Abstract
Genetic transformation has always been an important method for studying medical plant secondary metabolic regulation, among which stable transformation has a good reproducibility. However, it was time-consuming to obtain a stable transformed hairy root or transgenic plants, which was difficult to satisfy the great demand of researches on medical plant secondary metabolism–related genes. Moreover, Agrobacterium tumefaciens–mediated transient transformation has been extensively applied in studies of functional genes because of its simpleness, low cost, and short period. However, presently, researches on medical plant functional genes commonly used stable genetic transformation and some high-cost and high-difficulty transient transformation methods, such as gene gun and protoplast transformation. Thus, in this study, we selected the seedlings of Nicotiana benthamiana, Salvia miltiorrhiza, and Prunella vulgaris as the experimental material, with the methods of Agrobacterium tumefaciens injection, fast Agrobacterium-mediated seedling transformation (FAST), and FAST and mechanical damage. The results demonstrated that the injection transient transformation system of pCAMBIA1301 vector mediated by A. tumefaciens and the transient transformation of seedling system were not established in S. miltiorrhiza. In addition, the instantaneous transformation system of N. benthamiana and P. vulgaris seedlings was basically set up by FAST method. Besides, using the method of FAST and mechanical damage, the transient genetic transformation system of P. vulgaris seedlings was established for the first time. A. tumefaciens–mediated transient transformation of seedlings with pEAQ vectors provided an effective way and reference for the further study of functional genes of the medicinal plants N. benthamiana and P. vulgaris.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nicotiana benthamiana L. is a herbaceous plant belonging to the genus Nicotiana in the Solanaceae family, which is not only an essential industrial crop worldwide but also a model plant for biological mechanism studies due to its advantages of short generation time, disease susceptibility, and ease of genetic transformation (Udawat et al. 2016). Salvia miltiorrhiza Bunge and Prunella vulgaris L. belong to the family of Labiatae, which has important economic and medicinal value in China. Modern pharmacological studies exhibit that these medicinal plants have the properties of being anti-tumor, anti-virus, and anti-inflammatory (Tian et al. 2015; Ma et al. 2019; Zhang et al. 2018).
Transient transformation system, a new transformation method, has been able to replace the stable transformation (McIntosh et al. 2004). At present, there were two main means of transient transformation. One was protoplast transformation by electric shock or PEG penetration (Sheen 2001). The other was particle bombardment of plant epidermal cells or leaves (Sessa et al. 1998; Schweizer et al. 1999), also known as gene gun method. Due to the time-consuming and low success rate of protoplast culture and the need for special corollary equipment for gene gun, it was difficult for these two methods to be widely used. Compared with the two methods mentioned above, Agrobacterium-mediated transient transformation has the advantages of low cost, easy operation, and high success rate, which could be used in many research fields, including gene expression detection, gene silencing, subcellular localization, protein interaction analysis, and inhibitor function identification (Zhao et al. 2013). Agrobacterium tumefaciens, as a common tool for plant genetic transformation, mainly employed toothpick inoculation (Lu et al. 2003), injection (Fu et al. 2005), high pressure jet (Liu et al. 2002), and vacuum infiltration (Ekengren et al. 2003). These methods have been successfully used in many plant tissues, such as leaves of Arabidopsis thaliana (Clough and Bent 2010); petals of lilies and goldfish grass; leaves, petioles, and callus of Pinellia ternata (Jia et al. 2007); leaves and fruits of lettuce and tomato; leaves of grape (Yang et al. 2000); and fleshy fruits of fresh apples, pears, peaches, strawberries, and oranges (Zhao et al. 2013). Injection penetration transient transformation method was widely used to express exogenous genes in N. benthamiana leaves (Li and Zhang 2010). This was due to N. benthamiana has advantages like short growth cycle, weak disease resistance, or low rejection reaction that other medicinal plants do not have. However, in other medicinal plants, how to achieve effective instantaneous expression in a relatively short period of time has always been one of the bottlenecks of instantaneous means. Therefore, the selection of target plant materials (including growth status and tissue location) and A. tumefaciens strains was particularly important.
