Abstract
Cannabaceae is a known family because of the production of cannabinoids in laticifers and glandular trichomes of Cannabis sativa. Laticifers are latex-secreting structures, which in Cannabaceae were identified only in C. sativa and Humulus lupulus. This study aimed to expand the knowledge of laticifers in Cannabaceae by checking their structural type and distribution, and the main classes of substances in the latex of Celtis pubescens, Pteroceltis tatarinowii, and Trema micrantha. Such information is also updated for C. sativa. Samples of shoot apices, stems, leaves, and flowers were processed for anatomical, histochemical, ultrastructural, and cytochemical analyses. Laticifers are articulated unbranched in all species instead of non-articulated as previously described for the family. They occur in all sampled organs. They are thick-walled, multinucleate, with a large vacuole and a peripheral cytoplasm. The cytoplasm is rich in mitochondria, endoplasmic reticulum, dictyosomes, ribosomes, and plastids containing starch grains and oil drops. Pectinase and cellulase activities were detected in the laticifer wall and vacuole, confirming its articulated origin, described by first time in the family. These enzymes promote the complete dissolution of the laticifer terminal walls. The latex contains proteins, lipids, and polysaccharides in addition to phenolics (C. sativa) and terpenes (C. pubescens, T. micrantha). The presence of laticifers with similar distribution and morphology supports the recent insertion of Celtis, Pteroceltis, and Trema in Cannabaceae. The articulated type of laticifer found in Cannabaceae, Moraceae, and Urticaceae indicates that the separation of these families by having distinct laticifer types should be reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The secretory structures of Cannabaceae, especially of Cannabis sativa L., have aroused the interest of researchers since a long time ago because they are responsible for the production of a large amount of secondary metabolites of medicinal importance (Furr and Mahlberg 1981; Kim and Mahlberg 1991, 1997; Williamson and Evans 2000; Happyana et al. 2013). The latex of C. sativa, for example, is rich in cannabinoids and alkaloids (Furr and Mahlberg 1981), substances that have the medicinal potential to relieve symptoms related to the treatment of cancer, AIDS, and sclerosis (Ashton 2001; Honório et al. 2006; Hill et al. 2010). The latex is produced in laticifers, internal secretory structures that form organized systems (Fahn 1990), composed of one specialized cell (non-articulated type) or several cells forming a tube (articulated type) that produce an emulsion of many small particles dispersed in a liquid with a different refractive index (Fahn 1979).
Surprisingly, laticifers have been reported only in two species of Cannabaceae, Cannabis sativa (Furr and Mahlberg 1981; Mesquita and Dias 1984) and Humulus lupulus L. (Hagel et al. 2008), a small number of species if we consider that this family comprises ca. 109 species and 10 genera (Yang et al. 2013). The laticifers of Cannabis sativa (Furr and Mahlberg 1981; Mesquita and Dias 1984) and Humulus lupulus (Hagel et al. 2008) are currently classified as non-articulated and unbranched.
Cannabaceae belongs to the Urticalean Rosid clade that also comprises Moraceae, Ulmaceae, and Urticaceae (Sytsma et al. 2002). Differences in the distribution and morphology of laticifers have always been considered as important features for the diagnosis of the families of the Urticalean Rosid clade (Judd et al. 2009). For example, in Moraceae, the laticifers were described as non-articulated branched and distributed throughout the plant; in Urticaceae, the laticifers were described as non-articulated unbranched and present only in the bark; in Cannabaceae, the laticifers were also described as non-articulated unbranched and distributed throughout the plant vegetative body, and finally, Ulmaceae representatives do not present laticifers (Metcalfe 1966; Evert 2006; Fahn 1979). However, recent ontogenetic studies of laticifers have shown that their distribution and morphology in species of Moraceae and Urticaceae are similar, i.e., that species of these families present articulated branched (and anastomosed) laticifers with wide distribution in the plant (Marinho and Teixeira 2019a). The lack of studies on laticifer origin can be the cause of misinterpretations of the laticifer types (Demarco et al. 2006; Demarco and Castro 2008; Lopes et al. 2009; Canaveze and Machado 2016, Marinho and Teixeira 2019a), which means that other species of the Urticalean Rosid clade, including species of Cannabaceae, could also show articulated laticifers. Therefore, ontogenic studies are essential to a better laticifer classification in this clade. Even the latex exhibits different colors depending on the family, such as yellow-brown or colorless latex in Cannabaceae (Mahlberg 1993; Evert 2006), milky latex in Moraceae, and colorless latex in Urticaceae (Evert 2006). Thus, there appears to be a diversity of types of laticifers and of latex in the Urticalean rosids that can be confirmed by further studies.
Therefore, the objectives of the present study were to check the origin, detailed morphology, and distribution of laticifers, and the main classes of compounds of the latex in species of different genera of Cannabaceae (Celtis pubescens (Kunth) Spreng., Pteroceltis tatarinowii Maxim., and Trema micrantha (L.) Blume). Such data are also updated for Cannabis sativa. The cytochemical localization of cellulase and pectinase activities was also tested to better understand the formation of laticifers. We intend to contribute new data to help reviewing the synapomorphies established for the Urticalean Rosid clade.
Materials and methods
Samples of shoot and floral apices, stems, leaves, and flowers were obtained in the field or from herbarium specimens (Table 1). Vouchers were deposited in the SPFR herbarium (FFCLRP/USP) and in the CGMS herbarium (INBIO/UFMS).
