Abstract
The growth and development and metabolism of plants have different physiological responses to different light qualities. To study the influence of light qualities on green onions, the impacts of LED light treatment on the growth and development as well as the nutritional components and flavor substances in green onions were studied under controlled conditions. Leaf area, plant height, dry matter accumulation, Dickson’s quality index (DQI), nutritional content, and volatile compounds under different light quality treatments were determined. The results indicated that the white and blue combined light (W/B: 3/1) treatment was the most beneficial to growth and nutrient accumulation and led to higher levels of sulfur compounds in the green onions than the other treatments. This shows that it is possible to control the contents of compounds that affect consumer preferences by adjusting the lighting conditions and to thereby increase the value and quality of seasoning vegetables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Allium species are therapeutic herbs with bioactive ingredients. They can be used as medicinal plants for the treatment of diseases. Their roots, leaves, and juices are also valuable to human health (Khan et al. 2017). Green onion is a significant flavoring vegetable that is popular in dishes around the world and is valued for its medicinal uses. More than 200 compounds have been identified in Allium species, including various vitamins, sulfur compounds, amino acids, proteins, lipids, trace elements such as selenium and flavonoids, and various antioxidants (Štajner et al. 2006). The characteristic flavors and odors of Allium vegetables come from their sulfur-containing compounds, mainly S-(E)-1-propenyl cysteine sulfoxide (PeCSO), S-propyl cysteine sulfoxide (PCSO), and S-methyl cysteine sulfoxide (MCSO) (Imai et al. 2002). Research have indicated that these bioactive sulfides have anticancer effects, especially against gastric cancer (Nicastro et al. 2015). Volatile sulfides are therefore an important aspect of the sensory evaluation of green onion.
The flavor and aroma of plants are limited not only by genetic factors but also by environmental conditions and cultivation techniques (Wieczorek et al. 2018). In order to better match the surrounding environment, plants could continuously regulate their growth and physiology state. Plant individual development is very sensitive to changes in environmental factors. Illumination is not only a basic energy source for plant growth but also a major signal for plants to change from yellowing to non-yellowing state, which stimulates plant growth and development and affects plant life cycle and cell groups’ biosynthesis related to gene expression (Jiao et al. 2007). Light intensity directly affects the growth and development and structural characteristics of plants. The photoperiod mainly affects flower induction and floral differentiation of plants. The effect of light quality on plants is more important and complex, including physiological processes such as growth and secondary metabolism (Fan et al. 2019; Jing et al. 2018; Wang et al. 2018). Many plants’ processes and characteristics such as growth and development, photosynthesis, leaf anatomical structure, and chloroplast ultrastructure have different responses to different light qualities (Dong et al. 2014; Liu et al. 2011).
Light quality not only affected plant morphology, photosynthesis, and tissue structure but also affected the sensory quality of fruits and vegetables (Loughrin et al. 2003). It has been shown that light of different wavelengths could affect volatile compounds in petunias and fruits (such as strawberries, blueberries, and tomatoes) (Colquhoun et al. 2013). Studies by Fu et al. (2015) have shown that blue light and red light significantly increase the levels of most of the endogenous volatiles in preharvest tea compared to the levels under natural light or dark treatment. Carvalho et al. (2016) through experiments in basil founded that treatment in blue/red/far-red resulted in higher sesquiterpene volatile content, while in blue/red/yellow or blue/red/green treatment released higher levels of monoterpene volatiles.
Green onion (Allium fistulosum L.) is a very popular food that can be consumed fresh, dried, or in processed or medicinal preparations, and its surface wax can also play a role in its drought resistance (Liu et al. 2019). Its typical characteristics are its flavor and aroma. Although most experiments have attempted to alter vegetables’ or fruits’ aroma and taste through genetics and breeding, previous studies have shown that flavor substances may be regulated by the effects of light conditions (Loughrin and Kasperbauer 2001). After special light treatments, certain green onion traits, such as their aroma, may change. As green onions are a highly valuable horticultural crop, identifying the complex components of their aroma is essential. However, at present, investigations of the aroma characteristics and specific medicinal value of green onions are still limited. Therefore, this paper studied the changes in the aroma components of green onions under different qualities of light and provided theoretical and data support related to the secondary metabolism and medicinal value of green onions. This study explored the response of green onion growth and development as well as nutrient and volatile substance levels to different light treatments and demonstrated the effects of different qualities of light on plant size, plant height, dry matter accumulation, and nutrients as well as flavor and aromatic volatile compounds. These findings may provide ideas for crop producers trying to produce high-quality and high-value green onions.