FAST method is an A. tumefaciens–mediated transient transformation system based on plant seedlings (Li et al. 2009). Compared with A. tumefaciens injection method, FAST method has the advantages of simple operation, no special equipment, and could complete transformation and analysis in a very short experimental cycle (about a week). The acquisition of plant seedlings was usually fast, and the self-defense system of seedlings might not be fully mature, which provided favorable conditions for the invasion of A. tumefaciens and the expression of exogenous genes. Therefore, this technique was quickly applied by many researchers in different plant materials such as N. benthamiana (Ratcliff et al. 2001), tomato, rice (Li et al. 2009), orange, banana, lentil, sugarcane (Subramanyam et al. 2013), and a variety of woody plants (Zheng et al. 2012). In addition, using plants as expression systems to produce recombinant proteins has obvious advantages over traditional bacterial and yeast expression systems. Recombinant proteins produced in plants undergo post-translational modification by higher-level proteins, which have better safety and fidelity when used as drugs or in research (Kusnadi and Nikolov 1997; Lico et al. 2008). Moreover, some medicinal plants such as N. benthamiana could grow at high density in a short time and produce high biomass in a few weeks.
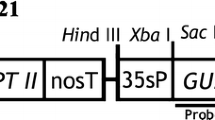
The pEAQ vector transient transformation system was based on cowpea mosaic virus (CPMV) RNA-2 (CPMV-HT) expression system, which added 5′-untranslated region (UTR) and 3′-UTR of CPMV virus to both sides of the target gene and co-expressed with virus silencing inhibitor P19 (Supplementary fig. 1), thus achieving the effect of large amount of expression of the target protein (Sainsbury et al. 2009). At present, pEAQ vectors have been successfully used to express various recombinant proteins, including human gastric lipase (hGL) (Vardakou et al. 2012) and recombinant human serum albumin (HSA) (Sun et al. 2011). Importantly, these exogenous proteins were expressed in N. benthamiana. Hence, we can speculate that the system can express one or more enzymatic genes related to secondary metabolite synthesis in N. benthamiana, S. miltiorrhiza, P. vulgaris, and other medicinal plants, so as to realize heterologous construction of secondary metabolite synthesis pathway.
Materials and methods
Plant materials and sample preparation
N. benthamiana, S. miltiorrhiza, and P. vulgaris seeds were collected from the Tianshili Plant Pharmaceutical (Shaanxi Province, China) Co., Ltd. When S. miltiorrhiza seeds germinated, they should be evenly sprayed in a culture dish covered with three layers of wet filter papers and placed in a light incubator. The incubation conditions in the light incubator were as follows: the temperature was at 25 ± 2 °C, the illumination time was about 12–14 h, and the light intensity was at 2000–3000 lx. The germination method of N. benthamiana and P. vulgaris seeds was the same as that of S. miltiorrhiza. After a week of germination, these seedlings were selected for Agrobacterium-mediated transient transformation of seedlings.
The cotyledons, stems, and roots of S. miltiorrhiza seedlings for total component analysis were prepared according to the following steps: (1) After the seeds of S. miltiorrhiza germinated on the gauze for 7 days, the cotyledons of the seedlings were carefully picked up with ophthalmic surgical scissors. The collected cotyledons were washed with distilled water and dried on filter paper then packed them into kraft envelope and dried in an oven at 40 °C until constant weight. (2) Used ophthalmic surgical scissors to carefully cut bare seedling stems along the gauze surface. And then dried and preserved them according to the treatment method of cotyledons. (3) The roots of the seedlings remaining on the back of the gauze were cut with ophthalmic surgical scissors. The treatment method was same with cotyledons, too. (4) The cotyledons, stems, and roots of S. miltiorrhiza seedlings dried to constant weight were ground into powder in a mortar for high-performance liquid chromatography (HPLC) analysis.