The samples collected in the field were fixed in buffered formalin (Lillie 1965) or in formalin-acetic acid-ethanol (70% FAA) for 48 h (Johansen 1940). The herbarium samples were rehydrated in heated distilled water and then treated overnight with 2% KOH (Smith and Smith 1942). Both types of samples were dehydrated through an ethanolic series, embedded in histological resin (Historesin, Leica), and sectioned in longitudinal planes (5 μm), using a rotary microtome (Leica RM 2245). The sections were stained with 0.1% Toluidine Blue in phosphate buffer, pH 6.8 (O’Brien et al. 1964), mounted in immersion oil, and analyzed under a light microscope.
Stem samples were also free-hand sectioned (in fresh material for Trema micrantha and Celtis pubescens and fixed material for Cannabis sativa and Pteroceltis tatarinowii), and the main compounds of the latex were investigated using the following reagents: Sudan IV for total lipids (Pearse 1985), Lugol for starch (Johansen 1940), ferric chloride for phenolic compounds (Johansen 1940), and Wagner’s reagent for alkaloids (Furr and Mahlberg 1981). Material embedded in historesin was stained with Toluidine Blue O for detection of phenolic compounds (O’Brien et al. 1964), period acid-Schiff (PAS) for neutral polysaccharides (Jensen 1962), and xylidine Ponceau for proteins (Vidal 1970). The presence of terpenes and tannins was tested in fresh samples of C. pubescens and T. micrantha free hand cut. The Nadi reagent was employed for terpenes (David and Carde 1964), and vanillin hydrochloric acid was employed for tannins (Mace and Howell 1974). Photomicrographs were obtained using a Leica DFC 295 digital camera coupled to a Leica DM 5000 B light microscope.
For the ultrastructural analysis, small pieces of the shoot apex of Celtis pubescens and Trema micrantha were fixed in Karnovsky’s solution (Karnovsky 1965) for 24 h, post-fixed in 1% osmium tetroxide in 0.1 M phosphate buffer, pH 7.2, washed in distilled water, dehydrated, and embedded in Araldite. Sections cut with a Leica Reichert Ultracut S ultramicrotome at 60–70 nm were collected on copper grids and contrasted with 2% uranyl acetate and lead citrate for 15 min. Transmission electron micrographs were obtained using a Jeol 100CXII instrument.
Cytochemical localization of cellulase and pectinase activities at the ultrastructural level was performed for Celtis pubescens and Trema micrantha. The shoot apices were collected, fixed in Karnovsky’s solution (Karnovsky 1965) for 24 h, washed 10 times in 0.1 M phosphate buffer, pH 7.2, and stored overnight in the buffer at 4 °C. For testing the cellulase activity, the samples were incubated in 0.05 M citrate buffer, pH 4.8, with 0.02% carboxymethylcellulose for 10 min at room temperature (Bal 1974). For pectinase activity, the samples were incubated in 0.1 M sodium acetate buffer, pH 5.0, with 0.5% pectin for 20 min at room temperature (Allen and Nessler 1984). Control samples were incubated in buffer respective of each test but without carboxymethylcellulose and pectin. Both treated and control samples were transferred to Benedict’s reagent heated to 80 °C for 10 min, washed in 0.1 M phosphate buffer, and post-fixed in 1% osmium tetroxide for 2 h. Then, the samples continued to be processed using the usual method for ultrastructural analysis.
Results
Laticifer distribution, origin, and morphology
Most of the species studied exhibit laticifers in all analised parts of the plant (Table 2, Fig. 1), such as leaf blade, petiole, stem, pedicel, sepal, filament, ovary, style, and stigma (Fig. 1a–h, Table 2). The exceptions are the sepals and filaments of Cannabis sativa flowers (Fig. 1a, b, Table 2).
Schematic drawings of longitudinal sections of flowers of Cannabaceae species showing the wide distribution of laticifers (hatched). a, bCannabis sativa: staminate (a) and pistillate flowers (b). c, dCeltis pubescens: staminate (c) and pistillate flowers (d). e, fPteroceltis tatarinowii: staminate (e) and pistillate flowers (f). g, hTrema micrantha: staminate (g) and pistillate flowers (h). Scale bars: a 1 mm; b 200 μm; c, d 500 μm; e, f 500 μm; g, h 500 μm
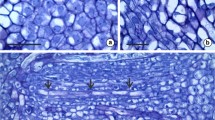
Laticifers are originated from ground meristem cells and are located externally to the phloem (Fig. 2). They are organized into a set of three or more laticifers (Fig. 2). The laticifer is articulated, formed by the addition of cells in the apex (Fig. 3a, b) and the disintegration of the terminal cell walls (Figs. 3c, d and 4a, b). Then, it elongates with the growth of the plant between the phloem and cortical parenchyma cells (Fig. 3b), or among other laticifers. The laticifers have thickened pecto-cellulosic cell walls (Figs. 2 and 3b), and usually possess nuclei of fusiform shape (Fig. 2).
