Abstract
Radial oxygen loss is a physical phenomenon that occurs naturally in aquatic plants. Typha domingensis was chosen as a model plant because it possesses basic morphological characteristics, such as a stem (rhizome) that produces leaves and adventitious roots, which are present in many aquatic plants. This study aimed to evaluate the following: the relevance of the anatomy of T. domingensis on gas diffusion among organs; the influence of plant parts on radial oxygen loss; the role of catalase in radial oxygen loss; and the proposition of a novel explanation for the downward diffusion of oxygen through the organs of this aquatic macrophyte and into the environment. Typha domingensis plants were cultivated in a greenhouse under different conditions: plants with intact leaves, plants with leaves cut in half, and plants without leaves. Furthermore, we evaluated the percentage of aerenchyma in different vegetative organs, the minimum pressure required for radial oxygen loss, the daily variations of dissolved oxygen, and the roots’ catalase activity. The results demonstrated that certain cellular features contributed to decreased oxygen diffusion among the organs, specifically, those found in the leaf-rhizome and root-rhizome interfaces as well as the suberin and lignin layers in these regions. Additionally, our experiments with a catalase activator and inhibitor validated that a significant amount of the oxygen released in radial oxygen loss could not, in fact, be exclusively supplied by the atmosphere. Thus, a complementary model is proposed in which catalase activity is an important component of radial oxygen loss.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic plants are important parts of wetlands, and they are distributed worldwide. These plants preserve the balance of aquatic ecosystems by constituting part of the local diversity (Bolduc et al. 2016). In addition, aquatic macrophytes have shown phytoremediation potential (Dai et al. 2017; Oliveira et al. 2017).
An important environmental function of aquatic macrophytes that warrants further study is their capacity to oxygenate their surroundings (Pang et al. 2016). To overcome the limitations imposed by low oxygen (O2) concentrations in wetland soils, macrophytes have developed effective adaptations, such as an efficient antioxidant system (Alam and Ghosh 2018) and a tissue specialized to store and distribute gases within the plant structure, the aerenchyma (Seago Jr et al. 2005; Colmer et al. 2006). It is well-known that O2 diffusion in an aqueous medium is 10,000 times slower than in a gaseous medium. Thus, the plants’ internal gas diffusion reservoir plays a key role in wetland ecosystems.
Flooding is a potential source of reactive oxygen species (ROS) in aquatic organisms. ROS participate in deleterious processes, such as damage to cell structures, including lipids, membranes, proteins, and DNA (Spengler et al. 2017); therefore, plants’ resistance to environmental pressures is linked to their antioxidant system. Catalase (CAT) is one of the main enzymes of this system, and it participates actively in the detoxification process consuming hydrogen peroxide (H2O2) (a ROS) and producing O2 and H2O (Alam and Ghosh 2018). This enzyme has been shown to be particularly efficient in aquatic macrophytes, such as T. domingensis (Corrêa et al. 2015).
Gas diffusion from intercellular spaces contributes to the supply of adequate amounts of O2 for processes such as photosynthesis and respiration (Retta et al. 2016; Miao et al. 2020). In submerged tissues such as those in macrophyte roots, the three-dimensional structural arrangement of cells forming the tissue is critical for gas diffusion (Armstrong and Beckett 2011; Ho et al. 2011). Some specialized tissues such as the aerenchyma exhibit high porosity; thus, they favor internal gas diffusion in plants (Kordyum et al. 2017). On the other hand, the cellular arrangement of meristematic tissues is characterized by the absence of intercellular spaces, which favors the reduction or even blockage of gas diffusion between this tissue and other parts of the plant (Brulé et al. 2016).
Radial oxygen loss (ROL) is defined by Armstrong (1980) as the O2 transfer from the root aerenchyma of aquatic plants to their rhizosphere. According to Dai et al. (2017), ROL is an important trait of aquatic plants and promotes tolerance to environments with low O2 content. Previous studies showed the importance of rhizosphere aeration for the uptake and transport of heavy metals by hyperaccumulator plants (White and Ganf 2000) for the maintenance of microorganisms that interact with plant roots (Ma et al. 2018) and for normal plant growth (Mano et al. 2006). Consequently, researchers have investigated ROL for a wide range of applications, such as environmental conservation, crop plant improvement, wastewater treatment, and heavy metal tolerance (Cheng et al. 2010; Rehman et al. 2017; Zhang et al. 2017). Some studies have tried to explain the origin and production of the O2 released in ROL (Zhang et al. 2014) and the mechanisms involved in gas diffusion and ROL (Colmer 2003).
Armstrong and Armstrong (1988), Armstrong (1980), and Brix (1993) investigated the physical principles involved in gas exchange dynamics and the mathematical models that could explain them. Furthermore, Colmer (2003) and Tanaka et al. (2007) re-examined and improved models for gas transport over long distances inside plants. These findings have contributed to the improvement of our understanding of gas diffusion dynamics and gas release by the plant; therefore, these models previously described can be improved by recent scientific findings. For instance, these models evaluate only the aerenchyma tissue, instead of the entire anatomical structure of the plant. Studies have demonstrated the presence of some tissues such as meristems (Kaul 1974) and the exodermis (Armstrong et al. 1992; Corrêa et al. 2015) in cattail. According to Brix et al. (1992), some species do not exhibit internal pressurization for convective flow, although there is evidence that connections between plant organs may permit airflow since meristems are not present as they can occlude lacunar spaces. Tornberg et al. (1994) estimated that the resistance in the leaf-rhizome connection is very high in Typha latifolia and T. angustifolia and both plant species have intercalary meristems at their leaf bases, which are related to increased resistance. However, previous studies did not provide any images of intercellular spaces in the connections. Additionally, the anatomical constraints of other macrophytes have not been sufficiently explored in previous studies; this is particularly true for research examining the anatomical connections among different plant organs, which is essentially nonexistent.
In this study, we hypothesized that the presence of tissues with few intercellular spaces will increase significantly the gas diffusion resistance among the different plant organs as well as into the soil. Additionally, we hypothesized that at least a significant part of the O2 released in ROL originates in CAT activity in the roots of macrophytes, such as T. domingensis.
Therefore, the aim of this study was to investigate the anatomical barriers for O2 diffusion and clarify the origin of O2 involved in ROL by using T. domingensis, an aquatic macrophyte distributed worldwide. The objectives of this study were (1) to investigate the role of anatomical traits in gas flow in T. domingensis, (2) to test the role of CAT in the O2 released from ROL in T. domingensis, and (3) to propose a complementary model to study the origin of O2 released from the roots in ROL.