Chemicals and reagents
4-methyl umbrella ketone (4-MU), 4-methyl umbrella ketone-D-glucuronide (MUG), X-Glucucuc, Silwet L-77, TaKaRa protein quantitative kit, 2 × buffer (0.1 M sodium citrate, 0.2 N HCl, pH 7.0), β-glucuronidase (GUS) tissue-staining reagent, GUS enzyme extract, GUS enzyme termination solution (0.2 M Na2CO3), surface disinfectant (0.05% sodium hypochlorite), cleaning buffer (10 mM MgCl2, 100 uM acetyleugenone), and co-culture buffer were obtained.
Strains and vectors
E. coli DH5α competent cells were purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd. A. tumefaciens EHA105 was preserved in our laboratory. The pEAQ vectors include plant expression vectors pCAMBIA1301 and pCAMBIA1304, which contain reporter genes GUS and green fluorescent protein (GFP) respectively.
Main instruments
Vacuum freeze dryer LL3000 (Somerfly Shier Company, USA), constant temperature shaker TSQ-280 (Shanghai Jinghong Experimental Equipment Co., Ltd., China), constant temperature incubator DNP-9082 (Shanghai Jinghong Experimental Equipment Co., Ltd., China), constant temperature incubator ZPW-350 (Heilongjiang dongtuo Instrument Manufacturing Co., Ltd., China), pH meter (Shanghai Instrument and Electrical Science Instrument Co., Ltd., China), electric heating constant temperature blast drying Box 101-2AB (Tianjin Tester Instruments Co., Ltd., China), electronic balance AUW120 (Shimadzu Corporation, Japan), electric converter (Eppendorf Company, Germany), high-performance liquid chromatography (Waters Company, USA), and high-intensity ultraviolet flaw detection lamp FC-100/FA (SPECTROLINE Company, USA) were required.
Analysis of active ingredients in S. miltiorrhiza leaves by HPLC
There were two main components of S. miltiorrhiza: rosmarinic acid and salvianolic acid B. The two active ingredients in the cotyledons, stems, and roots of S. miltiorrhiza were determined by HPLC. The method of extraction and detection has been slightly changed (Liang et al. 2012). When extracting the active ingredients, the powder of the above-mentioned dried sample should be weighed at 0.05 g, added 10 mL 70% methanol-water solution, ultrasounded for 60 min, centrifugated for 10 min at 12,000 rpm, and then the supernatant should be filtered through a 0.45-μm membrane for reserve. Waters 1525 binary chromatographic pump, waters 2996 PAD detector, and waters sun fire C18 (250 mm × 4.6 mm, 5 μm) were used in this experiment. The chromatography was performed under the following conditions: the flow rate was kept at 1 mL/min, the column temperature was maintained at 30 °C, and the injection volume was 20 μL. The detection wavelength was 288 nm (water-soluble component) and 270 nm (fat-soluble component). The mobile phase consisted of solvent A (0.02% phosphoric acid water, v/v) and solvent B (acetonitrile) was set in a gradient elution program: 0–10 min (5–20% B), 10–15 min (20–25% B), 15–20 min (25% B), 20–25 min (25–20% B), 25–28 min (20–25% B), 28–37 min (25–30% B), 37–45 min (30–45% B), 40–50 min (45–50% B), 50–60 min (50% B), 60–65 min (50–60% B), 65–70 min (60% B), 70–80 min (60–70% B), and 80–85 min (100% B).
