuolization was detected in meristematic cells of cucumber treated with BOA and DIBOA (Burgos et al. 2004), in radish seedlings exposed to coumarin (Aliotta et al. 1993), and in bean seedlings treated with S. deppei extract (Cruz-Ortega et al. 1998). As in the root cap cells, irregularly shaped nuclei were present also in apical meristem cells. Similar changes in numbers and shapes of the nuclei and nucleoli were reported for meristematic cells of bean (Phaseolus vulgaris) after treatment with S. deppei extract (Cruz-Ortega et al. 1998). In some seedlings treated with the aqueous extract of F. japonica here, we detected meristem cells with irregular triangular shapes and signs of stress, such as decomposed cell contents, and detached plasma membrane. All these ultrastructural effects again indicate changes related to cell death.
An additional reason for root growth suppression might be increased production and/or accumulation of ROS (Bogatek and Gniazdowska 2007). In the present study, we focused on H2O2, which acts as a signaling molecule and a damaging agent (Das and Roychoudhury 2014). Higher levels of H2O2 in the roots for the aqueous extract treatments are in agreement with the pronounced effects in root cells that were revealed by light and electron microscopy. Roots are the most sensitive of the plant organs, as they are the first to come into contact with other (potentially phytotoxic) molecules in the soil (Haugland and Brandsaeter 1996). Many allelopathic compounds increase the production and accumulation of ROS, such as H2O2 (Gechev et al. 2006).
To conclude, in the present study, we have shown that aqueous extracts of F. japonica and F. ×bohemica rhizomes suppress elongation of radish roots and increase their thickness. In the root tips, they caused ultrastructural damage to many cell organelles, which suggests they are involved in triggering cell death. Understanding the mechanism(s) of allelopathy and phytotoxicity is the key to explaining and managing knotweeds and other plant invaders. Therefore, further investigations of interactions between allelopathic compounds and roots are needed, including the physiological responses of the roots to single compounds and their mixtures, and the uptake of allelochemicals.
References
Abrahim D, Braguini WL, Kelmer-Bracht AM, Ishii-Iwamoto EL (2000) Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration in maize. J Chem Ecol 26(3):611–624
Aliotta G, Cafiero G, Fiorentino A, Strumia S (1993) Inhibition of radish germination and root growth by coumarin and phenylpropanoids. J Chem Ecol 19(2):175–183
Babula P, Vaverkova V, Poborilova Z, Ballova L, Masarik M, Provaznik I (2014) Phytotoxic action of naphthoquinone juglone demonstrated on lettuce seedlings roots. Plant Physiol Biochem 84:78–86
Bailey JP, Bímová K, Mandák B (2009) Asexual spread versus sexual reproduction and evolution in Japanese knotweed s.l. sets the stage for the Battle of the Clones. Biol Invasions 11:1189–1203
Bell PR, Mühlethaler K (1964) The degeneration and reappearance of mitochondria in the egg cells of a plant. J Cell Biol 20:235–248
Benitez-Alfonso Y, Faulkner C, Ritzenthaler C, Maule AJ (2010) Plasmodesmata: gateways to local and systemic virus infection. Mol Plant Microbe Intaract 23(11):1403–1412
Bímová K, Mandák B, Pyšek P (2003) Experimental study of vegetative regeneration of four invasive Reynoutria taxa (Polygonaceae). Plant Ecol 166:1–11
Bogatek R, Gniazdowska A (2007) ROS and phytohormones in plant-plant allelopathic interaction. Plant Signal Behav 2(4):3317–3318
Bonnett HT, Newcomb EB (1965) Polyribosomes and cisternal accumulations in root cells of radish. J Cell Biol 27:423–432
Burgos NR, Talbert RE, Kim KS, Kuk YI (2004) Growth inhibition and root ultrastructure of cucumber seedlings exposed to allelochemicals from rye (Secale cereale). J Chem Ecol 30(3):671–689
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Chen H, Tuck T, Ji X, Zhou X, Kelly G, Cuerrier A, Zhang J (2013) Quality assessment of Japanese knotweed (Fallopia japonica) grown on Prince Edward Island as a source of resveratrol. J Agric Food Chem 61:6383–6392
Cruz-Ortega R, Anaya AL, Hernández-Bautista BE, Laguna-Hernández G (1998) Effects of allelochemical stress produced by Sicyos deppei on seedling root ultrastructure of Phaseolus vulgaris and Cucurbita ficifolia. J Chem Ecol 24(12):2039–2057