The results of the present study showed that anatomical barriers constrained the O2 pathway from the shoot to the roots in aquatic macrophytes and that root CAT was sufficiently active and had adequate substrate to provide enough O2 to fill aerenchyma chambers. Thus, we suggested a complementary model for ROL in T. domingensis.
Material and methods
Plant material
Cattail plants (T. domingensis Pers. − Typhaceae) were collected from natural populations in wetlands located at Alfenas, Minas Gerais (21° 25′ 44′′ S, 45° 56′ 49′′ W) in the southeast region of Brazil. The collected plants contained rhizomes and approximately five leaves each (they were 1.5 m long). These plants were immersed into a 50% hypochlorite [commercial sodium hypochlorite (NaClO) solution and distilled water (v v−1); the final NaClO concentration was 3% (w v−1)] solution for 10 min and then washed with tap water before being cultivated in the greenhouse. The plants were grown in 60-L plastic pots containing 10 L of a nutrient solution (Hoagland and Arnon 1940) at 40% ionic strength to obtain acclimatized clone plants. The Hoagland and Arnon nutritive solution contained the following salts: NH4H2PO4, Ca(NO3)2, Mg(NO3)2, KNO3, K2SO4, FeSO4·7H2O, H2BO3, MnSO4·H2O, ZnSO4·7H2O, CuSO4·5H2O, and H2MoO4.H2O. All clone plants used in the experiments described below were of matching size and age (15 cm tall and 60 days old), and the rhizomes were 4.5 cm long and had a 0.9-cm diameter.
Experiments
Different experiments were conducted to investigate (1) the role the anatomy of T. domingensis played in gas diffusion in different plant organs, (2) the influence of plant parts in ROL, and (3) the role of CAT in ROL.
Anatomy and morphology of T. domingensis
We analyzed the anatomy of vegetative organs (leaves, rhizomes, and roots) while searching for tissues related to both enhanced (aerenchyma) and reduced gas diffusion (meristems and suberized tissues). The leaf base of Typha species has an intercalary meristem (Kaul 1974), and the roots of T. domingensis have an external cortex lacking intercellular spaces (Corrêa et al. 2017). The anatomical traits evaluated in roots were the area of the root section, the area occupied by aerenchyma chambers, and the percentage of aerenchyma chambers in the root (calculated as the ratio of the aerenchyma chambers area to the root area). Additionally, the anatomical traits evaluated in the rhizomes were the area of the rhizome section, the area occupied by aerenchyma chambers, and the percentage of aerenchyma chambers in the rhizome (calculated as the ratio of the aerenchyma chambers area to the rhizome area). Lastly, the anatomical traits evaluated in the leaves were the area of the leaf section, the area occupied by aerenchyma chambers, and the percentage of aerenchyma chambers in the leaf (calculated as the ratio of the aerenchyma chambers to the leaf area).
For anatomical analysis, plant parts were fixed in an FAA 70% solution (formaldehyde, acetic acid, and 70% ethanol at a 0.5:0.5:9 ratio) for 48 h and then stored in 70% ethanol until further analysis (Johansen 1940). Furthermore, the samples were dried with increasing ethanol concentrations (70%, 80%, 90%, and 100%) at 2-h intervals and embedded in historesin according to the manufacturer’s instructions (Leica Microsystems, Wetzlar, Germany). The application of an ethanol series, with increased concentrations of this compound, is an efficient method for dehydration (Johansen 1940) and is necessary to achieve the necessary water content to permit resin infiltration in the material. Transversal sections were obtained using a semi-automated rotary microtome (Yidi YD-335; Jinhua Yidi Medical Appliance CO., LTD, Zhejiang, China). Then, the sections were stained with toluidine blue 1% (w v−1) and mounted on slides with Entellan (Merck, Darmstadt, Germany). The slides were photographed using a microscope attached to an image capturing system (CX31; Olympus, Tokyo, Japan), and quantitative anatomical analysis was performed using UTHSCSA ImageTool software.
Fluorescence microscopy was performed to identify suberized or lignified tissues that may form barriers to gas diffusion. Cross and longitudinal sections of transition regions between the leaf and rhizome and the rhizome and root were placed in a solution containing distilled water and 0.1% berberine hemisulphate (w v−1) for 1 h and then washed in distilled water. Thereafter, the sections were kept in a 0.5% aniline blue (w v−1) solution for 30 min and then washed twice with distilled water. The sections were mounted in a 0.1% FeCl3 (w v−1) solution in 50% glycerol (w v−1) (Brundrett et al. 1988). Then, the slides were analyzed with a fluorescence microscope (BX60; Olympus) equipped with a cooled monochrome camera (Olympus). Images were captured with ultraviolet excitation/emission wavelengths of 358–461 nm (Brundrett et al. 1988).
In addition to the anatomical analysis, T. domingensis plants were analyzed to measure the total air volume that filled the aerenchyma spaces in different organs. The volumes of roots, rhizomes, and leaves of 10 plants were measured via the water displacement method using a measuring cylinder (data not shown). Based on the volume of each organ and its aerenchyma percentage, we calculated the air space volume in each plant part (the organ volume multiplied by the aerenchyma proportion). These air-filled spaces inside each organ are important for the calculation of the amount of gas necessary to provide enough pressure for gas movement across the barriers between plant organs (leaf-rhizome and rhizome-root interfaces) and from the root to the soil (mainly the exodermis and epidermis).
The anatomical analysis was used to identify the gas diffusion resistance along the entire plant structure. By incorporating some adaptations and considerations, we assumed a model similar to that used for CO2 diffusion in leaves, according to Terashima et al. (2011). In our model, the following assumptions regarding resistance to gas diffusion were necessary: (a) the intercellular spaces and aerenchyma cause low resistance; (b) tissues with primary cell walls and without lignin or suberin deposition (the epidermis, meristems, and the parenchyma) generate the greatest resistance on their plasma membranes, thin walls, and cytosol; (c) meristems and the epidermis generate high resistance owing to the absence of intercellular spaces; and (d) lignified or suberized tissues cause the highest resistance owing to their thick walls and the deposition of these substances on their secondary cell walls.
Determination of the pressure limit necessary for ROL
To determine the pressure limit required for noticeable ROL, plants were placed individually in plastic pots containing 3.5 L of deionized water and a sealed rubber hose that was attached to T. domingensis leaves and to an air compressor. These leaves were cut on the apical third, so that the pressurized air entered the leaf and passed through the leaf-rhizome and rhizome-root interfaces and from the root to the solution. A multiparameter probe (5565 MPS, version 1.12; YSI, Yellow Springs, Ohio, USA) that detects dissolved O2 in the water was used to determine ROL. The air pressure was increased in a gentle manner until the limit required for ROL detection was obtained, and it was then registered. The experiment was replicated 10 times, and the mean ± standard deviation (SD) was calculated for the experimental plants.