Electrical transformation of A. tumefaciens EHA105
The vectors pCAMBIA1301 and pCAMBIA1304 were transformed into A. tumefaciens EHA105 competent cells by electric shock. Firstly, 1-μL recombinant plasmid was added into the 100-μL EHA105 competent cells, gently mixed and ice-bathed for 30 min. The voltage of the electrometer was adjusted to 2.5 KV and electric shock to 5 ms. Secondly, the mixture of plasmid and competent cells was transferred to the bottom of the shock cup and quickly put into the electrometer. The electric pulse was started according to the set parameters. Then samples were taken out as soon as possible after electric shock, supplementing 1-mL fresh LB liquid medium. The treated solution was slightly shaken at 28 °C for 4–6 h. Subsequently, the bacterial solution was coated on solid medium containing rifampicin (50 mg/L) and kanamycin (50 mg/L) for 2–3 days in the darkness at 28 °C. Finally, the single colony was selected, the plasmid after shaking the bacteria was extracted, and the vector was verified by sequencing.
The A. tumefaciens injection and FAST method
The main method of A. tumefaciens injection was leaf injection. The above suspension of Agrobacterium with a sterile injection syringe of 1 mL was taken and then injected into the leaves.
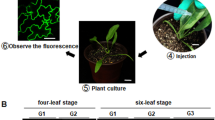
The FAST method was performed by the following steps (Li et al. 2009): (1) The monoclonal of A. tumefaciens was selected and cultured in 2-mL LB liquid medium, which contains 50 mg/mL Rif and 50 mg/mL Kan. The culture conditions were at 28 °C for 18–24 h on a shaker at 220 rpm; (2) the 2-mL bacterial solution was transferred into 100-mL LB liquid medium with the same antibiotics and grown at 28 °C on a shaker at 220 rpm until the concentration of bacterial solution was about OD600 = 0.5; (3) the bacterial solution was diluted with fresh antibiotic-free LB liquid medium to OD600 = 0.3 and cultured at the same conditions with step (2) until OD600 > 1.0 (about 10–12 h); (4) the bacterial liquid was transferred into 50-mL centrifugal tube with 5000 rpm centrifugation for 5 min, and LB liquid medium was discarded; (5) the suspension was washed with 10-mL cleaning buffer, centrifuged with 5000 rpm for 5 min, and discarded the supernatant; (6) the suspension was resuspended with 1-mL cleaning buffer; (7) 100-μL concentrated bacterial liquid which was obtained in step (6) was taken, diluted 10 times with cleaning buffer, and calculated the volume of concentrated bacterial liquid; (8) some 7-day plant seedlings (N. benthamiana, S. miltiorrhiza, P. vulgaris) were placed in sterile dishes and 20-mL co-culture buffer solution was added; (9) the calculated volume of concentrated bacterial liquid and adjusted OD600 = 0.5 (the calculation method is as follows: adding bacterial liquid volume = total volume of co-culture × 0.5/OD600 value of concentrated bacterial liquid) was added; (10) the cultured seedlings were slowly shaken at room temperature for 24–72 h (N. benthamiana was cultured for 24 h, P. vulgaris and S. miltiorrhiza were cultured for 40–72 h); (11) The co-cultured seedlings were soaked in surface disinfectant for 5 min, then washed repeatedly with sterile water for more than 5 times; (12) The seedlings were transferred to a culture dish containing 0.5 × MS liquid medium and cultured for 3 to 5 days for observation. The procedures of the negative control groups were the same as the above, but there was no concentrated bacterial solution added to the co-culture medium in step (9).
Mechanical damage treatment
Mechanical damage included cotyledon puncture and vacuum infiltration. The former was to place the seedlings in a culture dish and punctured cotyledon surface lightly with syringe needle (to avoid lateral force and scratch), each cotyledon has about five micro holes. The latter was to put the culture dish containing calculated volume of concentrated bacterial liquid in step (9) and seedlings into a vacuum dryer, vacuumed for 10 min with a vacuum pump, and then began the following infection steps.
GUS staining and quantitative detection
GUS staining and quantitative detection were according to the previous method (Jefferson 1987).
Data analysis
All experimental data were calculated by Microsoft Excel 2010.