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. https://doi.org/10.3389/fenvs.2014.00053
De Feo V, De Martino L, Quaranta E, Pizza C (2003) Isolation of phytotoxic compounds from tree-of-heaven (Ailanthus altissima Swingle). J Agric Food Chem 51:1177–1180
Dolenc Koce J, Šoln K (2018) Phytotoxic effects of Fallopia japonica and F. ×bohemica leaves. Phyton 57(1/2):47–57
Fan P, Hay AE, Marston A, Lou H, Hostettmann K (2009) Chemical variability of the invasive neophytes Polgonum cuspidatum Sieb and Zucc. and Polygonum sachalinensis F. Schmidt ex Maxim. Biochem Syst Ecol 37:24–34
Fan P, Hostettmann K, Lou H (2010) Allelochemicals of the invasive neophyte Polygonum cuspidatum Sieb. & Zucc. (Polygonaceae). Chemoecology 20:223–227
Frantík T, Kovářová M, Koblihová H, Bartůňková K, Nývltová Z, Vosátka M (2013) Production of medically valuable stilbenes and emodin in knotweed. Ind Crop Prod 50:237–243
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28(11):1091–1101
Gniazdowska A, Bogatek R (2005) Allelopathic interactions between plants. Multi site action of allelochemicals. Acta Physiol Plant 27(38):395–407
Gonzáles LF, Rojas MC (1999) Role of wall peroxidases in oat growth inhibition by DIMBOA. Phytochemisty 50:931–937
Gotté M, Ghosh R, Bernard S, Nguema-Ona E, Vicré-Gibouin M, Hara-Nishimura I, Driouich A (2015) Methyl jasmonate affects morphology, number and activity od endoplasmic reticulum bodies in Raphanus sativus root cells. Plant Cell Physiol 56(1):61–72
Hara-Nishimura I, Matsushima R (2003) A wound-inducible organelle derived from endoplasmic reticulum: a plant strategy against environmental stresses? Curr Opin Plant Biol 6:583–588
Haugland E, Brandsaeter LO (1996) Experiments on bioassay sensitivity in the study of allelopathy. J Chem Ecol 22(10):1845–1859
Huysmans M, Lema S, Coll NS, Nowack MK (2017) Dying two deaths – programmed cell death regulation in development and disease. Curr Opin Plant Biol 35:37–44
Jambunathan N (2010) Determination and detection of reactive oxygen species (ROS), lipid peroxidation and electrolyte leakage in plants. In: Sunkar R (ed) Plant stress tolerance, methods in molecular biology. Humana Press, Oklahoma, p 295
Kumpf RP, Nowack MK (2015) The root cap: a short story of life and death. J Exp Bot 66(19):5651–5662
Lee SH, Singh AP, Chung GC, Kim YS, Kong B (2002) Chilling root temperature causes rapid ultrastructural changes in cortical cells of cucumber (Cucumis sativus L.) root tips. J Exp Bot 53(378):2225–2237
Li P, Ding L, Zhang L, He J, Huan Z (2019) Weisiensin B inhibits primary and lateral root development by interfering with polar auxin transport in Arabidopsis thaliana. Plant Physiol Biochem 139:738–745
Lowe S, Browne M, Boudjelas S, De Porter M (2000) 100 of the world’s worst invasive alien species. A selection from the global invasive species database. The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), New Zealand, pp 12
Lupini A, Araniti F, Sunseri F, Abenavoli MR (2014) Coumarin interacts with auxin polar transport to modify root system architecture in Arabidopsis thaliana. Plant Growth Regul 74(1):23–31
Matsushima R, Hayashi Y, Kondo M, Shimada T, Nishimura M, Hara-Nishimura I (2002) An endoplasmic reticulum-derived structure that is induced under stress conditions in Arabidopsis. Plant Physiol 130:1807–1814
Miyazono Y, Hirashima S, Ishihara N, Kusukawa J, Nakamura K, Ohta K (2018) Uncoupled mitochondria quickly shorten along their long axis to form indented spheroids, instead of rings, in a fission-independent manner. Sci Rep. https://doi.org/10.1038/s41598-017-18582-6
Moravcová L, Pyšek P, Jarošík V, Zákravský P (2011) Potential phytotoxic and shading effects of invasive Fallopia (Polygonaceae) taxa on the germination of dominant native species. NeoBiota 9:31–47
Murrell C, Gerber E, Krebs C, Parepa M, Schaffner U, Bossdorf O (2011) Invasive knotweed affects native plants through allelopathy. Am J Bot 98(1):38–43
Nakano RT, Yamada K, Bednarek P, Nishimura M, Hara-Nishimura I (2014) ER bodies in plants of the Brassicales order: biogenesis and association with innate immunity. Front Plant Sci 5(73):1–17