Materials and methods
Plant material and cultivation conditions
The green onions (Allium fistulosum L.)’ variety tested was “Yuanzang,” and the seeds of the green onions were provided by Tai’an Taishan Seed Industry Technology Co., Ltd. The green onions were cultivated at Shandong Agricultural University (longitude: 117.12° E; latitude: 36.19° N) and were seeded in 50-cell trays. When the seedling height was approximately 15 cm, they were transferred to trays containing quartz sand and placed under LED lamps with the different light quality treatments (Fig. 1).
The transferred green onion plants were placed on the light culture rack in the artificial climate room. The light source is placed on the top of the culture rack. The outer layer of the culture rack is silver shading cloth to ensure that the LED light source is the only light source for plant growth. The distance between the green onions plants and the light source in each treatment is the same, ensuring that the light intensity of each treatment is 300 ± 10 μmol/m2 s, controlling the climate indoor temperature in the daytime 23–25 °C, night 18–20 °C, each treatment the illumination time starts from 6:00 AM, and the illumination is controlled by a timer for 12 h day−1. Each treatment contained 20 plants, and all treatments were repeated 3 times. The results represent two independent experiments.
LED lighting treatments
The lighting systems for the different light quality treatments were assembled on the culture rack. Each treatment consisted of 12 LED lamps, each of which was 1.2 m long, and each treatment was equipped with a dimmer connected in parallel (Huizhou Kedao Technology Co., Ltd.). This study used five different light qualities: white light as CK (W), a 3:1 combination of white:blue light (WB), a 3:1 combination of white:green light (WG), a 3:1 combination of white:yellow light (WY), and a 3:1 combination of white:red light (WR). The spectral characteristics of the LED light were measured by UNSPEC-DCTM (PP Systems, UK) in Fig. 1.
Data measurement method
Samples were taken on the 30th day of treatment. Five green onion plants were randomly selected in white light (W), 3:1 combination of white:blue light (WB), 3:1 combination of white:green light (WG), 3:1 combination of white:yellow light (WY), and 3:1 combination of white:red light (WR) treatments. The plant height and pseudostem diameter of each treatment green onion plant were measured with a ruler and a vernier caliper, respectively. The LI-3000C leaf area meter (LI-COR Biosciences, USA) was used to measure leaf area (LA) of all leaves of each green onion. The fresh green onion sample was divided into leaf, pseudostem, and root. After weighing, the fresh sample was heated in an oven at 105 °C for 15 min. Then, they were dried to constant weight at 75 °C, weighed separately, and the dry weight (DW) of shoots and roots and the total DW of the plant were calculated. The dry matter content was calculated as a constant weight percentage relative to the fresh matter weight. Then, following formula was used to calculate Dickson’s quality index (DQI) (Tsakaldimi, Ganatsas, & Jacobs, 2012):
At the time of harvest, 10 green onions were randomly selected from each tray, and the nutritional quality of the edible parts of the plants was determined. A 0.5-g sample of green onion leaf and pseudostem homogenate was accurately weighed; 10 ml of distilled water was added to the homogenate, and centrifuged at 10,000g for 10 min using a centrifuge. Then, the soluble sugar content was determined as described by Wan et al. (2014).
The cellulose content was determined by concentrated sulfuric acid hydrolysis and sugar determination (Viles and Silverman 1949) with slight modifications. Samples of 0.5 g of green onion leaf and pseudostem were accurately weighed and then digested with 60 ml of 60% sulfuric acid solution in a cold-water bath for 30 min. The mixture was brought to 100 ml with 60% sulfuric acid solution, shaken well, and filtered with a Buchner funnel. Then, 5 ml of the filtrate was placed in a 100-ml volumetric flask, shaken, brought to 100 ml with distilled water, and filtered. The filtrate was used as the cellulose extract to determine the cellulose content.
The pyruvic acid content was determined by 2,4-dinitrophenylhydrazine colorimetry (Schwimmer and Weston 1961) with slight modifications. Frozen green onion (0.5 g) was ground with 10-mL 8% trichloroacetic acid solution in an ice bath. After standing for 30 min, the mixture was centrifuged at 4000g at 4 °C for 10 min. After centrifugation, 1-ml supernatant was transferred to another 10-ml test tube, then added 2-ml 8% trichloroacetic acid, 1-ml 0.1% 2,4-dinitrophenylhydrazine, and 5-ml 1.5 mol/L NaOH solution. Then, the solution was shook, and the OD value was read at 520 nm after 10 min.
The soluble protein content was measured according to Paciolla et al. (2010) with slight modifications. One gram of frozen green onion was ground in 4 ml of a solution containing the following ingredients: 0.05 M Tris-HCl (pH 8.0), 0.001 M EDTA, 0.01 M MgCl2, and 0.05% cysteine. The homogenate was centrifuged at 20,000g for 20 min at 4 °C, and the resulting supernatant was tested for soluble protein content according to Bradford (1976) with serum albumin as the standard.