Results and discussion
Analysis of effective components in cotyledons, stems, and roots of S. miltiorrhiza seedlings
In order to comprehensively analyze the effects of A. tumefaciens–mediated transient infection on the secondary metabolites of S. miltiorrhiza seedlings, the active components of the cotyledons, stems, and roots of S. miltiorrhiza seedlings were detected by HPLC. At the same time, the same HPLC condition was used to measure the secondary metabolites in the seedlings of N. benthamiana and P. vulgaris. The results are shown in Fig. 1. In our study, it was found that there were significant differences in secondary metabolic species and contents among roots, stems, and cotyledons of S. miltiorrhiza seedlings. Rosmarinic acid was the main secondary metabolites of the three water-soluble components, but the content of rosmarinic acid was quite different, showing root > stem > cotyledon. Water-soluble salvianolic acid B was mainly found in the roots of S. miltiorrhiza seedlings, and a small amount of salvianolic acid B was detected in stems, but not in cotyledons, indicating that cotyledons were not the main accumulation sites of secondary metabolites in S. miltiorrhiza seedlings. The fat-soluble components were not accumulated in the roots, stems, and cotyledons of S. miltiorrhiza seedlings, which was consistent with previous experimenters (Li 2008). Therefore, it was suggested that water-soluble components should be the main research area if secondary metabolites of S. miltiorrhiza are considered, and the focus should be on the root. These two secondary metabolites were not detected in the seedlings of N. benthamiana and P. vulgaris.
Transient transformation with pCAMBIA1301 vector
To ensure the effective infection of A. tumefaciens EHA105 carrying binary vector pCAMBIA1301, which was containing GUS reporter gene, we first transformed N. benthamiana and S. miltiorrhiza leaves by A. tumefaciens injection. After GUS staining, the results are shown in Fig. 2. Compared with the control group, N. benthamiana leaves in the transformation experiment group reflected obvious blue color. It indicated that pCAMBIA1301 vector could successfully express GUS gene in N. benthamiana leaves. The staining results of S. miltiorrhiza leaves displayed that neither negative control nor infected experimental group has showed a blue staining. The research validated that pCAMBIA1301 vector could not be successfully transformed into S. miltiorrhiza leaves by Agrobacterium injection.
Therefore, we adopted FAST method to further investigate the reasons why the pCAMBIA1301 vector could not transform S. miltiorrhiza seedlings successfully. The result was demonstrated in Fig. 3. S. miltiorrhiza seedlings in the negative control group and pCAMBIA1301 transformation experiment group showed a blue color reaction on the stem, which might be due to the GUS background staining of S. miltiorrhiza seedlings. This characteristic of S. miltiorrhiza species was semblable with other plants (Richard 1987). Therefore, a specific GUS activity related to the transformation was not detected in the infection experimental group. The results were still the same after multiple repetitions, so it was judged that there were other unknown factors influencing the transient transformation of S. miltiorrhiza seedlings by plant expression vector pCAMBIA1301, which led to the fail transformation of S. miltiorrhiza seedlings. Consequently, the pCAMBIA1301 vector carried by A. tumefaciens EHA105 could not achieve effective transient transformation in S. miltiorrhiza leaves and seedlings by FAST method.
Transient transformation with pCAMBIA1304 vector
PCAMBIA1304 containing GFP reporter gene was used as transformation vector, and the seedlings of N. benthamiana and P. vulgaris were transformed by FAST method. N. benthamiana seedlings after infection were cultured in 0.5 × MS liquid medium for 3 days and observed under a fluorescence microscope with the excitation wavelength of 488 nm. GFP expression was observed in roots, stems, and cotyledons of N. benthamiana seedlings (Fig. 4). The green fluorescence was detected in all the seedlings of the infected group, and the infection efficiency was perfect. But there was no green fluorescence in the negative groups. These results indicated that pCAMBIA1304 vector could express exogenous genes in N. benthamiana by FAST method. Under the same infection conditions, most of the infected P. vulgaris seedlings did not show green fluorescence. Fluorescence signals were acquired only in the damaged parts of seedlings, such as root injured (Fig. 4c) and root tip injured (Fig. 4d). Therefore, we regarded that mechanical damage can improve transformation efficiency in the transient transformation of P. vulgaris seedlings.