Articulated laticifers of Cannabaceae species (longitudinal sections of the stem stained with Toluidine Blue). a Laticifers of Cannabis sativa (arrow) located between the cortical parenchyma and phloem. b Detail showing two laticifers; the arrow shows a nucleus. c Laticifers of Celtis pubescens (arrow) arranged into a set and located between the cortical parenchyma and phloem. d Detail of the laticifers showing a thick wall (arrow). e Multinucleate laticifers (arrow) of Pteroceltis tatarinowii located between the cortical parenchyma and phloem. f Detail showing laticifers with a nucleus and its nucleoli (arrow). g Laticifers of Trema micrantha (arrow) located between the cortical parenchyma and the phloem. h Detail showing laticifers with a thick wall (arrow). cp cortical parenchyma, ph phloem. Scale bars: a, c, e, g 50 μm, b, d, f, h 20 μm
Origin of the laticifers of Cannabaceae species (longitudinal sections; a, b stained with Toluidine Blue; c, d transmission electron microscope). a Vegetative meristem of Cannabis sativa. Note a series of laticifers cells with the terminal cell walls still present (arrows).b Laticifers of Celtis pubescens that elongate with the growth of the plant. c Laticifers of Celtis pubescens showing the thin terminal cell wall (arrow). d Disintegration process of the cell wall to form the articulated laticifer (arrows) in Celtis pubescens. Scale bars: a, b 20 μm, c 2 μm, d 1 μm
Ultrastructure of articulated laticifers of Celtis pubescens (TEM). a Three laticifers. Two mature laticifer (L1, L3) with peripheral cytoplasm showing a large vacuole and other small vacuoles near the wall, and part of the nucleus in the first. Developing laticifer; note two cells with a thin terminal wall (arrow) in degradation process (L2). b Detail of a disintegrating terminal wall (black arrow); note that the cytoplasm is rich in mitochondria, rough endoplasmic reticulum, and dictyosomes (close to the cell wall). c Cytoplasm with mitochondria, small vacuoles, rough endoplasmic reticulum, and osmiophilic bodies (*); note the thick wall and vesicles being added to it (arrow). d dictyosome, m mitochondria, n nucleus, rer rough endoplasmic reticulum, v vacuole, w wall. Scale bars: a, c 2 μm, b 1 μm
Ultrastructure
Subcellular characteristics of mature (Figs. 4a and 6a) and of developing laticifers (Figs. 4b–d, 5, 6c, d, and 7) present in the stems of Celtis pubescens (Figs. 4 and 5) and Trema micrantha (Figs. 6 and 7) were similar for these two species.
Ultrastructure of articulated laticifers of Celtis pubescens (TEM). a Plastid with starch grains and evident stacked thylakoids, and dictyosomes near the wall. b Plastid with stacked thylakoids containing lipid bodies and starch in degradation evidenced by electron-dense material (arrows). c Detail of plastids partially digested inside the vacuole. d Three laticifers arranged in parallel (L1, L2, L3) showing their thin walls; note the peripheral cytoplasm with osmiophilic bodies (*), a plastid, and the nucleus with two nucleoli. d dictyosomes, em electron-dense material, lb lipid bodies, pl plastid, n nucleus, nc nucleoli, s starch grains, w wall. Scale bars: a, c 1 μm, b 2 μm, d 3 μm
Ultrastructure of articulated laticifers of Trema micrantha (TEM). a Peripheral cytoplasm of a mature laticifer (arrows) showing a large vacuole containing latex substances. b Peripheral cytoplasm rich in mitochondria, rough endoplasmic reticulum, vacuoles formed by the endoplasmic reticulum, and osmiophilic bodies (*). c, d Laticifer in differentiation with active organelles. d Cytoplasm with mitochondria, dictyosomes, ribosomes, and lipophilic bodies; note the laticifer thick walls (arrow). d dictyosomes, lb lipophilic bodies, m mitochondria, rb ribosomes, rer rough endoplasmic reticulum, v vacuole. Scale bars: a 3 μm, b–d 1 μm
Ultrastructure of articulated laticifers of Trema micrantha (TEM). a Cytoplasm with mitochondria, rough endoplasmic reticulum, ribosomes, and lipophilic bodies; note the laticifer thick walls. b Cytoplasm with a mitochondria, dictyosomes releasing vesicles in the trans-Golgi face (arrow), ribosomes, and an osmiophilic body (*). c Plastid with starch grains (s). d Small vacuoles with latex substances. d dictyosomes, lb lipophilic bodies, m mitochondria, rer rough endoplasmic reticulum, rb ribosomes, v vacuole. Scale bars: a–c 1 μm, d 3 μm
The laticifer walls are thicker when in contact with adjacent parenchymatic cells and thinner when in contact with another laticifer wall (Fig. 4a), mainly in the terminal wall which are degraded during the laticifer formation (Fig. 4b, black arrows).
The mature laticifer has a large central vacuole, a peripheral cytoplasm (Figs. 4a and 6a), and small vacuoles close to the large vacuole (Fig. 4a). Developing laticifers show cytoplasm with dictyosomes (Figs. 4b, 5a, 6d, and 7b), plastids (Fig. 5a, b), amyloplasts (Fig. 7c), and numerous mitochondria with conspicuous cristae (Figs. 4b, c, 6b–d, and 7a, b), free ribosomes and polyribosomes (Figs. 4b, 6d, and 7a, b) as well as rough endoplasmic reticulum (Figs. 4b, c and 7a). The dictyosomes are formed of few cisterns and are usually located close to cell wall (Figs. 4b, 5a, and 6d). They are active and produce vesicles from the trans face of the trans-Golgi network that are released into the small vacuole (arrow, Fig. 7b). Some dictyosomes were found close to the endoplasmic reticulum. Plastids contain some thylakoids, oil droplets (Fig. 5b), and starch grains (Figs. 5a, b and 7c), which are partially consumed (electron-dense material, Fig. 5b). Plastids can also disintegrate and are found inside the small vacuole (Fig. 5c) with oil droplets, electron-dense material, and starch (asterisk, Figs. 5c, 6b, and 7b). The small vacuoles formed contain latex substances (Figs. 4a, c, 5c, 6a, b, and 7b, d) and join to the large central vacuole in the end of the laticifer development (Fig. 6a). Osmiophilic material is also present in the cytoplasm (asterisk, Figs. 4c, 5d, and 7b).