The free amino acid content was measured by the ninhydrin solution chromogenic method (Huang et al. 2010). Green onion leaves and pseudostem powder (0.4 g) were weighed out separately, placed in a 250-ml round-bottom flask, and boiled with water 3 times. Forty milliliters of water was added, boiled for 1 h, added 20 ml of water, boiled for 0.5 h, added 20-ml of water, boiled for 0.5 h, and filtered while hot after each boiling. The three filtrates were combined and placed in a 100-ml volumetric flask, diluted with water to 100 ml, and used as the test solution. Three milliliters of the test solution was placed in a 25-ml volumetric flask, 1.0 ml of sodium acetate buffered saline solution at pH 5.0 and 2.0 ml of 2.0% ninhydrin solution were added, and the solution was boiled in a 100 °C water bath for 19 min and then cooled for 10 min. The absorbance of the color system was measured at 568 nm. The free amino acid content was calculated according to the standard curve of Huang et al. (2010).
Vitamin C content was assessed spectrophotometrically (Samuoliene et al. 2012). Plant tissue (1.0 g) was accurately weighed out, and 10 ml of 5% oxalic acid was added to grind it into a homogenate. Then, it was centrifuged at 2000g for 5 min at 4 °C. It was added 2 ml of 0.1% methyl viologen and 2 ml of 2 M sodium hydroxide to 1 ml of supernatant, shook well, and let stand for 2 min. The OD was read at 600 nm with a spectrophotometer.
The root, pseudostem, and leaf samples of the green onion under different light quality treatments were collected as experimental materials. The volatiles of the green onion were determined by headspace solid phase microextraction (HS-SPME) with some modifications (D'Auria and Racioppi 2017). The experimental green onions were washed, and 1 g of green onion root, pseudostem, and leaves were separately measured out, chopped, and quickly placed in a 40-ml headspace sampling extraction bottle that was then sealed. An 85-μm CAR-PDMS extraction head (Millipore-Sigma) was installed into the SPME handle (Millipore-Sigma). The headspace bottle filled with the test sample was placed in a 45 °C water bath and fixed with an iron stand, and the SPME needle was inserted. In the headspace vial, the adsorption head was pushed out. The position of the extraction head in the headspace volume was adjusted and fixed in order to prevent the extraction head from touching the sample and contaminating the extraction head. After adsorption for 30 min of headspace extraction, the adsorption head was retracted, and the extraction head was quickly pulled out and directly inserted into the inlet of a GC-MS (QP-2010, Shimadzu, Japan) for volatile compound identification. After desorbing at 260 °C for 5 min, the SPME needle was removed.
The gas chromatography (GC) column was an RTX-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness; maximum temperature, 350 °C); initial column temperature 50 °C, for 2 min, increase at 5 °C/min up to 150 °C, then increase to 250 °C at 10 °C/min, and then hold for 5 min; inlet temperature, 260 °C; carrier gas flow (He), 0.8 ml/min; split ratio, 10:1. The mass spectrometry (MS) conditions were transmission line temperature, 280 °C; electron bombardment (EI) ion source; ionization voltage, 70 V; electron multiplier voltage, 1329 V; ion source temperature, 230 °C; quadrupole temperature, 150 °C; full-spectrum scanning; and mass scanning range 35~350 m/z.
The desorbed volatile components were analyzed and identified by GC-MS.
Qualitative method: By searching the NIST 17/Wiley standard spectral library and referring to the positive and negative matching degrees and the related literature query CAS codes, the positive and negative matching degrees greater than 800 were identified, and the volatile substances with relative contents greater than 1% were selected for qualitative analysis.
Quantitative method: The relative content of each component was obtained according to the peak area normalization method, and 2-octanol (0.819 μg·ml−1, 10 μL) was selected as the internal standard for accurate quantification. The content of each component (ng·g−1) was calculated as [(component area/peak area of the internal standard) × internal standard concentration (μg·mL−1) × 10 μL]/fresh weight (g).
Statistical analysis
The plant sampling in this study followed the principle of random sampling. Three replicates were performed per treatment. The results are representative of two independent experiments. We normalized the concentration of each compound to a logarithmic scale and established a developmental heat map (Heml 1.0). Principal component analysis (PCA) used origin9.1 software. Morphology, dry matter, and volatile matter content were statistically analyzed using one-way analysis of variance (ANOVA). There were significant differences between treatments using Duncan’s new multiple range test (P < 0.05). DPS software and Excel 2016 for statistical analysis and drawing were used.