FAST method in N. benthamiana and P. vulgaris seedlings with pCAMBIA1304. GFP was observed under a fluorescent microscope. a Root and stem of N. benthamiana seedlings. b Cotyledon of N. benthamiana seedlings. c The middle of P. vulgaris seedlings root. d The top of P. vulgaris seedlings root. e Overall comparison of N. benthamiana and P. vulgaris seedlings
Our research elucidated that the transformation of N. benthamiana seedlings and some damaged P. vulgaris seedlings could be successfully achieved by using Agrobacterium tumefaciens EHA105 and pCAMBIA1304 combined with FAST infection. The infection efficiency of N. benthamiana seedlings was higher than that of P. vulgaris seedlings. As shown in Fig. 4e, the individual comparison between week-old P. vulgaris seedlings and N. benthamiana seedlings showed that the discrepancy of infection efficiency might be closely related to the size of the two individuals. It was apparently reflected that there were significant divergences in the instantaneous infection efficiency of diversity plant materials (including different plant types and physiological states) when using the same strains and various carriers in the Agrobacterium-mediated transient transformation system. Furthermore, mechanical damage can improve the efficiency of infection, and similar verdict has been drawn by McIntosh’s experiment (McIntosh et al. 2004).
Transient transformation of P. vulgaris seedlings by FAST and mechanical damage
As described in the results from the “Transient transformation with pCAMBIA1304 vector” section, green fluorescent protein was expressed in damaged P. vulgaris seedlings. Therefore, we carried out the infection experiment of P. vulgaris seedlings by FAST and mechanical damage method. After co-cultured with A. tumefaciens for 72 h and 0.5 × MS liquid medium for 3 days, fluorescence microscopy was used to watch the reaction results. Clear and well reproducible green fluorescent protein expression was examined in cotyledons of P. vulgaris seedlings (Fig. 5a–c). It was displayed that FAST and mechanical damage method could successfully express exogenous genes in P. vulgaris seedlings. As reflected in Fig. 5e, f, a highly efficient green fluorescent protein expression was observed after injection of A. tumefaciens EHA105 carrying pCAMBIA1304 vector into the leaves of N. benthamiana as a positive control, which verified the effectiveness of pCAMBIA1304 vector. This exhibited that we have successfully established the transient transformation system of P. vulgaris seedlings, which provided an effective means for the study of functional genes in P. vulgaris, whereas the co-culture of bacterial solution and the addition of mechanical damage in the system were both a kind of injury stress for plant seedlings. It was implied and insinuated that the system was not suitable for some studies of stress-related functional genes (Jessica et al. 2014).
FAST and mechanical damage method in P. vulgaris seedlings with pCAMBIA1304. GFP was observed under a fluorescent microscope. a Cotyledon of P. vulgaris at 488-nm excitation light (× 50 magnification). b The same position with a in bright field. c Merge of a and b. d The cotyledon cells in a (expanded up to 8 times). e N. benthamiana leaf at 488-nm excitation light (× 50 magnification). f N. benthamiana leaf epidermal cells in e (expanded up to 8 times)
Conclusions
Genetic transformation has always been an important means to study the regulation of secondary metabolites in medicinal plants. Moreover, stable transformation has the advantage of good reproducibility. Nonetheless, obtaining stable hairy root system or transgenic plants was often time-consuming and laborious, which could not meet the needs of a large number of studies on secondary metabolic regulation–related functional genes of medicinal plants. Therefore, recently, Agrobacterium-mediated transient transformation system has been widely used in the study of plant functional genes owing to its easy operation, low cost, and short cycles.