The nucleus has one or two nucleoli (Fig. 5d).
Cytochemical localization of cellulases and pectinases
Positive reactions for cellulase (Fig. 8) and pectinase (Fig. 9) activities were found in the cell wall close to the middle lamella (Figs. 8a–c and 9a, c, d), vacuole (Figs. 8a, b and 9b), and endoplasmatic reticulum (Fig. 8a, b) by electron-dense crystalline inclusions. These electron-dense inclusions are reducing sugars, products of pectinase, and cellulase activities in the laticifers that react with Benedict’s reagent. The reaction products appear widespread (Figs. 8a, b and 9a, b), or densely accumulated, forming groups in the vacuole (Figs. 8a, b and 9b) or in the cell wall (Figs. 8c and 9c, d). In the adjacent cells to the laticifers, positive reaction was also observed but less intense. In the control samples that were boiled without pectin or carboxymethylcellulose, the activity of cellulase was positive but less intense than in the treated samples (Fig. 8d). However, the positive reaction of the pectinase activity was similar between the control and the treated samples for the enzyme activity in the cell wall (Fig. 9d).
Cytochemical localization of cellulase activity in the laticifers of Celtis pubescens (a, b, d) and Trema micrantha (c). a–c Laticifers incubated with carboxymethylcellulose. a, b Note the electron-dense reaction products in the middle lamella, vacuole, and endoplasmic reticulum (positive reaction—arrows). c Reaction product of the cellulase in the laticifer cell wall (positive reaction—arrow). d Laticifer of control specimen incubated without carboxymethylcellulose. Note the absence of reaction product. There are only small electron-dense products in the middle lamella (arrow). L laticifer, ml middle lamella, w cell wall. Scale bars: a–d 1 μm
Cytochemical localization of pectinase activity in the laticifers of Celtis pubescens (a, b) and Trema micrantha (c, d). a–c Laticifers incubated with pectin. a Electron-dense reaction products in the middle lamella (positive reaction—arrow). b Product reaction in the vacuole (positive reaction—arrow). c Reaction product of pectinase in the middle lamella (positive reaction—arrow). d Laticifer of control specimen incubated without pectin. Note that there is reaction product in the cell wall (positive reaction—arrow). L laticifer, ml middle lamella, er endoplasmic reticulum, w cell wall. Scale bars: a 2 μm, b–d 1 μm
Latex composition
The natural color of the latex is colorless in most species (Fig. 10a) except Cannabis sativa in which it is yellowish (Fig. 10b).
Histochemical analyses of the latex of Cannabaceae species (longitudinal sections). a Laticifers of Celtis pubescens without staining; note the colorless latex. b Laticifers of Cannabis sativa without staining; note the yellowish latex. c Positive reaction of the latex of Pteroceltis tatarinowii for proteins (stain: xylidine Ponceau). d Positive reaction of the latex of Celtis pubescens for proteins (stain: xylidine Ponceau). e Positive reaction of the latex of Trema micrantha for neutral polysaccharides (stain: PAS). f Positive reaction of the latex of Cannabis sativa for neutral polysaccharides (stain: PAS). g Positive reaction of the latex of Trema micrantha for total lipids (stain: Sudan IV). h Positive reaction of the latex of Cannabis sativa for total lipids (stain: Sudan IV). Scale bars 20 μm
The latex of the four species is similar in chemical composition, except for the presence of phenolic compounds, terpenes, and large starch grains (Table 3). It reacted positively for proteins with xylidine Ponceau (Fig. 10c, d), for neutral polysaccharides with PAS (Fig. 10e, f), and for total lipids with Sudan IV (Fig. 10g, h). Large starch grains were detected with Lugol (Fig. 10a, b) in the latex of C. pubescens, P. tatarinowii, and Trema micrantha, but not in C. sativa. Terpenes were detected with Nadi reagent in the latex of fresh samples of C. pubescens and T. micrantha (Fig. 11c, d). No tannins were found for these species with vanillin hydrochloric acid (Table 3). Phenolic compounds were only detected in the latex of C. sativa using ferric chloride (Fig. 11e) and Toluidine Blue (Fig. 11f). No alkaloids were detected in all studied species by using Wagner’s reagent (Table 3).
Histochemical analyses of the latex of Cannabaceae species (longitudinal sections). a Positive reaction of the latex of Trema micrantha for starch (stain: lugol). b Positive reaction of the latex of Celtis pubescens for starch (stain: lugol). c Positive reaction of the latex of Trema micrantha for terpenes (stain: Nadi reagent). d Positive reaction of the latex of Celtis pubescens for terpenes (stain: Nadi reagent). e, f Positive reactions of the latex of Cannabis sativa for phenolic compounds with ferric chloride (e) and Toluidine Blue (f). Scale bars 20 μm
Discussion
The present report of laticifers for Celtis, Pteroceltis, and Trema is a great novelty for the family because in a previous study of Sytsma et al. (2002), laticifers were considered to be absent in these genera that were recently inserted into Cannabaceae. Therefore, our results corroborate the insertion of these genera into Cannabaceae together with Cannabis and Humulus in which the presence of laticifers throughout the plant has been previously described (Meeuse 1942; Furr and Mahlberg 1981; Mesquita and Dias 1984; Hagel et al. 2008).