The influence of plant parts on ROL
One of the hypotheses that may explain ROL in macrophytes is that O2 enters the plant via stomata, broken stems, or leaves and then finds its way through leaves, towards the rhizome, then to the roots, and finally into the soil (Colmer 2003). Therefore, leaves and stems (shoots) may be crucial parts in the mechanism by which O2 enters the plant, if this is the only way that this process takes place. We examined T. domingensis plants in three distinct conditions to test this hypothesis: intact plants, leafless plants (all leaves were carefully removed leaving only rhizomes and roots, which were completely submerged), and plants with leaves cut in half (in the middle). Leafless plants were kept underwater for the entire duration of the experiment (they had no air contact). The dissolved O2 was measured using the multiparameter probe containing an O2 electrode (previously mentioned). All data sampling was performed in the morning between 7 a.m. and 9 a.m., while we implemented a 12-h photoperiod, and the daily mean air temperature and water temperature were 22.5 ± 1 °C and 14.5 ± 1 °C, respectively. The mean oxygen level was 4.21 mg L−1, while during the experiment, all measured values remained below the saturation level (calculated as 6.85 mg L−1). In order to stabilize the electrode, we kept it resting in the solution for 3 min before every measurement. To ensure sanitization and avoid the presence of microalgae, the pots were previously washed with deionized water and sodium hypochlorite; additionally, we avoided adding a nutrient solution during the 10-day duration of the experiment, which prevented algal growth. The experimental design was completely randomized with three treatments and 10 replicates. Dissolved O2 measurements were obtained for 10 consecutive days. The following equation was used for ROL calculation: ROL = O2D2 − O2D1, where O2D1 is the O2 concentration in a given day and O2D2 is the O2 concentration in the next day. ROL was expressed in mg L−1 day−1. During the 10 days of the evaluation, two main ROL events were detected, and calculations were performed when O2 concentration increases were observed. During the 10 days of the evaluation, four or five increases were detectable in consecutive days, and ROL calculations were performed for each increase. Data were further averaged for each replicate. Polynomial curves were predicted for O2 concentrations with periodic increases and depletions (Benson and Krause 1976; Benson et al. 1979; Wetzel 2001). Detectable increases in the O2 concentration were considered ROL events.
CAT activity in T. domingensis roots and its role in ROL
The CAT activity in Typha species is remarkably high (Corrêa et al. 2015), and O2 is a product of this enzyme’s reaction, whose substrate (H2O2) increases under flooding conditions (Voesenek and Bailey-Serres 2015). Thus, we performed experiments containing substances that modify CAT activity to determine the role of CAT as a complementary source of O2 for ROL. To this end, we used both intact and leafless plants (using a method similar to that previously described) and subjected them to three treatments: (1) sodium nitroprusside (SNP; Êxodo Científica, São Paulo, Brazil), described by Fu et al. (2016) as a CAT activator; (2) 3-amino-1,2,4-triazole (AT; Sigma Aldrich, St. Louis, MO, USA), described by Aver’yanov et al. (2015) as a CAT inhibitor; and (3) a control treatment (distilled water), without modifying CAT activity. All plants were placed in plastic pots containing 3.5 L of distilled water and either 0.1 mM SNP, 0.1 mM AT, or only distilled water (for the control plants). The experiment was conducted using a 2 × 3 factorial design with 10 replicates, and the entire experiment was repeated three times.
The O2 dissolved in the solution was measured using the multiparameter probe previously mentioned. Measurements were taken 12 h after the addition of CAT activity modifiers. Then, their difference was calculated and expressed as ΔO2.
The roots were collected 2 h after the application of CAT activity modifiers, placed in liquid nitrogen, and stored at − 80 °C until they were analyzed. CAT was extracted as follows, according to the method proposed by Biemelt et al. (1998): 0.2 g of fresh root mass was ground in liquid nitrogen and homogenized in 1.5 mL of an extraction buffer that contained 1.47 mL of potassium phosphate buffer (0.1 M; pH 7.0), 15 μL of EDTA (0.1 M; pH 7.0), 6 μL of dithiothreitol (DTT) (0.5 M), 12 μL of phenylmethylsulfonyl (PMSF) (0.1 M), ascorbic acid (0.001 M), and 22 mg of polyvinylpolypyrrolidone. The extract was centrifuged at 12,000 g for 30 min at 4 °C, and the supernatant was collected and stored at − 20 °C until further analysis. Moreover, CAT activity was evaluated according to Havir and McHale (1987) as follows: 10 μL aliquots of the enzyme extract was added to 170 μL of an incubation medium that contained 90 μL of potassium phosphate (200 mM; pH 7.0), 71 μL of water, and 9 μL of H2O2 (250 mM) and then incubated at 28 °C. The enzyme activity was determined by the decrease in absorbance at 240 nm measured every 15 s for 3 min, by monitoring the consumption of H2O2. The molar extinction coefficient used was 36 mM−1 cm−1. The specific activity of CAT was calculated based on the total amount of proteins in the samples determined according to Bradford (1976). We calculated the enzymatic activity in triplicate, and the mean was calculated for each replicate.
An additional experiment was conducted to test the direct effects of CAT modifiers (AT and SNP) on the enzymatic activity under in vitro conditions (to ensure these modifiers were acting on CAT extracted from T. domingensis roots). For this experiment, roots sampled from intact plants were used and subjected to CAT extraction as described above (Biemelt et al. 1998). Further, CAT activity was evaluated according to Havir and McHale (1987) using the same method described above. However, in addition to all the described components of the incubation medium, in this experiment, we applied three more treatments: (1) control (the incubation medium and the CAT extract), (2) the incubation medium and the CAT extract plus 0.1 mM of AT (CAT inhibitor), and (3) the incubation medium and the CAT extract plus 0.1 mM of SNP (CAT activator). The experiment was conducted on a completely randomized design with 10 replicates for each plant.
H2O2 was determined according to Velikova et al. (2000) as follows: 200 mg of fresh roots were ground in liquid nitrogen and 20% polyvinylpolypyrrolidone (w m−1) and further homogenized in 1.5 mL of trichloroacetic acid 0.1% (w v−1). The homogenate was centrifuged at 12,000 g for 15 min at 4 °C. The H2O2 content was determined measuring the absorbance at 390 nm in a reaction medium that contained 500 μL of extract, 500 μL of potassium phosphate buffer (10 mM; pH 7.0), and 1 mL of potassium iodide (1 M). We calculated the H2O2 content in the roots in duplicate, and the data were averaged to one replicate.