Results
Plant growth characteristic
After 30 days of light, the leaf number, leaf area, plant height, stem diameter, and pseudostem fresh weight of green onions under WB illumination were significantly higher than those of other illumination treatments. The stem dry matter content of the green onions treated with WB was slightly lower than that of the green onions treated with W, showing that in the WB treatment, the green onion has a higher water content. Dickson’s quality index (DQI) can be used to assess the quality and performance attributes of seedlings. We found that under different light conditions, the DQI of green onion seedlings decreased in the following order: WB > W > WR > WG > WY (Fig. 1, Table 1).
Nutrient ingredient
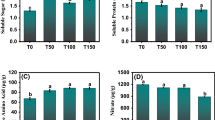
Table 2 shows the nutritional variations in the edible part of the green onions under different light quality treatments. From the data in the table, among the different green onion organs, except for pyruvate and soluble protein content, the nutrient content of the pseudostem was significantly higher than that of the leaves. For example, the soluble sugar content in the pseudostem was 41.03% higher than that in the leaves. In different light treatments, except for the Vc content, all test indicators for the quality of green onions in the WB were significantly higher than those in the other light treatments. Compared with WB treatment, the pyruvate content in green onions was 15.83%, 18.71%, 25.18%, and 38.85% lower in the W, WR, WG, and WY treatments, respectively.
Volatile contents
Based on empirical attempts to gather, dissociate, identify, and quantify 45 volatile substances by GC-MS in the various organs of green onion (Table S1), 27 compounds were detected in leaves, 28 compounds were detected in pseudostems, and 39 compounds were detected in roots. We established a heat map to better visualize the effects of different light qualities on various volatile compound types and a wide range of chemical classes in various green onions (see Table S2 for the complete statistical analysis). The results indicated that differences in the light environment have effects on all volatile substances (Fig. 2). The statistical comparison performed in duplicate between the two experiments showed that there are always important differences in certain volatile compounds in different light treatments. Figure 2 shows that the number of volatile components detected in the roots of green onions is the largest; the number of volatile components detected in leaves and pseudostems is the same, but different types of volatile compounds were detected. Of the different compound classes, all three organs showed the highest levels of sulfur compounds, especially trans-propenyl-propyl-disulfane (CAS: 23838-21-3), dipropyl disulfide (CAS: 629-19-6), dipropyl trisulfide (CAS: 6028-61-1), and 3,4-dimethyl thiophene (CAS: 632-15-5).
Individual volatile species. Heat map displayed relative levels of green onions volatile compounds in 30-day-old seedlings grown in the five enclosed chambers with artificial lighting and 3 organs; L leaf, P pseudostem, R root. Please refer to Table S1 for the chemical name represented by the CAS number. White indicates that no substance was detected. The results represent two independent experiments (n = 6)
In order to better visualize the subtle similarities and differences between the 5 light qualities and the 3 organs, we performed principal component analysis (PCA) on volatile compounds. PCA is an unsupervised clustering process for identifying patterns in data, via reducing the number of dimensions. It can define a limited number of principal components which describe independent variation in the results (Yang et al. 2013). In this study, the first two components PC1 and PC2 explained 35.5% and 24.8% of the total variance, respectively. The separation based on the type of treatment method cannot be observed from PCA, which indicated that the separation based on different organs was more important than the effect of light quality treatment. We found that the samples of the 3 organs were scattered in different quadrants, and there were obvious differences. Figure 3 shows that the compounds in green onion leaves aggregated under different light quality treatments, indicating that different light qualities have little effect on the compounds in the leaves. The grouping and the direction of each group is different for pseudostem (from positive PC1 & PC2 to smaller PC1 values and negative PC2) and root (from negative PC1 & positive PC2 to smaller PC1 and PC2 values). They show, in both cases, a clear separation of the WG and WY root and pseudostem samples.
Principal component analysis (PCA) graphs of volatiles extracted from the welsh onion. Different shapes represent different organs, and different colors represent different light quality treatments. Percentage indicates the proportion of variance explained by each axis. The volatiles are obtained from GC/MS data in the form of ng/g fresh weight. The results represent two independent experiments (n = 6)
The total volatiles from the different organs of 30-day-old green onions grown under the different light quality treatments were gathered and analyzed. The results showed that among the three organs of green onions, the content of sulfur compounds was the highest, followed by aldehydes and alcohols (Fig. 4). This model could be observed under the entire light conditions (Table S3). The content of sulfur compounds detected in the pseudostem was the highest of all the organs, and the leaves also had high levels of sulfur compounds. Ether and ketone volatiles were not detected in the pseudostem, and the roots showed the highest aldehyde content of all the organs. These findings indicate that the different organs accumulate different volatile substances. Figure 4 also shows the differences in volatiles among the different light quality treatments. In leaves, sulfur compounds were higher under the WB light treatment than under the other light treatments, while ester volatiles were not detected under the WG and WY light treatments. In the pseudostem, the aldehyde content was significantly higher under WG light than in the other treatments, while the ester compounds were only present under W and WB light. In the roots, sulfur compound levels under W and WR light were significantly higher than those under WB, WG, and WY light. These results indicate that the volatile substances in the same organ of green onion respond differently to different light qualities.