The laticifer structure of the Cannabaceae species studied so far is very similar. Our data of laticifer origin showed that they are articulated, contradicting previous studies that described non-articulated laticifers for Cannabis sativa and Humulus lupulus (Metcalfe 1966; Fahn 1979; Furr and Mahlberg 1981; Hagel et al. 2008). Likely, the lack of studies on the laticifer ontogeny has led to a misinterpretation of the laticifer types. Therefore, the classification of the laticifer types in the Urticalean rosid clade needs more attention, since in Moraceae and Urticaceae, there are also descriptions of both types of laticifers (non-articulated—Van Veenendaal and Den Outer 1990; Machado and Santos 2004; Quintanar et al. 2004; Jacomassi et al. 2007, 2010; Kitajima et al. 2012; and articulated—Milanez 1954; Topper and Koek-Noorman 1980; Marinho and Teixeira 2019a).
The laticifer distribution along the plant body is also similar because they are widely distributed in the vegetative and floral organs; the exception were the sepals of the pistillate apetalous flower of Cannabis sativa, which are structurally reduced with only three layers of cells. A counter-proposal is that laticifers were not observed because they can be extremely narrow and inconspicuous in such reduced organs.
The main compounds of the latex of Cannabaceae species are polysaccharides, proteins, and lipids (Furr and Mahlberg 1981; present study). Compounds such as starch grains (Pteroceltis tatarinowii, Celtis pubescens, and Trema micrantha) and terpenes (C. pubescens and T. micrantha) are reported here for the first time for the family. Alkaloids, previously found in Cannabis sativa by other researches (Furr and Mahlberg 1981), were not found in this study, even when using fresh samples of C. pubescens and T. micrantha. Terpenes and phenols were found in the cytoplasm of glandular trichomes (Mahlberg and Kim 2004) and in the latex (Furr and Mahlberg 1981, present study) of C. sativa. It is likely that such terpenes and phenols constitute the cannabinoids that are defined as a group of terpenophenolic compounds and are exclusively found in C. sativa (Mechoulam and Gaoni 1967; Croteau et al. 2000; Andre et al. 2016). Recent studies showed two biosynthetic pathways that form the precursors of the cannabinoids: one is the plastid pathway (produce—methylerythritol 4-phosphate (MEP)), and the other is the polyketide pathway (produce—olivetolic acid (OLA)) (Andre et al. 2016; Sirikantaramas and Taura 2017). It is noteworthy that the polyketide pathway is still uncertain in terms of location; preliminary analyses indicate that it occurs in the cytoplasm (Gagne et al. 2012). The presence of lipid bodies in the plastids of Trema micrantha and Celtis pubescens indicates the occurrence of the plastid pathway for the synthesis of terpenes in both species. Phenolic compounds may actually be absent or occur in an amount that it is not detectable by histochemical techniques and TEM analyses. Thus, it is difficult to identify a potential cannabinoid production in the latex of Celtis pubescens and Trema micrantha.
Cannabis sativa (Mesquita and Dias 1984), Celtis pubescens, and Trema micrantha (present study) show similar laticifer ultrastructure, while in Moraceae species, it is different (see Heinrich 1970; Rachmilevitz and Fahn 1982; Marinho and Teixeira 2019b). The Cannabaceae species differ only in the amount of osmiophilic material that is larger in C. sativa (Mesquita and Dias 1984) and lower in C. pubescens and T. micrantha (present study), because of their different latex composition. Therefore, not only the distribution of the laticifers is a conserved character in the family but also the subcellular morphology of the laticifers.
The abundant mitochondria with conspicuous cristae detected in the analyzed species are related to the energy supply for the synthesis of compounds in the secretory structures (Wilson and Mahlberg 1978; Evert 2006). Dictyosomes act on the secretion of polysaccharides (Fahn 1979, 1990; Dickison 2000; Evert 2006), and the plastids are involved in the production of terpenes and starch grains (Heinrich 1970; Wilson and Mahlberg 1978; Evert 2006). Beyond producing proteins and ribosomes (Evert 2006), the endoplasmic reticulum also participates in the formation of small vacuoles in the laticifers (Mesquita 1969; Nessler and Mahlberg 1977; Wilson and Mahlberg 1978; Mesquita and Dias 1984; Cai et al. 2009; present study).
The autophagy, the formation of a large vacuole from small vacuoles with participation of the endoplasmic reticulum followed by cytoplasm lysis, is usual in laticifers (i.e., Lupinus albus L., Mesquita 1969; Papaver soniferum L., Nessler and Mahlberg 1977; and Asclepia syriaca L., Wilson and Mahlberg 1978; Ficus carica, Rachmilevitz and Fahn 1982; Cannabis sativa, Mesquita and Dias 1984; Euphorbia kansui Liou, Cai et al. 2009; Zhang et al. 2018) and evident in the laticifers of Cannabaceae and Moraceae species (Heinrich 1970; Rachmilevitz and Fahn 1982; Mesquita and Dias 1984; present study), being considered an important process in the latex production and development of non-articulated and articulated laticifers (Evert 2006; Zhang et al. 2018). The hydrolysis renders the cytoplasm more transparent and forms small particles (Cai et al. 2009). Such particles, together with other compounds produced by the organelles such as starch, oil droplets, proteins, and phenolics before cytoplasm hydrolysis, compose the latex (Cai et al. 2009; present study).