Statistical analysis
The data were submitted to one-way or two-way ANOVA using the SISVAR 5.0 software (Ferreira 2011). Prior to parametric analysis, the data were tested for a normal distribution using the Shapiro-Wilk test, and the means were compared using the post hoc Scott-Knott test; p < 0.05 was considered statistically significant.
Results
Anatomy of T. domingensis
The leaves of T. domingensis comprised a single epidermal layer without intercellular spaces. Internally, 3–5 layers of palisade parenchyma were followed by 3–6 layers of large ground parenchyma cells, both having particularly small intercellular spaces (Fig. 1). According to our assumption, these three tissues represented limited gas diffusion capacity, and this pattern was found on both the adaxial and abaxial leaf sides. Large aerenchyma chambers were found on the central part of the leaf (Fig. 1a). Furthermore, the leaf-rhizome interface included an intercalary meristem that preserved the mitotic capability of the leaf base and promoted continuous growth (Fig. 1 c and d). However, this meristem and the region where cells were still differentiating lacked intercellular spaces, thereby limiting gas diffusion from the leaf aerenchyma to the rhizome (Fig. 1 c and d).
Anatomical structure of Typha domingensis leaves in cross sections (a and b) and leaf-rhizome connection in longitudinal sections (c and d). ade adaxial epidermis, abe abaxial epidermis, st stomata, pp palisade parenchyma, ae aerenchyma, gp ground parenchyma, vb vascular bundle, fb fibers, rhi rhizome, le leaf, asterisk leaf intercalary meristem. Bars = 300 μm (a), 50 μm (b), and 200 μm (c and d)
Similarly, the rhizome of T. domingensis comprised a single epidermal layer without intercellular spaces (Fig. 2 a and b). Internally, three or four exodermal layers, which had thick walls and lacked intercellular spaces, were detected. The aerenchyma was detected on the innermost part of the rhizome cortex; this area had small vascular bundles and parenchyma. The innermost part of the rhizome comprised an atactostelic cylinder with ground parenchyma and large, scattered vascular bundles; this part had few intercellular spaces (Fig. 2a).
Anatomical structure in cross sections of the rhizome (a and b), root (c and d), and the rhizome-root interface in longitudinal sections (e and f) of Typha domingensis. The bright areas on the F image show lignin/suberin deposition on fluorescence microscopy and staining with cyanide blue. Ep epidermis, ex exodermis, ae aerenchyma, gp ground parenchyma, vb vascular bundle, vc vascular cylinder, fb fibers, rhi rhizome, ro root, arrow rhizome-root interface. Bars = 300 μm (a, b, c, and e), 100 μm (d), and 200 μm (f)
Typha domingensis roots comprised a single epidermal layer without intercellular spaces. Internally, three layers of parenchyma cells with thick walls formed the exodermis (Fig. 2 c and d). The middle cortex consisted of large aerenchyma chambers, while the innermost part of the cortex contained parenchyma cells with few intercellular spaces. Moreover, the vascular cylinder (with the xylem and phloem) was found at the center of the roots. Intercellular spaces were nearly absent in this part of the root (Fig. 2b). The root-rhizome interface consisted of external root tissues as well as external rhizome parts, namely, the exodermis and epidermis, but lacked direct connections between the two organs along the root axis (Fig. 2e). This interface had scarce intercellular spaces (Fig. 2e), and fluorescence microscopy revealed a significant deposition of lignin and suberin on the exodermis of both the rhizome and the root (Fig. 2f).
The T. domingensis leaves had the highest aerenchyma percentage among the vegetative organs, followed by the root and the rhizome. In contrast, the low aerenchyma percentage found in the rhizome indicated that few parts were filled with air, although this organ had a large area section measuring 6.1 mm2. In fact, the largest and smallest air spaces were found on the leaves and rhizomes, respectively, of T. domingensis (Table 1). The leaf pressure threshold required to promote ROL was 0.08 ± 0.01 (SD) MPa.
We found miniscule intercellular spaces and deposition of compounds restrictive to air diffusion in the leaf-rhizome and rhizome-root interfaces. Furthermore, the deposition of lignin and suberin was remarkably abundant in the cell walls of the root exodermis and in the external layers of the rhizome. The connection between the root and rhizome included large areas with lignified and suberized tissues and lacked intercellular spaces (Fig. 2 e and f). Additionally, the leaf base had scarce intercellular spaces because of the intercalary meristem found in this region (Fig. 1 c and d).
The influence of plant parts on ROL
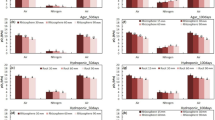
The T. domingensis plants subjected to different experimental conditions regarding their leaves (the plants were either intact, leafless, or with their leaves cut) exhibited ROL; however, the differences among them were not statistically significant (p = 0.08) (Fig. 3a). In addition, the O2 concentration did not increase constantly throughout the experiment; two main ROL events were detected for all plant conditions in days with increased dissolved O2 levels followed by days with decreased O2 levels (Fig. 3 b, c, and d).
Root oxygen loss (ROL) expressed as the mean difference of the dissolved oxygen between two consecutive days on the solution containing Typha domingensis plants that were either intact, leafless, or with their leaves cut (a). Dissolved oxygen content and ROL events throughout the 10-day evaluation of plants that were intact (b), leafless (c), and with their leaves cut (d). Bars indicate a p value ≤ 0.05. Columns with the same letters do not differ according to the Scott-Knott test at p < 0.05
CAT activity on T. domingensis roots and its role in ROL
No interaction was found between the effect of the plant parts and enzyme modifiers (p = 0.98), and no effect of intact or leafless plants was found (p = 0.05); however, a significant effect of the CAT modifiers was detected (p < 0.01). Both intact and leafless plants showed similar rates of ROL (Fig. 4a). The highest and lowest ROL means were detected in plants treated with SNP and AT, respectively, whereas the means of control plants were in the intermediate range (Fig. 4b).
Root oxygen loss (ROL) expressed as the mean difference of the dissolved oxygen 12 h after the application of the treatments. The ROL was calculated in the solution containing intact and leafless Typha domingensis plants subjected to 3-amino-1,2,4-triazole (AT) [catalase (CAT) inhibitor] and sodium nitroprusside (SNP) (CAT activator). As no significant effect was detected (p = 0.92), data are shown for different plant types (a) and CAT modifiers (b). Bars indicate a p value of ≤ 0.05. Columns with the same letters do not differ according to the Scott-Knott test at p < 0.05
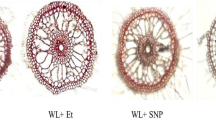
The CAT activity of intact plants was modified after the application of SNP and AT. In order to demonstrate the efficiency of the modifiers, the in vitro experiment (which proved the effects of both SNP and AT) showed that SNP increased CAT activity by 3.3 times compared to the control treatment, and AT reduced the CAT activity to values close to zero (Fig. 5a). In addition, intact plants always showed higher CAT activity than leafless plants (Fig. 5b). The H2O2 concentration on T. domingensis roots was not significantly different between leafless and intact plants. However, plants subjected to the AT treatment had the highest H2O2 levels in their roots (Fig. 5c), which was later consumed as the substrate for CAT under this treatment.