The research suggested that the Agrobacterium-mediated instantaneous transformation system had distinct infection efficiency in different plant seedling materials. In this experiment, S. miltiorrhiza as a material was not successful in the transient transformation of pEAQ vector mediated by A. tumefaciens and the transient transformation of seedlings. This might be associated to the defense mechanism of S. miltiorrhiza against the transient expression of exogenous genes. The data of instantaneous transformation between N. benthamiana and P. vulgaris seedlings also implied that the discrepancy of seedlings’ instantaneous infection efficiency might be linked to the diversity of individual size and tissue density of seedlings. GFP and GUS exogenous genes were validly expressed in N. benthamiana leaves by pEAQ vector. This provided a powerful approach for rapid and efficient expression of exogenous genes in N. benthamiana and further probe of their functions. The highly efficient co-expression of multiple exogenous genes also offered the probability of heterologous construction of secondary metabolic pathways in N. benthamiana. At the same time, the instantaneous transformation system of P. vulgaris seedlings was preliminarily established by FAST and mechanical damage method, which exhibited a significant means for the study of functional genes in P. vulgaris. However, the co-culture of material and bacterial solution and the addition of mechanical damage in the system were all stress treatments for plant seedlings. These factors restricted the utilization of this system in the study of stress-related functional genes.
Abbreviations
- FAST:
-
Fast Agrobacterium-mediated seedling transformation
References
Clough SJ, Bent AF (2010) Floral dip: a simplified method for Agrobacterium -mediated transformation of arabidopsis thaliana. Plant J 16:735–743
Ekengren SK, Liu Y, Schiff M, Dinesh-Kumar SP, Martin GB (2003) Two mapk cascades, npr1, and tga transcription factors play a role in pto-mediated disease resistance in tomato. Plant J 36:905–917
Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB (2005) Virus-induced gene silencing in tomato fruit. Plant J 43:299–308
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. Embo J 6:3901–3907
Jessica W, Sheba G, Rizvi N, Cram EJ, Lee P, Carolyn WT (2014) Optimizing the transient Fast Agro-mediated seedling transformation (FAST) method in Catharanthus roseus seedlings. Plant Cell Rep 33:89–97
Jia Y, Ma Y, Guo Y, Li M (2007) Research on the transient express of gus gene by Agrobacterium tumefaciens in pinellia ternata breit. Acta Agric Boreali Sin:42–45
Kusnadi A R, Nikolov Z L, 1997. Howard J A, Production of recombinant proteins in transgenic plants: practical considerations. Biotechnol Bioeng 56, 473–484
Li JT 2008 Histochemical localization and biological operation of secondary metabolites of Salvia miltiorrhiza. Master's Degree Thesis
Li Y, Zhang YH (2010) Study on Agrobacterium tumefaciens-mediated transient transformation of tobacco by infiltration. Exp Technol Manag 27:50–52
Li JF, Park E, von Arnim AG, Nebenführ A (2009) The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods 5:6
Liang ZS, Yang DF, Liang X, Zhang YJ, Liu Y, Liu FH (2012) Roles of reactive oxygen species in methyl jasmonate and nitric oxide-induced tanshinone production in Salvia miltiorrhiza hairy roots. Plant Cell Rep 31:873–883
Lico C, Chen Q, Santi L (2008) Viral vectors for production of recombinant proteins in plants. J Cell Physiol 216:366–377
Liu Y, Schiff MP, Dinesh-Kumar S (2002) Virus-induced gene silencing in tomato. Plant J Cell Mol Biol 31:777–786
Lu R, Malcuit I, Moffett P, Ruiz MT, Peart J, Wu AJ, Rathjen JP, Bendahmane A, Day L, Baulcombe DC (2003) High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J 22:5690–5699