The latex composition of Cannabaceae species (Furr and Mahlberg 1981; present study) indicates that laticifers act in plant defense against herbivores. This defense includes preventing the insect from feeding on the plant and the accumulation of gums, gel, or phenols that form tyloses, suggesting an increased resistance, as observed in elm trees (Ulmaceae, Dickison 2000). The laticifer distribution on the plant body is another criterion that cannot be neglected in the inference of functions for such an interesting and complex secretory structure. In Ficus species, laticifers have been considered to act in promoting the pollination by protecting the galled flowers (flowers where the wasp offspring emerges) against attack by non-pollinating wasps (Marinho et al. 2018). Cannabaceae consist of exclusively wind-pollinated species (Miller 1970; Barth et al. 1975; Arruda and Sazima 1988; Culley et al. 2002); thus, different selective pressures should act on laticifer distribution along the flower. Differently from Ficus, the flowers of Cannabaceae are exposed favoring wind pollination but also exposed to UV radiation, insects, or other animals. Thus, protection appears to be the main function of laticifers in Cannabaceae. This can be illustrated by the finding of laticifers in the stigmatic region of the species studied, which is an important part of the flower for the reproductive success of wind-pollinated species (Culley et al. 2002; Friedman and Barrett 2009) with rare reports of laticifers.
Pectinase and cellulase activities were found in the wall and vacuole of Celtis pubescens and Trema micrantha; thus, these enzymes participate in the formation of its articulated laticifers, by the complete dissolution of cellulose and pectin in the terminal walls (Nessler and Mahlberg 1981; Allen and Nessler 1984). Pectinase and cellulase activities were reported for articulated laticifers (Sheldrake 1969; Nessler and Mahlberg 1981; Pilatzke-Wunderlich and Nessler 2001; Marinho and Teixeira 2019b), while there are only reports of pectinases for non-articulated laticifers (Wilson et al. 1976; Allen and Nessler 1984). Therefore, the presence of these enzymes is an important tool to confirm the occurrence of articulated laticifers in Cannabaceae.
The reaction product also shows pectinase and cellulase activities in the lateral region of the laticifer wall, suggesting therefore that these enzymes can also be important in the wall lateral expansion (Allen and Nessler 1984; Marinho and Teixeira 2019b, present study). Interestingly, pectinase activity is found inclusive in the control test, but only in the cell wall. Similar results were obtained for nonarticulated laticifers of Nerium oleander (Allen and Nessler 1984) in that the saturation of the pectinase by endogenous pectin of the middle lamella and the addition of exogenous pectin do not alter the density of the reaction product in this region.
The results suggest that the cellulase and pectinase enzymes are synthesized in the endoplasmic reticulum and then are released to the cell wall through exocytosis (Liang et al. 2009; Yu et al. 2004; Wang et al. 1998; Marinho and Teixeira 2019b). Likely, the reaction product presence in the vacuole indicates the occurrence of endocytosis as a result of the translocation of the products of the degraded cell wall to the vacuole (Giordani 1980; Demarco and Castro 2008).
In conclusion, we suggest that the presence of articulated anastomosing laticifers (sensu Ramos et al. 2019) can be a synapomorphy for Cannabaceae. In addition, we believe that the vast majority of the laticiferous species of the Urticalean rosid clade are likely to have articulated laticifers, and thus, the separation of these families by having distinct laticifer types should be reviewed. The wide distribution of laticifers in vegetative and floral organs is reported here for the first time for the family, as well as the occurrence of large starch grains and terpenes in the latex. The similar laticifer ultrastructure of C. sativa, C. pubescens, and T. micrantha should indicate that these species produce the same chemical classes of compounds but in different amounts. The wide distribution of laticifers in the floral organs of Cannabaceae expands our knowledge about this secretory structure and suggests that they have an important function in the protection of floral organs in this family. Therefore, we emphasize the importance of more ecological studies to better understand the role of laticifers in flowers.
References
Allen RD, Nessler CL (1984) Cytochemical localization of pectinase activity in laticifers of Nerium oleander L. Protoplasma 119:74–78
Andre CM, Hausman J-F, Guerriero G (2016) Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci 7:1–17
Arruda VLV, Sazima M (1988) Polinização e reprodução de Celtis iguanaea (Jacq.) Sarg. (Ulmaceae), uma espécie anemófila. Brazilian Journal Botany 11:113–122
Ashton CH (2001) Pharmacology and effects of Cannabis: a brief review. Br J Psychiatry 178:101–106
Bal AK (1974) Cellulase. In: Haya MA (ed) Electron microscopy of enzymes, vol 3. Van Nostrand Reinhold, New York, pp 68–79
Barth O, Macieira E, Corte-Real S (1975) Morfologia do polen anemofilo alergisante no Brasil. Memorias Instituto Oswaldo Cruz 73:141–150