Volatile profiles. (a) Heat map for means of 8 subclasses, with quantification within the 5 light qualities and 3 organs. (b) Quantification of total volatiles and 8 subclasses in 30-day-old green onions seedlings grown in the five enclosed chambers with artificial lighting and 3 organs. Numbers are represented based on internal standard after normalization of the total peak. L leaf, P pseudostem, R root. White means no detected substance. Results are representative of two independent experiments (n = 6)
Main sulfur compounds
Figure 2 and Fig. 4 show that sulfur compounds are the main volatiles in green onions. Among all the sulfur compounds, trans-propenyl-propyl-disulfane (CAS: 23838-21-3), dipropyl disulfide (CAS: 629-19-6), dipropyl trisulfide (CAS: 6028-61-1), and 3,4-dimethyl thiophene (CAS: 632-15-5) played major roles. Figure 5 shows that there are differences in the contents of these four substances in the three organs of green onion, with the highest levels in pseudostems and similar levels in leaves and roots, indicating that the different organs in green onion have different accumulation capacities for these volatile substances. The compounds accumulated significantly differently in the three organs under the different light quality treatments, indicating that the light quality affects the accumulation of flavor substances.
Main sulfur compounds. Quantification of main sulfur compounds in 30-day-old seedlings grown in the five enclosed chambers with artificial lighting and 3 organs. Numbers are represented based on internal standard after normalization of the total peak. L leaf, P pseudostem, R root. Error bars represent standard error. Results are representative of two independent experiments (n = 6). Different letters (a, b, c, d) in the same column indicate significant differences among treatments at P < 0.05 according to Duncan’s new multiple range test
In green onions, the leaves and pseudostems are the edible parts. In the leaves, the contents of the sulfur compounds trans-propenyl-propyl-disulfane (CAS: 23838-21-3), dipropyl disulfide (CAS: 629-19-6), dipropyl trisulfide (CAS: 6028-61-1), and 3,4-dimethyl thiophene (CAS: 632-15-5) at WB treatment were significantly higher than other light qualities. The contents of the sulfur compounds trans-propenyl-propyl-disulfane (CAS: 23838-21-3), dipropyl disulfide (CAS: 629-19-6), dipropyl trisulfide (CAS: 6028-61-1), and 3,4-dimethyl thiophene (CAS: 632-15-5) in the pseudostems were significantly higher than leaves and roots. And there were significant differences among light quality treatments, for example, the content of dipropyl disulfide (CAS: 629-19-6) under the WB light treatment reached 2455.66 ng·g−1, and the dipropyl disulfide contents under the W, WG, WY, and WR treatments were 5.80%, 59.04%, 40.10%, and 36.45% lower, respectively, than that under the WB treatment.
Discussion
As we all know, light is a necessary environmental factor for plant growth and development and photosynthesis. The intensity and quality of light can act as a signaling factor during plant development and directly affect the morphology of the plant (Yang et al. 2015). Zuo et al. (2012) studies have shown that photoreceptors in plants could respond by sensing the surrounding light environment. Plants use this information to predict accurate indicators of future competition, and it can even induce morphological reactions.
In our research, under different LED lighting conditions, there were significant differences in the physiology morphology and growth situation of green onions (Table 1, Fig. 1). We noticed that, compared with the W treatment, the WB treatment significantly promoted the growth of green onion, while the WY and WG treatments significantly inhibited the growth of green onion; this result was inconsistent with the results of Li and Kubota (2009) in lettuce. In addition, compared with full-spectrum W light, monochromatic light (B, G, Y, R) treatment significantly inhibited the growth of green onion (Gao et al. 2020). However, Wang et al. (2016) also showed that red and blue mixed light with different ratios led to significant differences in lettuce growth and morphology. This suggests that the responses of different plants to light qualities and the effects of mixed light qualities in different proportions on plant growth and development are not the cumulative effects of individual light types on plants. Rather, more complex regulatory mechanisms are involved. The origin of the light and the light environment of plant species breeding locations are inseparable.