Catalase (CAT) activity and H2O2 concentration in the roots of Typha domingensis after the application of 3-amino-1,2,4-triazole (AT) (CAT inhibitor) and sodium nitroprusside (SNP) (CAT activator) or neither (control). (a) In vitro activity of CAT, (b) CAT activity for the experiment performed ex vitro with samples taken 2 h after the application of CAT modifiers, and (c) H2O2 activity for the experiment performed ex vitro with samples taken 2 h after the application of CAT modifiers. Bars indicate a p value of ≤ 0.05. Columns with the same letters do not differ according to the Scott-Knott test at p < 0.05
Discussion
Does ROL depend on macrophyte shoots?
The models that have been proposed to explain ROL argue that O2 enters the plant via broken shoots, then diffuses throughout the rhizome and roots, and is finally released into the soil (Armstrong and Armstrong 1988; Armstrong 1980; Konnerup et al. 2011). According to Colmer (2003), O2 enters the plants via stomata or broken shoots and then diffuses from the shoot to the roots, where ROL occurs. However, our results indicated that the origin of O2 may not rely exclusively on the shoot air uptake, because even leafless and submerged plants exhibited ROL. In addition, the pressure threshold to promote noticeable ROL on T. domingensis (0.08 MPa) was considerably higher than the estimated values of internal pressure in other macrophytes; for instance, Konnerup et al. (2011) found an average internal pressure of 0.0004 MPa in Cyperus L. species. It seems unlikely that the high pressure on the leaf mesophyll necessary to promote ROL could be reached only by the air uptake of leaf stomata.
In this study, we measured O2 concentrations for 10 days for each experiment, which certainly exceeded the time required to deplete the aerenchyma-stored gas; two ROL events were detectable, and the second event that occurred from the sixth to the eighth day was higher. In fact, the average amount of O2 released was 1.15 ± 0.3 mg L−1 day−1, which was calculated as the mean of all treatments at the second ROL event (Fig. 3). This mean was too high to be sourced only from aerenchyma-stored gas. In this experiment, the van’t Hoff equation could be used to estimate the amount of O2 in T. domingensis roots: n = PV/RT, where P is the pressure for ROL in standard atmospheres (atm), V is the aerenchyma gas volume in liters (L), R is the gas constant, and T is the temperature in kelvin degrees (K). In our experimental conditions, we could consider the values for the pressure for ROL (measured), root aerenchyma volume (Table 1), average temperature, and gas constant to be 0.7 atm (0.07 MPa), 0.00056 L (where 0.0001288, or 23%, is the estimated O2), 293.15 K (20 °C), and 0.082, respectively. Therefore, n = (0.7 × 0.00012)/(0.082 × 293.1), which is approximately equal to 0.0000375 M of O2; this suggests that approximately 1.2 mg of O2 was present in the roots. Notably, this O2 amount is released on a daily basis (1.15 ± 0.3 mg L−1), which means the entirety of O2 present in the roots could be exhausted in a single day. Furthermore, if we consider pressure values as those predicted by convective models for internal pressurization that reach up to 800 Pa in two Typha species (Tornberg et al. 1994), which equal 0.0079 atm, the van’t Hoff equation will be: n = (0.0079 × 0.00012)/(0.082 × 293.1). The results of this calculation will be 0.00000004 M of O2, which is equal to 0.00128 mg of O2 present in the roots. This calculation, considering convective flow pressures, means that the entirety of O2 present in the roots will be exhausted in just 1 min and 26 s. Consequently, the total amount of O2 stored in the roots of T. domingensis will be insufficient to provide constant O2 release for more than 1 day, thereby implying that, particularly in leafless plants, O2 in the root aerenchyma must be replenished by a mechanism different from those previously proposed (shoot uptake and transport).
The ROL was not affected by the removal of the shoot and was measured as the difference of dissolved O2 in the solution between consecutive days (Fig. 4). In addition, no differences were detected between intact and leafless plants (Figs. 3 and 4). The analysis of these results showed that ROL occurred independently of the shoot integrity.
Conclusively, our results showed that ROL was independent of macrophyte shoots and occurred even in the absence of this part of the plant. Moreover, these results suggest that a mechanism different from shoot air diffusion is necessary to replenish the O2 released in ROL.
Anatomical barriers for O2 diffusion throughout the shoot-root-soil continuum
Most studies concerning O2 diffusion through the plant and its role in ROL disregard many anatomical traits that could significantly limit the plant’s internal airflow. Different plant tissues show variable permeability to O2 depending on the abundance of intercellular spaces. The resistance to gas diffusion in intercellular spaces is low, but it increases greatly in the presence of cell membranes and cell liquid compartments (Terashima et al. 2011). Thus, tissues that typically have a low percentage of intercellular spaces show a high resistance to O2 diffusion.
Anatomical barriers present in specific tissues of T. domingensis have been previously reported; however, their role in O2 diffusion has not been discussed or considered when suggesting the models to explain ROL. For instance, Kaul (1974) identified the intercalary meristem on the leaf base. Furthermore, the root exodermis of several aquatic plants limits O2 diffusion from the roots in order to allow its use in root respiration (Armstrong and Armstrong 1988; Armstrong et al. 1992; Brix and Schierup 1990; Corrêa et al. 2017). According to Pi et al. (2009), gas diffusion occurs naturally under low resistance conditions within one of the plant’s organs containing abundant intercellular spaces. The high percentage of aerenchyma present and the volume of air contained in it provided the necessary conditions for gas distribution in the roots and leaves of T. domingensis. However, the aerenchyma percentage found in the rhizome was significantly lower than that in roots and leaves; thus, this organ has a limited gas diffusion capacity.
The multiseriate exodermis in the roots and the suberin deposition in the root-rhizome interface further impede O2 diffusion. The hydrophobic property of suberin (Song et al. 2011) is another factor that limits (or even blocks) gas diffusion through the root-rhizome interface as well as the release of O2 from the external root cortex and the epidermis.