Cai X, Li W, Yin L (2009) Ultrastructure and cytochemical localization of acid phosphatase of laticifers in Euphorbia kansui Liou. Protoplasma 238:3–10
Canaveze Y, Machado SR (2016) The occurrence of intrusive growth associated with articulated laticifers in Tabernaemontana catharinensis a. DC., a new record for Apocynaceae. Int J Plant Sci 177:458–467
Croteau R, Kutchan TM, Lewis NG (2000) Natural products (secondary metabolites). In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. Wiley, Hoboken, pp 1250–1318
Culley TM, Weller SG, Sakai AK (2002) The evolution of wind pollintion in angiosperms. Trends Ecol Evol 17:361–369
David R, Carde JP (1964) Coloration différentielle des inclusions lipidiques et terpeniques des pseudophylles du pin maritime au moyen du reactif Nadi. C R Acad Sci 258:1338–1340
Demarco D, Castro MM (2008) Laticíferos articulados anastomosados em espécies de Asclepiadeae (Asclepiadoideae, Apocynaceae) e suas implicações ecológicas. Rev Bras Bot 31:701–713
Demarco D, Kinoshita LS, Castro MM (2006) Laticíferos articulados anastomosados: novos registros para Apocynaceae. Rev Bras Bot 29:133–144
Dickison W (2000) Integrative plant anatomy. Academic Press, San Diego
Evert RF (2006) Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. Wiley, Hoboken
Fahn A (1979) Secretory tissues in plants. Academic Press, London
Fahn A (1990) Plant Anatomy. Butterworth-Heinemann, London
Friedman J, Barrett SCH (2009) Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann Bot 103:1515–1527
Furr M, Mahlberg PG (1981) Histochemical analyses of laticifers and glandular trichomes in Cannabis sativa. J Nat Prod 44:153–159
Gagne SJ, Stout JM, Liu E, Boubakir Z, Clark SM, Page JE (2012) Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. PNAS 31:12811–12816
Giordani R (1980) Dislocation du plasmalemme et libération de vésicules pariétales lors de la degradation des parois terminales durant la différenciation des laticifères articulés. Biol Cell 38:231–236
Hagel JM, Yeung EC, Facchini PJ (2008) Got milk? The secret life of laticifers. Trends Plant Sci:1360–1385
Happyana N, Agnolet S, Muntendam R, Van Dam A, Schneider B, Kayser O (2013) Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 87:51–59
Heinrich G (1970) Elektronenmikroskopische Untersuchung der Milchrohren von Ficus elastica. Protoplasma 70:317–323
Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS (2010) Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci 30:14980–14986
Honório KM, Arroio A, Silva ABF (2006) Aspectos terapêuticos de compostos da planta Cannabis sativa. Química Nova 29:318–325
Jacomassi E, Moscheta IS, Machado SR (2007) Morfoanatomia e histoquímica de Brosimum gaudichaudii Trécul (Moraceae). Acta Bot Bras 21:575–597
Jacomassi E, Moscheta IS, Machado SR (2010) Morfoanatomia e histoquímica de órgãos reprodutivos de Brosimum gaudichaudii (Moraceae). Braz J Bot 33:115–129
Jensen WE (1962) Botanical histochemistry: principles and practice. W. H. Freeman and Co, San Francisco
Johansen DA (1940) Plant Microtechnique. McGraw-Hill Book Co. Inc, New York
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (2009) Sistemática vegetal: um enfoque filogenético, 3ª edn. Artmed, Porto Alegre
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of hight osmolality for use in electron microscopy. J Cell Biol 27:137–138
Kim E-S, Mahlberg PG (1991) Secretory cavity development glandular trichomes of Cannabis sativa L. (Cannabaceae). Am J Bot 78:220–229
Kim E-S, Mahlberg PG (1997) Immunochemical localization of tetrahydrocannabinol of (THC) in cryofixed glandular trichomes of Cannabis (Cannabaceae). Am J Bot 84:336–342
Kitajima S, Taira T, Oda K, Yamato KT, Inukai Y, Hori Y (2012) Comparative study of gene expression and major proteins function of laticifers in lignified and unlignified organs of mulberry. Planta 235:589–601
Liang S, Wang H, Yang M, Wu H (2009) Sequential actions of pectinases and cellulases during secretory cavity formation in Citrus fruits. Trees 23:19–27
Lillie RD (1965) Histopathologic technic and practical histochemistry. McGraw-Hill Book Company, New York
Lopes KLB, Thadeo M, Azevedo AA, Soares AA, Meira RMSA (2009) Articulated laticifers in the vegetative organs of Mandevilla atroviolacea (Apocynaceae, Apocynoideae). Botany 87:202–209
Mace M, Howell C (1974) Histochemistry and identification of condensed tannin precursors in roots of cotton seedlings. Can J Bot 52:2423–2426
Machado AV, Santos M (2004) Morfo-anatomia foliar comparativa de espécies conhecidas como espinheira-santa: Maytenus ilicifolia (Celastraceae), Sorocea bonplandii (Moraceae) e Zollernia ilicifolia (Leguminosae). Insula 33:01–19
Mahlberg PG (1993) Laticifers: an historical perspective. Bot Rev 59:1–23
Mahlberg PG, Kim ES (2004) Accumulation of cannabinoids in glandular Trichomes of Cannabis (Cannabaceae). Journal Industrial Hemp 9(1):15–35
Marinho CR, Teixeira SP (2019a) Novel reports of laticifers in Moraceae and Urticaceae: revisiting synapomorphies. Plant Syst Evol 305(1):13–31
Marinho CR, Teixeira SP (2019b) Cellulases and pectinases act together on the development of articulated laticifers in Ficus montana and Maclura tinctoria (Moraceae). Protoplasma 256:1093–1107