Some studies have shown that the influences of light qualities on the nutrient levels in different crops are not consistent. Lin et al. (2013) showed that the soluble sugar content in hydroponic lettuce under a red/blue/white (1/1/1) light treatment was considerably higher than that under a red/blue (1/1) light treatment. Red light can significantly increase the soluble sugar content of tomato fruits, while under blue light, the soluble sugar content is lower, but the vitamin C content is significantly increased (Li et al. 2017). This experiment found that WB led to the highest green onion quality, and its dry matter accumulation and protein, free amino acid, and soluble sugar contents were higher than those of the other treatments, indicating that increasing the blue light ratio is not only beneficial to green onion protein accumulation but also promotes carbohydrate synthesis (Table 2).
By combining GC/MS analysis and cluster analysis, we classified the important chemical components of the different green onion organs. The heat maps we build were effective tools for observing the behavior of these components. We found that the main volatile compounds in the three green onion organs were sulfur compounds and aldols. This is basically consistent with the measurement results of Kuo and Ho (1992). The accumulation of volatiles in green onion showed obvious plasticity based on the ambient light environment. Increasing the proportion of blue light to white light induced the accumulation of sulfur compounds and ethers in the leaves of green onion. Increasing the proportion of green light to white light induced the accumulation of alcohols and aldehydes in the pseudostems of the green onions but reduced the accumulation of ethers in the roots. For the most important category—sulfur compounds—the biosynthesis of sulfur compounds in the roots of green onion was inhibited by an increase in the proportion of green light or yellow light (Fig. 4). This shows that light quality can regulate the synthesis of volatile substances in green onions. This may be because the different light qualities stimulate the peltate glands on the leaf surface to synthesize and store volatiles (Gang et al. 2001) or because different light qualities affect the enzyme activity of specific volatile metabolism pathways (Iijima et al. 2004). High-intensity green light has been reported to stimulate the phenylpropane pathway in lettuce (Johkan et al. 2012).
The main flavor substance in Allium is organic sulfide. Onions species were regarded as food and medicine primarily due to the flavors and medicinal properties of their sulfur compounds (McCallum et al. 2005). In this study, we found that the content of sulfur compounds trans-propenyl-propyl-disulfane, dipropyl disulfide, dipropyl trisulfide, and 3,4-dimethyl thiophene in the three organs of green onion was higher than the content of other volatile compounds. Among them, dipropyl trisulfide is consistent with previous studies, while trans-propenyl-propyl-disulfane, dipropyl disulfide, and 3,4-dimethyl thiophene are not inconsistent (Farag et al. 2017). This may be due to the difference in onion species between the two experiments. Volatile compounds in Allium plants have been shown to have potential human health benefits, such as antispasmodic, antifungal, hemolytic, anti-inflammatory, cholesterol-lowering, and cytotoxic activities (Lanzotti et al. 2014). As an important indicator of the nutritional quality of Allium plants, the content of organic sulfide can be represented by the content of its decomposition product, pyruvate. In this study, the pyruvate content was the highest in green onions in the WB treatment, which is consistent with the result that the highest sulfur compound content was found in green onion leaves in the WB treatment (Fig. 4b). Kong et al. (2013) also suggested that the supply level of nitrogen and sulfur was significantly positively correlated with the content of spicy substances in green onions. Therefore, further research is necessary to determine whether light quality regulates mineral element metabolism in green onions.
Conclusion