Ma YH, Wang YX, Liu X, Yang LH, Yu LT (2019) Research progress in pharmacological activities of Salvia miltiorrhiza. J Jilin Med Univ 40:440–442
McIntosh KB, Hulm JL, Young LW (2004) A rapid Agrobacterium-mediated Arabidopsis thaliana transient assay system. Plant Mol Biol Report 22:53–61
Ratcliff F, Martin-Hernandez AM, Baulcombe DC (2001) Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J 25:237–245
Sainsbury F, Thuenemann EC, Lomonossoff GP (2009) Peaq: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol J 7:682–693
Schweizer PJ, Abderhalden O, Dudler R (1999) A transient assay system for the functional assessment of defense-related genes in wheat. Mol Plant-Microbe Interact 12:647–654
Sessa G, Borello U, Morelli G, Ruberti I (1998) A transient assay for rapid functional analysis of transcription factors in arabidopsis. Plant Mol Biol Report 16:191
Sheen J (2001) Signal transduction in maize and arabidopsis mesophyll protoplasts. Plant Physiol 127:1466–1475
Subramanyam K, Rajesh M, Jaganath B, Vasuki A, Theboral J, Elayaraja D, Karthik S, Manickavasagam M, Ganapathi A (2013) Assessment of factors influencing the Agrobacterium-mediated in planta seed transformation of brinjal (Solanum melongena L.). Appl Biochem Biotechnol 171:450–468
Sun QY, Ding LW, Lomonossoff GP, Sun YB, Luo M, Li CQ, Jiang L, Xu ZF (2011) Improved expression and purification of recombinant human serum albumin from transgenic tobacco suspension culture. J Biotechnol 155:164–172
Tian YQ, Ding P, Zhang YQ (2015) Overview on researches of medicinal use of tobacco. China Pharm 24:126–128
Udawat P, Jha RK, Sinha D, Mishra A, Jha B (2016) Overexpression of a cytosolic abiotic stress responsive universal stress protein (SbUSP) mitigates salt and osmotic stress in transgenic tobacco plants. Front Plant Sci 7:518
Vardakou M, Sainsbury F, Rigby N, Mulholland F, Lomonossoff GP (2012) Expression of active recombinant human gastric lipase in Nicotiana benthamiana using the cpmv-ht transient expression system. Protein Expr Purif 81:69–74
Yang ZN, Ingelbrecht IL, Louzada E, Skaria M, Mirkov TE (2000) Agrobacterium-mediated transformation of the commercially important grapefruit cultivar rio red (citrus paradisi macf.). Plant Cell Rep 19:1203–1211
Zhang JH, Qiu JN, Wang L, Zhang S, Cheng FF, Liu B, Jiang YY (2018) Research progress on chemical constituents and pharmacological effects of Prunella vulgaris. Chin Tradit Herbal Drugs 49:3432–3440
Zhao WT, Wei JH, Liu XD, Gao ZH (2013) Main methods and application progress of plant instantaneous expression technology. Biotechnol Commun:294–300
Zheng L, Liu G, Meng X, Li Y, Wang Y (2012) A versatile Agrobacterium-mediated transient gene expression system for herbaceous plants and trees. Biochem Genet 50:761–769
Funding
This work was supported by National Natural Science Foundation of China (81703641, 31871694), Zhejiang Province Public Welfare Technology Application Research Project (CN) (LGN19H280004), China Postdoctoral Science Foundation (2019 M652146) and Fundamental Research Funds of Zhejiang Sci-Tech University (2019Q046).
Author information
Authors and Affiliations
Contributions
P.X. and Z.L. conceived and designed the research. W.H., T.L., and P.X. analyzed the results. D.Y. collected the plant samples. T.L. and P.X. preformed the experiments. W.H. and P.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary figure 1
The pattern of pEAQ-GFP-HT (PNG 161 kb)
Rights and permissions
About this article
Cite this article
Xia, P., Hu, W., Liang, T. et al. An attempt to establish an Agrobacterium-mediated transient expression system in medicinal plants. Protoplasma 257, 1497–1505 (2020). https://doi.org/10.1007/s00709-020-01524-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01524-x