Marinho CR, Pereira RAS, Peng Y-Q, Teixeira SP (2018) Laticifer distribution in fig inflorescence and its potential role in the fig-fig wasp mutualism. Acta Oecol 90:160–167
Mechoulam R, Gaoni Y (1967) Recent advances in the chemistry of hashish. Fortschr Chem Org Naturst 25:175–213
Meeuse ADJ (1942) A study of intercellular relationships among vegetable cells with special reference to sliding growth and to cell shape. Recueil des Travaux Botaniques Neerlandais 38:18–140
Mesquita JF (1969) Electron microscope study of the origin and development of the vacuoles in root-tip cells of Lupinus albus L. J Ultrastruct Res 26:242–250
Mesquita JF, Dias JDS (1984) Ultrastructural and cytochemical study of the laticifers of Cannabis sativa L. Bol Soc Brot 57:337–356
Metcalfe CR (1966) Distribution of latex in the plant kingdom. Econ Bot 21:115–127
Milanez FR (1954) Sobre os laticíferos foliares de Ficus retusa. Rodriguésia 28(29):159–192
Miller N (1970) The genera of Cannabaceae in the southeastern United States. Journal of the Arnold Arboretum 51:185–203
Nessler C, Mahlberg P (1977) Ontogeny and cytochemistry of alkaloidal vesicles in laticifers of Papaver somniferum L. (Papaveraceae). Am J Bot 64:541–551
Nessler CL, Mahlberg PG (1981) Cytochemical localization of cellulase activity in articulated, anastomosing laticifers of Papaver somniferum L. (Papaveraceae). Am J Bot 68:730–732
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Pearse AGE (1985) Histochemistry: theoretical and applied. C. Livingstone, Edinburgh
Pilatzke-Wunderlich I, Nessler CL (2001) Expression and activity of cell-wall-degrading enzymes in the latex of opium poppy, Papaver somniferum L. Plant Mol Biol 45:567–576
Quintanar A, Castrejón JLZ, Lopéz C, Salgado-Ugarte IH (2004) Anatomía e histoquímica de la corteza de cinco especies de Moraceae. Polibotánica:15–38
Rachmilevitz T, Fahn A (1982) Ultrastructure and development of the laticifers of Ficus carica L. Ann Bot 49:13–22
Ramos MV, Demarco D, Souza ICC, Freitas CDT (2019) Laticifers, Latex, and Their Role in Plant Defense. Trends Plant Sci 24(6): 553–567
Sheldrake AR (1969) Cellulase in latex and its possible significance in cell differentiation. Planta 89:82–84
Sirikantaramas S, Taura F (2017) Cannabinoids: biosynthesis and biotechnological applications. In: Chandra S, Lata H, ElSohly M (eds) Cannabis sativa L. Botany and Biotechnology. Springer, Cham, pp 183–206
Smith FH, Smith EC (1942) Anatomy of the inferior ovary of Darbya. Am J Bot 29(6):464–471
Sytsma KJ, Morawetz J, Pires JC, Nepokroeff M, Conti E, Zjhra M, Hall JC, Chase MW (2002) Urticalean Rosids: circumscription, Rosid ancestry, and phylogenetics based on rbcL, trnL-F, and ndhF sequences. Am J Bot 89(9):1531–1546
Topper SMC, Koek-Noorman J (1980) The occurrence of axial latex tubes in the secondary xylem of some species of Artocarpus J.R. & G. Forster (Moraceae). IAWA Journal 1:113–119
Van Veenendaal WLH, Den Outer RW (1990) Distribution and development of the non-articulated branched laticifers on Morus nigra L. (Moraceae). Acta Botanica Neerlandica 39:285–296
Vidal BC (1970) Dichroism in collagen bundles stained with xylidine-Ponceau 2R. Ann Histochim 15:289–296
Wang XY, Guo GQ, Nie XW, Zheng GC (1998) Cytochemical localization of cellulase activity in pollen mother cells of David lily during meiotic prophase I and its relation to secondary formation of plasmodesmata. Protoplasma 204:128–138
Williamson EM, Evans FJ (2000) Cannabinoids in clinical practice. Drugs 60:1303–1314
Wilson K, Mahlberg P (1978) Ultrastructure of non-articulated laticifers in mature embryos and seedlings of Asclepias syriaca L. (Asclepiadaceae). Am J Bot 65:98–109
Wilson KJ, Nessler CL, Mahlberg PG (1976) Pectinase in Asclepias latex and its possible role in Laticifer growth and development. Am J Bot 63(8):1140–1144
Yang M-Q, Van Velzen R, Bakker FT, Sattarian A, Li D, Yi T (2013) Molecular phylogenetics and character evolution of Cannabaceae. Taxon 62:473–485
Yu CH, Guo GQ, Nie XW, Zheng GC (2004) Cytochemical localization of pectinase activity in pollen mother cells of tobacco during meiotic prophase I and its relation to the formation of secondary plasmodesmata and cytoplasmic channels. Acta Bot Sin 46:1443–1453
Zhang Q, Wang D, Zhang H, Wang M, Li P, Fang X, Cai X (2018) Detection of autophagy processes during the development of nonarticulated laticifers in Euphorbia kansui Liou. Planta 247:845–861
Acknowledgments
The authors thank Edimárcio da Silva Campos (FCFRP/USP), Maria Dolores Seabra Ferreira, and José Augusto Maulin (FMRP/USP) for technical assistance and Elettra Greene for English review.
Funding
This work was supported by Sao Paulo Research Foundation (FAPESP) (process number 2014/07453-3, 2018/03691-8), National Council for Scientific and Technological Development (CNPq) (process number 303493/2015-1; 156,025/2017-5), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (code number 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Alexander Schulz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leme, F.M., Borella, P.H., Marinho, C.R. et al. Expanding the laticifer knowledge in Cannabaceae: distribution, morphology, origin, and latex composition. Protoplasma 257, 1183–1199 (2020). https://doi.org/10.1007/s00709-020-01500-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01500-5