Light quality can have a profound effect on the growth and development of green onion. Therefore, it is not surprising that light signals can also affect the abundance of secondary metabolites. Here, we found that WB treatment promoted the growth of green onion and WB was conducive to the accumulation of nutrients such as soluble sugar, soluble protein, crude cellulose, and pyruvate content. Different light conditions affected the volatile components and morphology in different organs of green onion. WY and WG significantly affected the synthesis of pseudostem and root compounds of green onion, and WB treatment increased the content of important sulfur compounds in green onion leaves. Looking to the future, through simple design, using safe and cheap light treatment procedures to adjust the lighting conditions of plants may improve their taste and aroma, and then control the content of compounds that consumers prefer to improve the value and quality of herbs.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carvalho S D, Schwieterman M L, Abrahan C E, Colquhoun T A, Folta K M (2016) Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Front Plant Sci 7: 1328. 13https://doi.org/10.3389/fpls.2016.01328
Colquhoun TA, Schwieterman ML, Gilbert JL, Jaworski EA, Langer KM, Jones CR, Rushing GV, Hunter TM, Olmstead J, Clark DG, Folta KM (2013) Light modulation of volatile organic compounds from petunia flowers and select fruits. Postharvest Biol Technol 86:37–44
D'Auria M, Racioppi R (2017) HS-SPME-GC-MS analysis of onion (Allium cepa L.) and shallot (Allium ascalonicum L.). Food Research 1:161–165
Dong C, Fu Y, Liu G, Liu H (2014) Growth, photosynthetic characteristics, antioxidant capacity and biomass yield and quality of wheat (Triticum aestivumL) exposed to LED light sources with different spectra combinations Journal of Agronomy and Crop Science 200: 219–230
Fan Y, Zhao X, Wang H, Tian Y, Xiang Q, Zhang L (2019) Effects of light intensity on metabolism of light-harvesting pigment and photosynthetic system in Camellia sinensis L. cultivar ‘Huangjinya’. Environ Exp Bot 166:103796. https://doi.org/103710.101016/j.envexpbot.102019.103706.103009
Farag M A, Ali S E, Hodaya R H, El-Seedi H R, Sultani H N, Laub A, Eissa T F, Abou-Zaid F O F, Wessjohann L A (2017) Phytochemical profiles and antimicrobial activities of Allium cepa Red cv and A sativum subjected to different drying methods: a comparative MS-based metabolomics Molecules 22:
Fu X, Chen Y, Mei X, Katsuno T, Kobayashi E, Dong F, Watanabe N, Yang Z (2015) Regulation of formation of volatile compounds of tea (Camellia sinensis) leaves by single light wavelength. Sci Rep 5: 16858. 168https://doi.org/10.11038/srep16858
Gang D R, Wang J, Dudareva N, Nam K H, Simon J E, Lewinsohn E, Pichersky E (2001) An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol 125: 539. 5 https://doi.org/10.1104/pp.1125.1102.1539
Gao S, Liu X, Liu Y, Cao B, Chen Z, Xu K (2020) Photosynthetic characteristics and chloroplast ultrastructure of welsh onion (Allium fistulosum L.) grown under different LED wavelengths. BMC Plant Biol 20: 78. https://doi.org/10.1186/s12870-12020-12282-12870
Huang S, Wu Y, Liu M (2010) Quantitative determination of total free-amino acid in Nervilia fordii (Hance) Schltr. by ninhydrin colorimetric method. Chinese Journal of Information on Traditional Chinese Medicine 17:50–52
Iijima Y, Davidovich-Rikanati R, Fridman E, Gang DR, Bar E, Lewinsohn E, Pichersky E (2004) The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol 136:3724–3736
Imai S, Tsuge N, Tomotake M, Nagatome Y, Sawada H, Nagata T, Kumagai H (2002) An onion enzyme that makes the eyes water. Nature 419:685–685
Jiao Y, Lau OS, Deng XW (2007) Light-regulated transcriptional networks in higher plants. Nat Rev Genet 8:217–230
Jing X, Wang H, Gong B, Liu S, Wei M, Ai X, Li Y, Shi Q (2018) Secondary and sucrose metabolism regulated by different light quality combinations involved in melon tolerance to powdery mildew. Plant Physiol Biochem 124:77–87
Johkan M, Shoji K, Goto F, Hahida S, Yoshihara T (2012) Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ Exp Bot 75:128–133
Khan SA, Jameel M, Kanwal S, Shahid S (2017) Medicinal importance of Allium species: a current review. International Journal of Pharmaceutical Science and Research 2:29–39
Kong L, Xu K, Wang L, He P, Zhang Y (2013) Influence of nitrogen and sulfur interaction on growth and quality of Chinese spring onion. Journal of Plant Nutrition and Fertilizer 19:1272–1278
Kuo MC, Ho CT (1992) Volatile constituents of the solvent extracts of Welsh onions (Allium fistulosum L. variety maichuon) and scallions (A. fistulosum L. variety caespitosum). J Agric Food Chem 40:1906–1910
Lanzotti V, Scala F, Bonanomi G (2014) Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem Rev 13:769–791
Li Q, Kubota C (2009) Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ Exp Bot 67:59–64
Li Y, Wang L, Wen L, Wei M, Shi Q, Yang F, Wang X (2017) Effects of red and blue light qualities on main fruit quality of tomato during color-turning period. Acta Horticulturae Sinica 44:2372–2382
Lin K-H, Huang M-Y, Huang W-D, Hsu M-H, Yang Z-W, Yang C-M (2013) The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci Hortic 150:86–91