Pacifici E, Di Mambro R, Dello Ioio R, Costantino P, Sabatini S (2018) Acidic acid elongation drives cell differentiation in the Arabidopsis root. EMBO J 37:e99134
Piqueras A, Olmos E, Hellín E (1994) Cytological changes related with salt tolerance in embryogenic callus of Citrus limon. Plant Cell Tiss Org 39:13–18
Ponomareva AA, Polygalova OO (2012) Changes in mitochondrial shape in wheat root cells exposed to mitochondrial poisons. Russ J Plant Physiol 59(3):428–433
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK (2007) Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12(3):98–105
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Siemens TJ, Blossey B (2007) An evaluation of mechanisms preventing growth and survival of two native species in invasive Bohemian knotweed (Fallopia ×bohemica, Polygonaceae). Am J Bot 94(5):776–783
Šoln K, Likar M, Dolenc Koce J (2021) Effects of rhizome extracts from invasive knotweed species Fallopia japonica and F. ×bohemica on radish seed germination and root growth of seedlings. Allelopath J 52(1):103–118
Soltys D, Rudzinska-Langwald A, Kurek W, Gniazdowska A, Sliwinska E, Bogatek R (2011) Cyanamide mode of action during inhibition of onion (Allium cepa L.) root growth involves disturbances in cell division and cytoskeleton formation. Planta 254:609–621
Strgulc Krajšek S, Dolenc Koce J (2015) Sexual reproduction of knotweed (Fallopia sect. Reynoutria) in Slovenia. Preslia 87(1):17–30
Takahashi L, Aparacida Sert M, Kelmer-Bracht AM, Bracht A, Ishii-Iwamoto EL (1998) Effects of rutin and quercetin on mitochondrial metabolism and on ATP level in germinating tissues of Glycine max. Plant Physiol Biochem 36(7):495–501
Tucker Serniak L (2016) Comparison of the allelopathic effects and uptake of Fallopia japonica phytochemicaly by Raphanus sativus. Weed Res 56:97–101
van Doorn WG, Beers EP, Dangl JL, Franklin-Tong VE, Gallois P, Hara-Nishimura I, Jones AM, Kawai-Yamada M, Lam E, Mundy J, Mur LJA, Petersen M, Smertenko A, Taliansky M, Van Breusegem F, Wolpert T, Woltering E, Zhivotovsky B, Bozhkov PV (2011) Morphological classification of plant cell deaths. Cell Death Differ 18:1241–1246
Yamada K, Goto-Yamada S, Nakazaki A, Kunieda T, Kuwata K, Nagano AJ, Nishimura M, Hara-Nishimura I (2020) Endoplasmic reticulum-derived bodies enable a single-cell chemical defense in Brassicaceae plants. Commun Biol. https://doi.org/10.1038/s42003-019-0739-1
Acknowledgements
The authors are thankful to Dr. Aleš Kladnik for the suggestions of image analysis, Dr. Maks Merela and his co-workers for rhizome grinding, and Dr. Chris Berrie for thorough reading of the manuscript and English editing. We are also deeply grateful to the editor and the anonymous reviewers for valuable comments and suggestions.
Availability of data and material
The authors declare availability of data and material in personal laboratory diaries.
Code availability
Not applicable.
Funding
This study was financially supported by the Slovenian Research Agency (grant no. P1-0212).
Author information
Authors and Affiliations
Contributions
All authors contributed critically to the manuscript and approved the final manuscript. KŠ and NŽ performed the experiment. JDK contributed to data and image analysis.
Corresponding author
Ethics declarations
Ethics approval
No ethical approval was needed.
Consent to participate
The authors declare consent to participate.
Consent for publication
The authors declare consent for publication in Journal of Chemical Ecology.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Radish seedling stained with diaminobenzidine after 5 days for control seedling (a) and seedling exposed to the aqueous extracts of Fallopia japonica (b) and F. × bohemica (c) rhizomes. Bar: 1 cm. (PNG 450 kb)
ESM 2
Radish seedling stained with diaminobenzidine after 7 days for control seedling (a) and seedling exposed to the aqueous extracts of Fallopia japonica (b) and F. × bohemica (c) rhizomes. Bar: 1 cm. (PNG 8321 kb)
Rights and permissions
About this article
Cite this article
Šoln, K., Žnidaršič, N. & Dolenc Koce, J. Root growth inhibition and ultrastructural changes in radish root tips after treatment with aqueous extracts of Fallopia japonica and F. ×bohemica rhizomes. Protoplasma 259, 343–355 (2022). https://doi.org/10.1007/s00709-021-01668-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01668-4