In addition to the aforementioned anatomical barriers hindering O2 diffusion throughout the plant organs, leaf tissues also present difficulties for the air uptake to reach the aerenchyma and for the gas diffusion between consecutive chambers. For instance, stomatal resistance is very well-known (Terashima et al. 2011) and is largely reduced when stomatal pores open. This is the way air enters leaves by providing carbon dioxide (CO2) for photosynthesis. However, in T. domingensis leaves, the stomata are located just above the palisade parenchyma that lacks (or has unnoticeable) intercellular spaces (Fig. 1), which constitutes a second resistance barrier for air diffusing into the aerenchyma. Successively, ground parenchyma creates a third resistance barrier. The sum of these resistances comprises a strong barrier for air to fill the aerenchyma chambers sufficiently to reach the high pressure required for ROL (0.08 MPa).
Figure 6 indicates the locations on the structure of T. domingensis where significant resistance occurred. Considering the main tissue and organ interfaces, the total resistance for O2 diffusion through the plant body could be defined as follows: RTO2 = Rl + Rt + Rlri + Rriro + Ree, where RTO2 is the total resistance to O2 diffusion, Rl is the leaf resistance, Rt is the trabecular resistance, Rlri is the resistance of the leaf-rhizome interface, Rriro is the resistance of the rhizome-root interface, and Ree is the resistance of the root exodermis and epidermis. Consequently, these accumulated resistances may limit severely the process in the current O2 diffusion model (Colmer 2003). Thus, the anatomy of aquatic macrophytes (such as T. domingensis) is adapted to store O2 in the aerenchyma and to diffuse it within an organ; nonetheless, the diffusion between organs is limited by barriers in their interfaces.
Scheme of the path to O2 diffusion from the atmosphere to the soil throughout the plant body, when considering anatomical resistance. RTO2 total resistance to O2 diffusion, Rl leaf resistance, Rt trabecular resistance, Rlri resistance of the leaf-rhizome interface, Rriro resistance of the rhizome-root interface, Ree resistance of the exodermis and epidermis of the roots
CAT activity is an O2 source for ROL
The CAT enzyme consumes H2O2 to produce O2 and H2O; its activity is associated with the scavenging of H2O2 (a ROS) to protect plant cells against oxidative stress (Møller 2001). Flooding causes O2 deprivation that promotes ROS formation; thus, the aerenchyma tissue has evolved to store O2 to avoid this effect (Voesenek and Bailey-Serres 2015). Therefore, aquatic macrophytes experience constant O2 deficiency, thereby promoting the increase of H2O2 in roots, which are detoxified by CAT activity. This provides a continuous source of H2O2 for CAT. Consequently, this enzyme is able to operate steadily in the roots of aquatic macrophytes, such as T. domingensis.
In aquatic macrophytes, CAT activity is increased by several environmental factors such as heavy metals (Pereira et al. 2014), population density (Corrêa et al. 2015), UV radiation (Xu et al. 2014), and chemical oxygen demand (Xu et al. 2011). Thus, this enzyme is exceptionally responsive to environmental conditions. The CAT modifiers used in this study were efficient in altering this enzyme’s activity (in vitro assay shown in Fig. 5a), and these changes were sufficient to shift the levels of ROL in the plants (Fig. 4). The role of this enzyme in ROL was evidenced in a convincing manner by the 81.9% increase and 49.5% reduction of ROL obtained from treatments with a CAT activator and inhibitor, respectively. In fact, changes in CAT activity were proportionally related to ROL.
These results support the effects of CAT modifiers and the enzyme’s use of H2O2 as a substrate. CAT activity was reduced by its inhibitor (AT) as is shown in Fig. 5, and during the effect of the latter, H2O2 was accumulated in T. domingensis tissues. Then, the excess H2O2 in the roots increased CAT activity. Therefore, the plants treated with the inhibitor showed the highest CAT activity, 2 h after application, because the enzyme’s substrate accumulated, which confirm the Michaelis-Menten kinetics model that states that enzyme activity rates depend on the substrate concentration (Reuveni et al. 2014).
Another issue was whether CAT would be able to provide sufficient O2 to match the levels of ROL that we measured (1.15 mg L−1). The average CAT activity for control plants was 0.8 μmol H2O2 min−1 μg−1 protein (data not shown). The method to assess CAT activity is based on H2O2 consumption; however, the analysis of the equilibrated equation showed that for every mol of this substrate, 0.5 mol of O2 would be produced (Møller 2001). Thus, 0.4 μmol O2 min−1 μg−1 protein is produced from CAT activity in T. domingensis roots. Furthermore, 12 g of fresh mass was the average mass of the root system of the T. domingensis experimental plants measured at a similar growth stage and size (data not shown). The protein proportion found on T. domingensis roots was 0.08 mg protein g−1 fresh mass. Therefore, the total protein in the root system of these plants was 0.96 mg (960 μg−1). Furthermore, the O2 production capacity of the T. domingensis root system was 384 μmol O2 min−1, which is equivalent to 12.3 mg O2 min−1 of enzyme activity. Thus, this enzyme has an exceptional capacity to produce O2, and a single minute of its operation could provide sufficient O2 to maintain the ROL measured in T. domingensis roots. Additionally, this enzyme activity rate was similar to those previously reported for Typha species and for other aquatic macrophyte species (Corrêa et al. 2015; Yang and Ye 2015). This shows that, in aquatic macrophyte roots, CAT could be a reliable source of O2 to supplement the aerenchyma and the root’s aerobic metabolism. In addition, CAT could support ROL rates found in aquatic macrophyte roots.
Complementary model to explain the aerenchyma O2 origin and ROL
The O2 supplied via transport from the shoots may be insufficient for ROL because of anatomical barriers. Complementary CAT acting as an O2 source in the roots may be a plausible way to replenish the gas lost in ROL. Moreover, O2 produced directly in the roots avoided all anatomical barriers except for the root exodermis (Ree resistance), which facilitated substantially O2 in reaching the soil. Consequently, we propose a complementary model.
Conclusion
In summary, anatomical barriers consisting of tissues lacking intercellular spaces constrain the O2 pathway from the shoot to the roots in aquatic macrophytes. The root CAT was sufficiently active and had adequate substrate to provide enough O2 to fill aerenchyma chambers and to support ROL. Moreover, the O2 released by aquatic macrophytes in wetlands was partially originated in CAT activity. Typha domingensis roots produced sufficient O2 for ROL, independently of the aerial plant part examined.