Liu X, Gao S, Liu Y, Cao B, Chen Z, Xu K (2019) Comparative analysis of the chemical composition and water permeability of the cuticular wax barrier in Welsh onion (Allium fistulosum L.). Protoplasma1-8
Liu X, Guo S, Xu Z, Jiao X, Takafumi T (2011) Regulation of chloroplast ultrastructure, cross-section anatomy of leaves, and morphology of stomata of cherry tomato by different light irradiations of light-emitting diodes. HortScience 46:217–221
Loughrin JH, Kasperbauer MJ (2001) Light reflected from colored mulches affects aroma and phenol content of sweet basil (Ocimum basilicum L.) leaves. J Agric Food Chem 49:1331–1335
Loughrin JH, Kasperbauer MJ (2003) Aroma content of fresh basil (Ocimum basilicum L.) leaves is affected by light reflected from colored mulches. J Agric Food Chem 51:2272–2276
McCallum J, Porter N, Searle B, Shaw M, Bettjeman B, McManus M (2005) Sulfur and nitrogen fertility affects flavour of field-grown onions. Plant Soil 269:151–158
Nicastro HL, Ross SA, Milner JA (2015) Garlic and onions: their cancer prevention properties. Cancer Prev Res 8:181–189
Paciolla C, D’Emerico S, Tommasi F, Scrugli A (2010) Karyomorphological and biochemical studies in Glebionis coronaria (L.) Spach and Glebionis segetum (L.) Fourreau from Italy. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 144:563–567
Samuoliene G, Sirtautas R, Brazaityte A, Duchovskis P (2012) LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem 134:1494–1499
Schwimmer S, Weston W (1961) Onion flavor and odor, enzymatic development of pyruvic acid in onion as a measure of pungency. J Agric Food Chem 9:301–304
Štajner D, Milić N, Čanadanović-Brunet J, Kapor A, Štajner M, Popović BM (2006) Exploring Allium species as a source of potential medicinal agents. Phytother Res 20:581–584
Tsakaldimi M, Ganatsas P, Jacobs DF (2012) Prediction of planted seedling survival of five Mediterranean species based on initial seedling morphology. New For 44:327–339
Viles FJ, Silverman L (1949) Determination of starch and cellulose with anthrone. Anal Chem 21:950–953
Wan Y-Y, Zhang Y, Zhang L, Zhou Z-Q, Li X, Shi Q, Wang X-J, Bai J-G (2014) Caffeic acid protects cucumber against chilling stress by regulating antioxidant enzyme activity and proline and soluble sugar contents. Acta Physiologiae Plantarum 37: 1706. 17 https://doi.org/10.1007/s11738-11014-11706-11736
Wang J, Lu W, Tong Y, Yang Q (2016) Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Frontiers in plant science 7: 250. 2 https://doi.org/10.3389/fpls.2016.00250
Wang X, Fu X, Chen M, Huan L, Liu W, Qi Y, Gao Y, Xiao W, Chen X, Li L, Gao D (2018) Ultraviolet B irradiation influences the fruit quality and sucrose metabolism of peach (Prunus persica L.). Environ Exp Bot 153:286–301
Wieczorek MN, Walczak M, Skrzypczak-Zielińska M, Jeleń HH (2018) Bitter taste of Brassica vegetables: the role of genetic factors, receptors, isothiocyanates, glucosinolates, and flavor context. Crit Rev Food Sci Nutr 58:3130–3140
Yang B, Yang H, Chen F, Hua Y, Jiang Y (2013) Phytochemical analyses of Ziziphus jujuba Mill. var. spinosa seed by ultrahigh performance liquid chromatography-tandem mass spectrometry and gas chromatography-mass spectrometry. Analyst 138:6881–6888
Yang F, Wang X, Liao D, Lu F, Gao R, Liu W, Yong T, Wu X, Du J, Liu J, Yang W (2015) Yield response to different planting geometries in maize–soybean relay strip intercropping systems. Agron J 107:296–304
Zuo Z-C, Meng Y-Y, Yu X-H, Zhang Z-L, Feng D-S, Sun S-F, Liu B, Lin C-T (2012) A study of the blue-light-dependent phosphorylation, degradation, and photobody formation of arabidopsis CRY2. Mol Plant 5:726–733
Funding
This study was supported by the Shandong Province’s dual-class discipline construction project (Grant No. SYL2017YSTD06), the Agricultural application technology innovation project in Shandong Province of China (Grant No. 310131), and the China Agriculture Research System (Grant No. CARS-24-A-09).
Author information
Authors and Affiliations
Contributions
Song Gao: conceptualization, methodology, software, formal analysis, investigation, data curation, writing—original draft, visualization. Xuena Liu, Ying Liu: validation, investigation. Bili Cao, Zijing Chen: supervision, project administration. Kun Xu: conceptualization, resources, writing—review & editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2463 kb)
Rights and permissions
About this article
Cite this article
Gao, S., Liu, X., Liu, Y. et al. Comparison of the effects of LED light quality combination on growth and nutrient accumulation in green onion (Allium fistulosum L.). Protoplasma 258, 753–763 (2021). https://doi.org/10.1007/s00709-020-01593-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01593-y