References
Alam NB, Ghosh A (2018) Comprehensive analysis and transcript profiling of Arabidopsis thaliana and Oryza sativa catalase gene family suggests their specific roles in development and stress responses. Plant Physiol Biochem 123:54–64. https://doi.org/10.1016/j.plaphy.2017.11.018
Armstrong W (1980) Aeration in higher plants. Adv Bot Res 7:225–332. https://doi.org/10.1016/S0065-2296(08)60089-0
Armstrong BYJ, Armstrong W (1988) Phragmites australis - a preliminary study of soil-oxidizing sites and internal gas transport pathways. New Phytol 108:373–382
Armstrong W, Beckett PM (2011) Experimental and modelling data contradict the idea of respiratory down-regulation in plant tissues at an internal [O2] substantially above the critical oxygen pressure for cytochrome oxidase. New Phytol 190:431–441. https://doi.org/10.1111/j.1469-8137.2010.03537.x
Armstrong J, Armstrong W, Beckett PM (1992) Phragmites australis: Venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytol 120:197–207. https://doi.org/10.1111/j.1469-8137.1992.tb05655.x
Aver’yanov AA, Pasechnik TD, Lapikova VP, Romanova TS, Baker CJ (2015) Systemic reduction of rice blast by inhibitors of antioxidant enzymes. Russ. J. Plant Physiol 62:586–594. https://doi.org/10.1134/S1021443715050052
Benson BB, Krause D (1976) Empirical laws for dilute aqueous solutions of nonpolar gases: J. Chem Phys 64:689–709. https://doi.org/10.1063/1.432215
Benson BB, Krause D, Peterson MA (1979) The solubility and isotopic fractionation of gases in dilute aqueous solution I. Oxygen: J Solution Chem 8:655–690. https://doi.org/10.1007/BF01033696
Biemelt S, Keetman U, Albrecht G (1998) Re-aeration following hypoxia or anoxia leads to activation of the antioxidative defense system in roots of wheat seedlings. Plant Physiol 116:2651–2658. https://doi.org/10.1104/pp.116.2.651
Bolduc P, Bertolo A, Pinel-Alloul B (2016) Does submerged aquatic vegetation shape zooplankton community structure and functional diversity? A test with a shallow fluvial lake system. Hydrobiologia 778:151–165. https://doi.org/10.1007/s10750-016-2663-4
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein biding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brix H (1993) Macrophyte-mediated oxygen transfer in wetlands: transport mechanisms and rates. In: Constructed Wetlands for Water Quality Improvement. Edited by Moshiri, G.a. pp. 391–398. Lewis publishers
Brix H, Schierup HH (1990) Soil oxygenation in constructed reed beds: the role of macrophyte and soil-atmosphere interface oxygen transport. In: Constructed Wetlands in Water pollution control, Proceedings of the International Conference on the Use of Constructed Wetlands in Water Pollution Control 161, Cambridge, UK, pp. 53–66
Brix H, Sorrel BK, Orr P (1992) Internal pressurization and convective gas flow in some emergent freshwater macrophytes. Limnol Oceanogr 37:1420–1433. https://doi.org/10.4319/lo.1992.37.7.1420
Brulé V, Rafsanjani A, Pasini D, Westerna TL (2016) Hierarchies of plant stiffness. Plant Sci 250:79–96. https://doi.org/10.1016/j.plantsci.2016.06.002
Brundrett MC, Enstone DE, Peterson CA (1988) A berberine–aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 146:133–142
Cheng H, Liu Y, Tam NF, Wang X, Li SY, Chen GZ, Ye ZH (2010) The role of radial oxygen loss and root anatomy on zinc uptake and tolerance in mangrove seedlings. Environ Pollut 158:1189–1196. https://doi.org/10.1016/j.envpol.2010.01.025
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36. https://doi.org/10.1046/j.1365-3040.2003.00846.x
Colmer TD, Cox MCH, Voesenek LACJ (2006) Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytol 170:767–777. https://doi.org/10.1111/j.1469-8137.2006.01725.x
Corrêa FF, Madail RH, Barbosa S, Pereira MP, Castro EM, Soriano CTG, Pereira FJ (2015) Anatomy and physiology os cattail as related to different population densities. Planta Daninha 33:1–12. https://doi.org/10.1590/S0100-83582015000100001
Corrêa FF, Pereira MP, Madail RH, Santos BR, Barbosa S, Castro EM, Pereira FJ (2017) Anatomical traits related to stress in high density populations of Typha angustifolia L. (Typhaceae). Braz J Biol 77:52–59. https://doi.org/10.1590/1519-6984.09715
Dai M, Liu J, Liu W, Lu W, Jia H, Hong H, Yan C (2017) Phosphorus effects on radial oxygen loss, root porosity and iron plaque in two mangrove seedlings under cadmium stress. Marine Poll Bull 119:262–269. https://doi.org/10.1016/j.marpolbul.2017.04.013
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciênc Agrotec 35:1039–1042. https://doi.org/10.1590/S1413-70542011000600001
Fu JJ, Chu XT, Sun YF, Xu YF, Hu TM (2016) Involvement of nitric oxide in 5-aminolevulinic acid-induced antioxidant defense in roots of Elymus nutans exposed to cold stress. Biol Plant 60:585–594. https://doi.org/10.1007/10535-016-0635-1
Havir EA, Mchale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455. https://doi.org/10.1104/pp.84.2.450
Ho QT, Verboven P, Verlinden BE, Herremans E, Wevers M, Carmeliet J, Nicolai BB (2011) A three-dimensional multiscale model for gas exchange in fruit. Plant Physiol 155:1158–1168. https://doi.org/10.1104/pp.110.169391
Hoagland DR, Arnon DI (1940) Crop production in artificial culture solutions and in soils with special reference to factors influencing yield absorption of inorganic nutrients. Soil Sci 50:463–485
Johansen DA (1940) Plant microtechnique. Tata McGraw-Hill Book Company, New York, p 523
Kaul RB (1974) Ontogeny of foliar diaphragms in Typha latifolia. Am J Bot 61:318–323
Konnerup D, Sorrell BK, Brix H (2011) Do tropical wetland plants possess convective gas flow mechanisms? New Phytol 190:379–386. https://doi.org/10.1111/j.1469-8137.2010.03585.x
Kordyum E, Kozeko L, Ovcharenko Y, Brykov V (2017) Assessment of alcohol dehydrogenase synthesis and aerenchyma formation in the tolerance of Sium L. species (Apiaceae) to water-logging. Aquat Bot 142:71–77. https://doi.org/10.1016/j.aquabot.2017.07.001
Ma X, Zarebanadkouki M, Kuzyakov Y, Blagodatskaya E, Pausch J, Razavi BS (2018) Spatial patterns of enzyme activities in the rhizosphere: effects of root hairs and root radius. Soil Biol Biochem 118:69–78. https://doi.org/10.1016/j.soilbio.2017.12.009
Mano Y, Omori F, Takamizo T, Kindiger B, Bird M, Loaisiga CH (2006) Variation for root aerenchyma formation in flooded and non-flooded maize and teosinte seedlings. Plant Soil 281:269–279. https://doi.org/10.1007/s11104-005-4268-y
Miao Y, Bi Q, Qin H, Zhang X, Tan N (2020) Moderate drought followed by re-watering initiates beneficial changes in the photosynthesis, biomass production and Rubiaceae-type cyclopeptides (RAs) accumulation of Rubia yunnanensis. Ind Crop Prod 148:112–123. https://doi.org/10.1016/j.indcrop.2020.112284
Møller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52:561–591. https://doi.org/10.1146/annurev.arplant.52.1.561
Oliveira JPV, Pereira MP, Duarte VP, Corrêa FF, Castro EM, Pereira FJ (2017) Cadmium tolerance of Typha domingensis Pers . (Typhaceae ) as related to growth and leaf morphophysiology. Braz J Biol 78:509–516. https://doi.org/10.1590/1519-6984.171961
Pang S, Zhang S, Lv XY, Han B, Liu K, Qiu C, Wang C, Wang P, Toland H, He Z (2016) Characterization of bacterial community in biofilm and sediments of wetlands dominated by aquatic macrophytes. Ecol Eng 97:242–250. https://doi.org/10.1016/j.ecoleng.2016.10.011
Pereira FJ, Castro EM, Olivera CD, Pires MF, Pereira MP, Ramos S, Faquin V (2014) Lead tolerance of water hyacinth. An Acad Bras Ciênc 86:1423–1433. https://doi.org/10.1590/0001-3765201420140079
Pi N, Tam NFY, Wu Y, Wong MH (2009) Root anatomy and spatial pattern of radial oxygen loss of eight true mangrove species. Aquat Bot 90:222–230. https://doi.org/10.1016/j.aquabot.2008.10.002
Rehman F, Pervez A, Mahmood Q, Nawa B (2017) Wastewater remediation by optimum dissolve oxygen enhanced by macrophytes in constructed wetlands. Ecol Eng 102:112–126. https://doi.org/10.1016/j.ecoleng.2017.01.030
Retta M, Ho QT, Yin X, Verboven P, Berghuijs H, Struik PC, Nicolai BM (2016) A two-dimensional microscale model of gas exchange during photosynthesis in maize (Zea mays L.) leaves. Plant Sci 246:37–51. https://doi.org/10.1016/j.plantsci.2016.02.003
Reuveni S, Urbakh M, Klafter J (2014) Role of substrate unbinding in Michaelis-Menten enzymatic reactions. Proc Natl Acad Sci U S A 111:4391–4396. https://doi.org/10.1073/pnas.1318122111
Seago JL Jr, Marsh LC, Stevens KJ, Soukup A, Votrubova O, Estone D (2005) A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Ann Bot 96:565–579. https://doi.org/10.1093/aob/mci211
Song Y, Ye L, Nii N (2011) Effects of soil water availability on development of suberin lamellae in the endodermis and exodermis and on cortical cell wall thickening in red bayberry (Myrica rubra Sieb. et Zucc.) tree roots. Sci Hortic 129:554–560. https://doi.org/10.1016/j.scienta.2011.04.005
Spengler A, Wanninger L, Pflugmacher S (2017) Oxidative stress mediated toxicity of TiO2 nanoparticles after a concentration and time dependent exposure of the aquatic macrophyte Hydrilla verticillata. Aquat Toxicol 190:32–39. https://doi.org/10.1016/j.aquatox.2017.06.006
Tanaka N, Yutani K, Aye T, Jinadasa KBSN (2007) Effect of broken dead culms of Phragmites australis on radial oxygen loss in relation to radiation and temperature. Hydrobiologia 583:165–172. https://doi.org/10.1007/s10750-006-0483-7
Terashima I, Hanba YT, Tholen D, Niinemts U (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155:108–116. https://doi.org/10.1104/pp.110.165472
Tornberg T, Bendix M, Brix H (1994) Internal gas transport in Typha latifolia L. and Typha angustifolia L. 2. Convective throughflow pathways and ecological significance. Aquat Bot 49:91–105. https://doi.org/10.1016/0304-3770(94)90031-0
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Voesenek LACJ, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206:57–73. https://doi.org/10.1111/nph.13209
Wetzel RG (2001) Limnology: Lake and river ecosystems. Springer Academic press, Third edition, 1006 p
White SD, Ganf GG (2000) Influence of stomatal conductance on the efficiency of internal pressurisation in Typha domingensis. Aquat Bot 67:1–11. https://doi.org/10.1016/S0304-3770(99)00090-X
Xu J, Li C, Yang F, Dong Z, Zhang J, Zhao Y, Qi P, Hu Z (2011) Typha angustifolia stress tolerance to wastewater with different levels of chemical oxygen demand. Desalination 280:58–62. https://doi.org/10.1016/j.desal.2011.06.050
Xu D, Wu Y, Howard A, Jiang X, Guan Y, Gao Y (2014) Influence of UV radiation on chlorophyll, and antioxidant enzymes of wetland plants in different types of constructed wetland. Environ Sci Pollut Res 21:10108–10119. https://doi.org/10.1007/s11356-014-2909-5
Yang J, Ye Z (2015) Antioxidant enzymes and proteins of wetland plants: their relation to Pb tolerance and accumulation. Environ Sci Pollut Res 22:1931–1939. https://doi.org/10.1007/s11356-014-3610-4
Zhang J, Wu H, Hu Z, Fan J (2014) Examination of oxygen release from plants in constructed wetlands in different stages of wetland plant life cycle. Environ Sci Pollut Res 21:9709–9716. https://doi.org/10.1007/s11356-014-2905-9
Zhang L, Yang Q, Wang S, Li W, Jiang S, Liu Y (2017) Influence of silicon treatment on antimony uptake and translocation in rice genotypes with different radial oxygen loss. Ecotoxicol Environ Saf 144:572–577. https://doi.org/10.1016/j.ecoenv.2017.06.076
Funding
The authors thank CNPq [Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Counsel of Technological and Scientific Development)], CAPES [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordination for the Improvement of Higher Education Personnel)], and FAPEMIG [Fundação de Amparo à Pesquisa do estado de Minas Gerais (Minas Gerais State Research Foundation)] for funding and research grants awarded to complete the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling Editor: Muhammad Mujtaba
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duarte, V.P., Pereira, M.P., Corrêa, F.F. et al. Aerenchyma, gas diffusion, and catalase activity in Typha domingensis: a complementary model for radial oxygen loss. Protoplasma 258, 765–777 (2021). https://doi.org/10.1007/s00709-020-01597-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01597-8